* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organometallics II
Ring-closing metathesis wikipedia , lookup
Bottromycin wikipedia , lookup
Discodermolide wikipedia , lookup
Aldol reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Elias James Corey wikipedia , lookup
Petasis reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Stille reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Kinetic resolution wikipedia , lookup
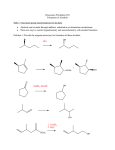
Grignard reagents act as nucleophiles toward the carbonyl group δ– Synthesis of Alcohols R diethyl ether δ+ C R + • • MgX •O• •• – MgX O •• •• Grignard or Organolithium Reagents C H3O+ Organolithium reagents react with carbonyls in the same general way that Grignard reagents do. two-step sequence gives an alcohol as the isolated product Grignard reagents react with: R C • OH • •• Grignard reagents react with: formaldehyde to give primary alcohols formaldehyde to give primary alcohols other aldehydes to give secondary alcohols ketones to give tertiary alcohols esters to give tertiary alcohols Example Grignard reagents react with formaldehyde δ– H R δ+ H C diethyl ether Mg H R C •• O •• •• – MgX O •• •• Cl H + MgCl H MgX H3O+ product is a primary alcohol diethyl ether C H R C • OH • •• O H H CH2OH (64-69%) H3O+ CH2OMgCl Grignard reagents react with: Grignard reagents react with aldehydes formaldehyde to give primary alcohols H δ– R aldehydes to give secondary alcohols δ+ R' C diethyl ether H R C R' + MgX •• O •• •• – MgX O •• •• H3O+ product is a secondary alcohol H R C • OH • •• Example Question Mg CH3(CH2)4CH2Br CH3(CH2)4CH2MgBr diethyl ether H 3C C O H CH3(CH2)4CH2CHCH3 H3O+ CH3(CH2)4CH2CHCH3 OH OMgBr • What is the product of the reaction of ethylmagnesium bromide (CH3CH2MgBr) with butanal (CH3CH2CH2CH=O) followed by dilute acid? • A) 2-hexanol • B) 1-butanol • C) 3-hexanol • D) 3-pentanol (84%) Example H 2C O CHLi + CH 1. diethyl ether 2. H3O+ CHCH OH (76%) CH2 Grignard reagents react with: formaldehyde to give primary alcohols aldehydes to give secondary alcohols ketones to give tertiary alcohols R' Grignard reagents react with ketones R" δ– R δ+ R' C diethyl ether Question R" R C + •• O •• •• – MgX O •• •• • What is the product of the reaction shown at the right? R' MgX H3O+ product is a tertiary alcohol R" R C R' • A) B) • C) D) • OH • •• Example Question Mg CH3Cl HO CH3 diethyl ether H3O+ CH3MgCl • Two alcohols, each having the molecular formula C11H22O, are formed in the reaction of methyl lithium with 3-(R)-tertbutylcyclohexanone. These two alcohols are • A) constitutional isomers. • B) enantiomers in equal amounts. • C) enantiomers in unequal amounts. • D) diastereomers. O ClMgO CH3 (62%) Remember: Sodium Salts of Acetylenes NaNH 2 HC CH Synthesis of Acetylenic Alcohols HC NH3 CNa O HC CNa + HO C 1. NH3 2. H3O+ (65-75%) CH Using Acetylenic Grignard Reagents Question CH + CH3CH2MgBr MgBr CH3(CH2)3C • What compound will be obtained from the reaction shown below? diethyl ether CH3(CH2)3C • • A) B) • C) D) + CH3CH3 CMgBr MgBr 1. H2C O 2. H3O+ CH3(CH2)3C CCH2OH (82%) Question • Which one of the compounds below will be produced when methylmagnesium bromide is added to propanoic acid (CH3CH2CO2H)? • A) CH3CH2COCH3 • B) CH3CH2CO2-MgBr • C) CH3CH2CH(OH)CH3 • D) CH3CH2CH2CH3 Preparation of Tertiary Alcohols From Esters and Grignard Reagents Grignard reagents react with esters δ– R' R •• δ+ OCH3 •• C MgX O •• •• diethyl ether Grignard reagents react with esters R' R C •• OCH3 •• • O • + MgX • • •• – but species formed is unstable and dissociates under the reaction conditions to form a ketone δ– R' R •• δ+ OCH3 •• C R' diethyl ether R MgX O •• •• this ketone then goes on to react with a second mole of the Grignard reagent to give a tertiary alcohol C •• OCH3 •• • O • + MgX • • •• – –CH3OMgX R C O •• •• R' Example O CHCOCH3 2 CH3MgBr + (CH3)2CHCOCH 1. diethyl ether 2. H3O+ OH (CH3)2CHCCH CHCCH3 CH3 (73%) Two of the groups attached to the tertiary carbon come from the Grignard reagent Question • Which one of the compounds below will be produced when methylmagnesium bromide is added to methyl propanoate (CH3CH2CO2CH3) followed by acid? • A) CH3CH2COCH3 • B) CH3CH2CO2-MgBr • C) CH3CH2CH(OH)CH3 • D) CH3CH2CH2CH3 • E) 2-methyl-2-butanol







![Group Activity 3 [10 PTS]](http://s1.studyres.com/store/data/010780770_1-3445600a9b56e890a0f283c789afe8fb-150x150.png)