* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ultrastructure of cell types of the olfactory epithelium in a catfish
Tissue engineering wikipedia , lookup
Cell membrane wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Programmed cell death wikipedia , lookup
Cell growth wikipedia , lookup
Signal transduction wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Endomembrane system wikipedia , lookup
Cytokinesis wikipedia , lookup
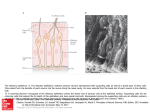
J. Biosci., Vol. 22, Number 2, March 1997, pp 233– 245. © Printed in India. Ultrastructure of cell types of the olfactory epithelium in a catfish, Heteropneustesfossilis (Bloch) N C DATTA* and SHOVAN BANDOPADHYAY Fishery and Ecology Research Unit, Department of Calcutta, 35, Ballygunge Circular Road, Calcutta 700019, India Zoology, University of MS received 21 May 1996; revised 19 December 1996 Abstract. Transmission electron microscopical study of olfactory epithelium of a mud-dwelling catfish, Heteropneustes fossilis (Bloch) shows receptor, supporting, goblet and basal cells. The receptor cells are of ciliated and microvillous type. Both ciliated and microvillous receptor cells are provided with olfactory knob. The dendrite of all the receptor cells bears many longitudinally arranged microtubules. Occurrence of the rod cell and its function is quite debatable. Specialized juctional complexes between the receptor and adjacent cells are clearly noted. The supporting cells are both ciliated and nonciliated. The ciliated supporting cells are responsible for water ventilation in the olfactory chamber as well as in the inter-lamellar spaces. This facilitates better perception of odours by the receptor cells. In addition to providing mechanical support to other cells, the nonciliated supporting cells also have a secretory function which is evident from the present study. The different stages of maturity of goblet cells are well documented. The presence of white cells in the olfactory epithelium is a very rare finding. Keywords. Catfish; olfactory epithelium; receptor cell; rod cell; white cell. Introduction Heteropneustes fossilis (Bloch) is primarily a mud dwelling fish of the freshwater bodies of Indian subcontinent. D ue to this peculiar habitat, its dependence on chemoreceptors is obvious. Literature survey indicates that a number of workers (Trujillo Cenoz 1961; Bannister 1965; Kleerekoper 1969; Theisen 1972; Jakubowski 1981; Zielinski and H ara 1992; Zeiske et al 1992; Singh et al 1995) have described different cell types of the olfactory epithelium in a number of fishes through the transmission electron microscopy (TEM). The present study portrays the Ultrastructure of the cell types in the olfactory epithelium of H. fossilis and an attempt has also been made to ascribe the possible function of the cells. Materials and methods Previously acclimatized in the laboratory, adult specimens of H. fossilis (OrderSiluriformes, Family Heteropneustidae) measuring from 15-20 cm long were taken *Corresponding author. 233 234 N C Datta and Shovan K Bandyopadhyay for TEM study. After dissecting out the olfactory rosettes, the lamellae were severed into small pieces and fixed in 2.5% ice cold glutaraldehyde buffered at 7.4 pH with 0·1 phosphate buffer for two hours. After rinsing with the same buffer the tissues were further fixed in 1% OsO4 in 0·1 phosphate buffer (pH 7·4) for l h at 4°C. During dehydration, pre staining of the tissues was done in saturated solution of uranyl acetate in 70% alcohol for 15 min. Ultrathin sections of embedded tissues were cut with a diamond knife on LKB Nova ultrotome and were then contrasted with uranyl acetate for 5 min and lead citrate for 6 min. The sections were examined in JEOL 100 CX transmission electron microscope operated at emissive mood at 60 KV. The details of the structural components of various cell types were then studied and described. 3. Results The olfactory epithelium of H. fossilis is pseudostratified and therefore the receptor, supporting, goblet and basal cells are not aligned in the olfactory mucosa.The unusual white cells (Evans et al 1982) are scantly distributed at the middle position of the epithelium. The receptor cells are morphologically differentiated into ciliated and microvillous type. A peculiar rod cell is also present sporadically in the mucosa. Two types of supporting cells are distinguished: nonciliated and ciliated type (figure 1). 3.1 Receptor cell The receptor cells are typical bipolar neurons. The free surface of the dendrite bulges out to form a small flask shaped terminal swelling called the olfactory knob (figure 2), which is more prominent in the microvillous neuron (figure 3). The microvilli on the olfactory knob range from 0·1 um to 0·15 µm in diameter. The olfactory knob of ciliated receptor cell distally bears cilia which show the typical 9 + 2 arrangement of the microtubules. in cross section (figure 2). The diameter of the cilia is approximately 0·3 µm to 0·4 µm. The basal body of the cilia is clearly discernible. The length of both cilia and microvilli could not be measured because full length of them were not available in the section. The dendrites of the receptor cells are elongated and their width usually increase gradually towards the nucleus. The dendritic regions are mostly separated from each other by the supporting cells. The axon of the receptor cells does not extend towards the basement membrane, instead it passes obliquely through the intercellular spaces between the supporting and basal cells (figure 1). The dendrite contains some logitudinally arranged microtubules (figure 6). The nuclei of the receptor cells are usually deeply stained and situated in the lower third of the epithelium towards basement membrane. These are oval to elongated in shape (figure 7). The heterochromatin material remains scattered and also distributed along the periphery of the nucleus. Mitochondria are mostly aggregated at the apical part of the dendrite. The shape varies from round to oblong. Free ribosomes and golgi bodies are observed. The junctional complex appears to be zonula occludens and present between receptor and adjacent supporting cells (figures 2,3). Ultrastructure of olfactory epithelium of H. fossilis 235 Figure 1. H. fossilis. A diagrammatic view of the olfactory epithelium showing different types of cell, bb, basal body; be, basal cell; bm, basement membrane; c, cilium; cf, collagen fibre; crc, ciliated receptor cells; esc, ciliated supporting cell; edp, electron dense particle; eov, electron opaque vesicle; g, golgi complex; gc, goblet cell; id, interdigitation; jc, junctional complex; m, mitochondria; mrc, microvillous receptor cell; mt, microtubules; mv, microvillous; nsc, nonciliated supporting cell; ok, olfactory knob; r, rod; rer, rough endoplasmic reticulum; re, rod cell; ser, smooth endoplasmic reticulum; sv, secretory vesicle; tm, translucent microvesicles; we, white cell. 3.2 Rod cell The rod cell is occasionally observed in the sensory area of the lamellae. A firm rod like cilia occupies almost the entire free margin (figure 4) of the cell. The base of the rod is wide and the diameter is about 1·15 µm. The rod gradually becomes pointed towards the free end.The length of the rod is 8·15 µm approximately. It contains a number of parallely oriented microtubules along its long axis (figure 5). The arrangement of these 236 N C Datta and Shovan K Bandyopadhyay Figures 2-5. (2) TEM of surface region of the olfactory epithelium in longitudinal section. Arrow shows the cross section of the cilia and also the discharging of the secretory products. (Bar = 0·5 µm). (3) TEM of surface region of the olfactory epithelium in longitudinal section. (Bar = 1 µm). (b, basal body; c, cilia; cr, ciliated receptor cell; eov, electron opaque vesicle; m, microvilli; mr, microvillous receptor cell; ok, olfactory knob; sc, supporting cell; z, zonula occludens). (4) TEM of the olfactory epithelium in oblique section showing rod cell (rc) with a rod (r). (Bar = 4 µm). (5) A magnified view of the base of the rod. Arrows indicate the microtubules. (Bar = 0 5 µm). Ultrastructure of olfactory epithelium of H. fossilis 237 Figure 6. TEM of the dendrite of microvillous receptor cell (mr) surrounded by supporting cell (sc). Arrows indicate the microtubules. (Bar = 1 µm). microtubules could not be ascertained because the transverse sectional view of the rod could not be encountered in the section. The basal bodies of the cilia are very prominent. The upper cytoplasmic part of the cell resembles dendritic extensions of the receptor cells. The entire cell takes a deep stain. Aggregation of mitochondria at this area is also prevalent. The nucleus is placed at the lower third of the cell. Granulated endoplasmic reticulum is present near the nucleus. Free ribosomes are scattered in the cytoplasm. The junctional complex form a firm attachment with the adjacent cell. 3 3 Supporting cell The nonciliated supporting cells are fairly distributed in the olfactory epithelium. The cells are columnar which extend from epithelial free margin to the basement membrane. Usually the cells are broad at the apical region although some narrow cells are also discernible (figures 1, 2, 8). The distal surface is flat and covered with some irregular small projections. 238 N C Datta and Shovan K Bandyopadhyay Figures 7-11. (7) TEM showing elongated nucleus of receptor cell. (Bar = 1 µm). (8) TEM shows a broad supporting cell (sc) with endoplasmic reticulum over the nucleus. (Bar = 1 µm). (9) TEM of the longitudinal section of the olfactory epithelium showing ciliated supporting cell. (Bar = 1 µm). (10) Apical margin of the ciliated supporting cell. Arrows indicate the translucent microvesicles. (Bar = 1 µm). (11) Longitudinal section of the apical region of the olfactory epithelium showing the immature goblet cell. (Bar = 1·5 µm). (g, golgi complex; id, interdigitation). Ultrastructure of olfactory epithelium of H. fossilis 239 The nucleus is almost round and placed at the lower third area of the cell (figures 1, 8). The amount of euchromatin is comparatively more than that of heterochromatin and the latter is disposed more near the nuclear envelope. Mitochondria of various shapes are heaped at the apical region of the cell which consist of a dense matrix with compact cristae. The endoplasmic reticulum is arranged in the supranuclear region. Some electron opaque vesicles are present in the marginal area of some of these cells (figures 1, 2). The size of the vesicles varies approximately from 0·4 µm to 0·7 µm. These vesicles possess some secretory granules that are released from time to time (figure 2). The ciliated supporting cells are mainly distributed in the middle region of the lamella. These cells are also columnar and the free broad end is provided with a number of cilia (figures 1, 9). The length of the cilia could not be measured but the diameter is about 0·25 µm. These are typical kinocilia possessing 9 + 2 axial pattern of microtubules. The basal bodies are present but the rootlets are not observed. A few translucent micro vesicles with smooth surface are scattered at the apical margin of the cell (figure 10). The nucleus is oval with well marked heterochromatin. Mitochondria are distributed at the apical region of the cell and the shape varies from round to elongate. 3.4 Goblet cell These cells are distributed in the distal part of the lamella as well as on the surface layer of raphe. Immature and mature stages of goblet cells have been identified. In the immature stage the cell has a prominent golgi complex at the supranuclear region Figure 12. TEM of a portion of mature goblet cell showing interdigitation (id) and huge secretory vesicle (sv). (Bar = 1 µm). 240 N C Datta and Shovan K Bandyopadhyay (figure 11). The vesicles with the secretion move towards the free end of the cell and gradually become accumulated giving a globoid shape to the cell (figures 1,12). Due to heavy accumulation of the secretory granules the nucleus along with other cell organelles are pushed towards the bottom of the cell. The nucleus is oval. At the subsurface area the interdigitation of the cell membrane of both immature and mature goblet cells with the adjacent supporting cell is quite prominent (figures 1,11,12). 3.5 Basal cell Basal cells are loosely arranged at the base of the olfactory epithelium and form a discontinuous layer over the basement membrane. These cells make way to the axons of the receptor cells to reach the basement membrane. The cells are usually round, oval or pearshaped (figure 13) and remain interspersed with the bases of receptor, supporting and goblet cells. Nucleus is also of various shapes. Cytoplasm is dense with scattered mitochondria, rough endoplasmic reticulum and golgi complex (figure 14). 3.6 White cell It is the most weakly stained cell present in the epithelium intervening the supporting cell. The cell is polygonal (figures 1,15). The nucleus is comparatively large with well defined nuclear membrane. The nucleolus is enlarged and dense but the karyoplasmic particles are very scanty. The cytoplasmic granules are also less. The mitochondria are swollen and cristae are in degenerated condition. The endoplasmic reticulum is dialated and shows signs of deterioration (figure 15). 4. Discussion The ciliated and microvillous receptor cells together with rod cell rarely occur in an olfactory epithelium in H. fossilis. Yamamoto and Ueda (1977, 1978) reported the occurrence of three types of cell in salmon and in a catfish, Parasilurus asotus. The identity of rod cell as a receptor neuron has been questioned by Ichikawa and Ueda (1977) as the cells were found unaffected by the olfactory nerve transection. On the contrary, Yamamoto and Ueda (1979a, b) suggested that the rod cell is a transformed ciliated neuron (type 2 ciliated cell) because they observed intermediate form in Cheilopogon agoo and three species of Perciformes. In a recent study Singh et al (1995) regarded the rod cell as the receptor cell in Schizothoraichthys progastus and Schizothorax richardsonii. They have also observed the intermediate stage of rod cell. The scarcity of the rod cell in the sensory epithelium ofH. fossilis indicates that it is not a regular type of cell. From the available literature it is evident that the formation of the rod may be due to the result of fusion of cilia of ciliated neuron. According to Hernadi (1993) the density of rod cell increases after increased C d2+ exposure which probably indicates that the environmental factor regulates the density of this kind of cell. Rowley and Moran (1985) were also of the opinion that the rod cells are the artefacts of ciliated receptor cell and such transformation is either due to water pollution or fixation. H. fossilis, a mud dweller, may have to encounter the environmental stress which may necessitate the formation of the rod cell. Ultrastructure of olfactory epithelium of H. fossilis Figures 13-15. (13) A basal region of the olfactory epithelium. (Bar = 2 µm). (bc, basal cell; bm, basement membrane). (14) A magnified portion of the basal cell. Arrows show the rough endoplasmic reticulum. (Bar = 0·5 µm). (15) TEM of a white cell in the olfactory epithelium. (Bar= 1µm). (er, endoplasmic reticulum; m, mitochondria; , nucleus; nl, nucleolus; nm, nuclear membrane). 241 242 N C Datta and Shovan K Bandyopadhyay Earlier Thornhill (1967) and Wilson and Westerman (1967) believed it as a degenerating sensory cell. Theisen et al (1980) with SEM and TEM micrographs showed some thick protrusions combining with the cilia in the sensory epithelium of Belone belone. These protrusions simulate rod cell and they (op. cit.) considered this cell as a degenerated one. But the present study puts a question to its degenerated status because it has been observed that the rod cell have well defined nucleus, rough endoplasmic reticulum and densely populated mitochondria indicating its functional state, although its real function is yet to be ascertained. As in other catfishes (Yamamoto and Ueda 1978; Erickson and Caprio 1984; Theisen et al 1991) the microvillous and ciliated receptor cells are distributed in the sensory area of the olfactory epithelium ofH. fossilis. The independency of ciliated and microvillous receptors is now established through a developmental study in rainbow trout by Zielinski and Hara (1988). They stated that the ciliated receptor cell ontogenetically precedes the microvular receptor. But previously Theisen et al (1986) mentioned that the ciliated and microvillous receptor cell develop from identical stem cell and the development of the centrioles is arrested in microvillous receptors. In H. fossilis no such centrioles is discernible in microvillous receptors. Hence it can be stated that ciliated and microvillous neurons develop separately from undifferentiated cell and also function separately. It is supported by the fact that in some teleosts the ciliated receptors are altogether absent (Bannister 1965). In H. fossilis all the receptors have microtubules in their dendrite. Such a condition has also been reported in many fishes (Andres 1969; Theisen 1972). These microtubules are probably responsible for maintaining the shape of the dendritic process and channalizing different transport materials to the particular site. However, Behnke and Forer (1967) suggested its supportive function and involvement in cytoplasmic streaming. It is generally accepted that the olfactory receptor macromolecules are associated with the membrane of cilia (Rhein and Cagan 1981; Hara 1992). In H. fossilis it is evident that not only the cilia and microvilli but also the membrane of the olfactory knob has the receptor site. This view is supported by the finding of H ara and Zielinski (1989) that the olfactory knob develops earlier than the cilia or microvilli. The mechanical support to the surrounding receptor cell by the supporting cell is now an established phenomena. But in H. fossilis some vesicles filled with secretory granules suggest the secretory function of the supporting cell. The micrograph clearly shows the discharging of the secretory material (figure 2). The secretory function of the supporting cell was also proposed earlier by Thornhill (1967), Bertmar (1973) and Zeiske et al (1979), although Yamamoto (1982) had a doubt on such function due to lack of evidence. The ciliated supporting cell in H. fossilis can easily be distinguished from the ciliated receptor cell as it does not have any olfactory knob. The long cilia are inclined at the same direction indicating their role in water ventilation in the olfactory chamber as well as in the interlamellar spaces which obviously promotes better odour perception by the receptor cells. Aggregation of mitochondria at the apical region of the cell indicates that they are providing the required energy for the movement of cilia. The immature and mature state of goblet cells of H. fossilis have been clearly identified. The interdigitation of goblet cell with the adjacent supporting cell helps it to be in its position firmly during profuse secretion. But it is interesting to note that no report of such type of interdigitation is available. However, Thornhill (1967) mentioned the interdigitation of supporting cell with basement membrane, other supporting cell, Ultrastructure of olfactory epithelium of H. fossilis 243 basal or sensory cell. Gemne and Doving (1969) and Zeiske et al (1979) reported the interdigitation between supporting and receptor cell. Regarding the function, Hornung and Mozell (1981) proposed that the secretion helps in facilitating the odorant removal. In H. fossilis, as it is a mud dweller the secretion of the goblet cell helps in binding the microscopic debris that enter in the olfactory chamber with water current and finally it exit through the nasal aperture. It also helps in decreasing the friction of water in the chamber as well as protecting the epithelium from coming in contact with the hazardous material to some extent. The junctional complex in H. fossilis, the zonula occludens is prominent between receptor and supporting cell which is also reported by Gemne and Doving (1969) and Theisen (1972). Thornhill (1967) mentioned the junctional complex in lamprey as zonula adherens and stated it acts as a barrier to prevent the influx and eflux of substances into and out of the interlamellar spaces. But Gemne and Doving (1969) stated that the "Z onula occludentes" protects the epithelial lining in the fresh water fish, Lota lota against the osmotic pressure. The basal cell is believed as the progenitor of receptor or the supporting cell as its mitotic condition is observed by Breucker et al (1979) and Zeiske et al (1992). The rough endoplasmic reticulum and mitochondria also support its functional state. Occurrence of white cell is reported by Evans et al (1982) in the neuroepithelium of rainbow trout. A similar kind of cell named X cell is also described by Peters et al (1984) in the epithelial tumour tissue of Platichthys stellatus. Although the microanatomical description of the X cell is very similar to that of the white cell, it is assumed that the white cell is quite different from the X cell. This is because the X cells are densely distributed in the epithelial tumours (Peters et al 1984) where as the white cell is scarcely found in the olfactory epithelium ofH. fossilis. As all its organelles are found in degenerating condition it is obviously a degenerating cell. However, presence of degenerating cell in the olfactory epithelium of teleosts and other vertebrates is a very common feature as mentioned by Thornhill (1967) and Bertmar (1973) which is corroborated by the present study. Acknowledgements Thanks are due to Dr (Mrs) S Majumdar and Mr Sailen Dey, Biophysics Division, Indian Institute of Chemical Biology, Calcutta for the technical assistance in the transmission electron microscopical study. Prof. S G Pal and Dr Biswas, Biophysics Laboratory of the Calcutta University also deserve thanks for valuable suggestions. References Andres K H 1969 Der olfaktorische Saum der Katze; . Zellforsch. 96 250 274 Bannister L H 1965 The fine structure of the olfactory surface of teleostean fishes; Q. J- . Microsc, Sci. 106 333 342 Behnke O and F orer A 1967 Evidence for four classes of micro tubules in individual cells; J. Cell Sci. 2 169 192 Bertmar G 1973 U ltrastructure of the olfactory mucosa in the homing Baltic Sea trout Salmo trutta trutta; Mar. Biol. 19 74 88 Breucker H, Zeiske and Melinkat R 1979 Development of the olfactory organ in the rainbow fish Nematocentris maccullochi (Atheriniformes, Melanotaeniidae); Cell Tissue Res. 200 53 68 244 N C Datta and Shovan K Bandyopadhyay Erickson J R and Caprio J 1984 The spatial distribution of ciliated and microvillous olfactory receptor neurons in the channel catfish is not matched by a differential specificity to amino acid and bile salt stimuli; Chem. Sens. 9 127-142 Evans R E, Zielinski Β and Hara Τ J 1982 Development and regeneration of the olfactory organ in rainbow trout; in Chemoreception in fishes (ed.) Τ J Hara (Amsterdam: Elsevier) pp 15-38 Gemne G and Doving Κ Β 1969 Ultrastructural properties of primary olfactory neurons in fish, Lota lota L Am. J. Anat. 126 457-476 Hara Τ J (ed.) 1992 Mechanism of olfaction; in Fish chemoreception (London: Chapman and Hall) pp 150-170 Hara Τ J and Zielinski Β 1989 structural and functional development of the olfactory orgon in teleosts; Trans. Am. Fish. Soc. 118 183-194 Hernadi L 1993 Fine structural characterisation of the olfactory epithelium and its response to divalent cations cadmium in the fish Alburnus alburnus (teleostei, Cyprinidae): A Scanning and transmission electron microscopic study; Neurobiology (BP) 1 11-31 Hornung D Ε and Mozell Μ Μ 1981 Accessibility of odorant molecules to the receptors; in Biochemistry of taste and olfaction (eds) R Η Cagan and Μ R Kare (New York: Academic Press) pp 33-45 Ichikawa Μ and Ueda Κ1977 Fine structure of the olfactory epithelium in goldfish, Carassius auratus: Study of retrograde degeneration; Cell Tissue Res. 183 445-455 Jakubowski Μ 1981 Ultrastructure scanning electron microscopy, transmission electron microscopy of the olfactory epithelium in the weis, Silurus glanis L. (Siluridae, Pisces); Z. Mikrosk. Ant. Forsch. 95 337-352 Kleerekoper Η 1969 Olfaction in fishes (Bloomington: Indiana Univ. Press) pp 222 Peters Ν, Schmidt W, Kranz Η, Watermann Β and Stich Η F1984 Das X-Zellen-Problem; Mitt. Hamb. Zool. Mus. Inst. 80 53-65 Rhein L D and Cagan R Η 1981 Role of cilia in olfactory recognition; in Biochemistry of taste and olfaction (eds) R Η Cagan and Μ R Kare (New York: Academic Press) pp 47-68 Rowley J C and Moran D Τ 1985 HRP applied to transected trout olfactory nerves fills ciliated receptors, microvillar receptors and some basal cells; Chem. Sens. 10 392-393 Singh N, Bhatt Κ C, Bahuguna Mukesh Κ and Kumar Dharmendra 1995 Fine structure of olfactory epithelium in Schizothoraichthys progastus McClelland and Schizothorax richardsonii Gray (Cyprinidae: Teleostei) from Garhwal Himalaya (India); J. Biosci. 20 385-396 Theisen Β 1972 Ultrastructure of the olfactory epithelium in the Australian lungfish Νeoceratodus forsteri; , Acta Zool. (Stockholm) 53 205-218 Theisen Β, Breucker Η, Zeiske Ε and Melinkat R 1980 Structure and development of the olfactory organ in the garfish Belone belone (L) (Teleostei, Atheriniformes); Acta Zool. (Stockholm) 61 161-170 Theisen Β, Zeiske Ε and Breucker Η 1986 Functional morphology of the olfactory organs in the spiny dogfish (Squalus acanthias L) and the small-spotted catshark (Scyliorhinus canicula (L); Acta Zool. (Stockholm) 67 73-86 Theisen Β, Zeiske Ε, Silver W L, Marui Τ and Caprio J 1991 Morphological and physiological studies on the olfactory organ of the striped eel catfish, Plotosus lineatus; Mar. Biol 110 127-135 Thornhill R A 1967 The Ultrastructure of the olfactory epithelium of the lamprey Lampetrafluviatilis; J. Cell Sci. 2 591-602 Trujillo-Cenoz Ο 1961 Electron microscope observations on chemo and mechano receptor cells of fishes; Z. Zellforsch. 54 654-676 Wilson J A F and Westerman R A 1967 The fine structure of the olfactory mucosa and nerve in the teleosts CarassiuscarassiusL;Ζ.Zellforsch. 83196-206 Yamamoto Μ 1982 Comparative morphology of the peripheral olfactory organ in teleosts; in Chemoreception in fishes (ed.) Τ J Hara (Amsterdam: Elsevier) pp 39-60 Yamamoto Μ and Ueda Κ 1977 Comparative morphology offish olfactory epithelium. I Salmoniformes; Bull Jpn. Soc. Sci. Fish. 43 1163-1174 Yamamoto Μ and Ueda Κ 1978 Comparative morphology of fish olfactory epithelium. VI Siluriformes; Zool. Mag. (Tokyo) 87 254-261 Yamamoto Μ and Ueda Κ 1979a Comparative morphology of fish olfactory epithelium. VIII Atheriniformes; Zool Mag. (Tokyo) 88 155-164 Yamamoto Μ and Ueda Κ 1979b Comparative morphology offish olfactory epithelium. IX Tetraodontiformes; Zool. Mag. (Tokyo) 88 210-218 Zeiske Ε, Breucker Η and Melinkat R 1979 Gross morphology and fine structure of the olfactory organ of rainbow fish (Atheriniformes, Melanotaeniidae); Acta Zool. (Stockholm) 60 173-186 Ultrastructure of olfactory epithelium of H. fossilis 245 Zeiske E, Theisen Β and Breucker Η 1992 Structure, development and evolutionary aspects of the peripheral olfactory system; in Fish chemoreception (ed.) Τ J Hara (London: Chapman and Hall) pp 13-39 Zielinski Β and Hara Τ J 1988 Morphological and physiological development of olfactory receptor cells in rainbow trout (Salmo gairdneri) embryos; J. Comp. Neurol. 271 300-311 Zielinski Β and Hara Τ J 1992 Ciliated and microvillar receptor cells degenerate and then differentiate in olfactory epithelium of rainbow trout following olfactory nerve section; Microsc. Res. Tech. 23 22-27 Corresponding editor: SAMIR BHATTACHARYA