* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF Mynark - American Kinesiology Association
Nonsynaptic plasticity wikipedia , lookup
Neuroregeneration wikipedia , lookup
Neural engineering wikipedia , lookup
Embodied language processing wikipedia , lookup
Development of the nervous system wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroscience in space wikipedia , lookup
Proprioception wikipedia , lookup
End-plate potential wikipedia , lookup
Evoked potential wikipedia , lookup
Electromyography wikipedia , lookup
Central pattern generator wikipedia , lookup
Muscle memory wikipedia , lookup
Aging brain wikipedia , lookup
Microneurography wikipedia , lookup
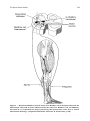
188 INVITED REVIEW Mynark and Koceja JOURNAL OF APPLIED BIOMECHANICS, 2001, 17, 188-203 © 2001 by Human Kinetics Publishers, Inc. Effects of Age on the Spinal Stretch Reflex Richard G. Mynark and David M. Koceja The spinal stretch reflex consists of a relatively simple neuronal network. The Ia afferent fiber of the muscle spindle communicates to the alpha motoneuron via a single synapse. This basic pathway has been studied extensively over the past century, yet considerable information continues to emerge concerning the manner in which this pathway adapts to aging. It is well accepted that the amplitude of the spinal stretch reflex declines with normal aging, and it is intuitively agreed that these changes have a detrimental impact on the motor output of aging individuals. Understanding the changes observed in the spinal stretch reflex pathway due to aging requires a recognition of the changes that can occur in each component of this spinal network. This review will address these changes by following the spinal stretch reflex from initiation to completion. The components that result in the sensory input to the motoneuron will be covered first, followed by a review of the physiological changes that can occur to the motoneuron soma that can affect the processing of the sensory input. The output of the motoneuron encompasses the remaining components from the motor axon itself, to the neuromuscular junction, and then to the characteristic changes in the muscle. Finally, the functional effect that these changes have on the reflex as a fundamental motor behavior will be addressed, especially in terms of its impact on posture and balance. Key Words: aging, spinal cord, neuromuscular Introduction Understanding the changes observed in the spinal stretch reflex due to aging requires a recognition of the changes that can occur in each component of the reflex. The spinal stretch reflex is elicited when the mechanical stretch of a tendon or muscle is detected by the muscle spindle. The amplitude and rate of that length change is coded and relayed to the central nervous system by way of the Ia afferent pathway. This information is passed monosynaptically to the homonymous alpha-motoneuron pool. This powerful stimulus usually leads to the initiation of a motor volley to the neuromuscular junction, resulting in a corrective contraction of the stretched muscle. This circuitry is shown in Figure 1. R.G. Mynark is with the Dept. of Exercise and Sport Science, University of North Carolina, Chapel Hill, NC 27599; D.M. Koceja is with the Motor Control Laboratory, Dept. of Kinesiology, Indiana University, Bloomington, IN 47405. 188 The Spinal Stretch Reflex 189 Figure 1 — Spinal stretch reflex pathway, depictinjg the muscle spindle afferent neuron and the monosynaptic connection to the alpha motoneuron. (Adapted from Barr & Kiernan, 1983, p. 76) Two basic experimental designs have been used to stimulate and measure the spinal stretch reflex (SSR) arc: (a) the traditional tendon-tap methodology and (b) the Hoffmann or H-reflex. Measuring the SSR via the tendon-tap involves a mechanical perturbation to the tendon of the target muscle. EMG activity can be recorded as a result of the increased Ia fiber activity, and the resultant muscle contraction elicited by this perturbation. The methodology of the tendon-tap reflex is shown in Figure 2. Measurement of the SSR via the H-reflex involves an electrical stimulation of the Ia fibers. The reflex-elicited contraction of the muscle, as a result of electrical stimulation of the Ia fibers, provides insight into the central mechanisms that mediate motoneuron excitability, independent of the peripheral muscle spindle receptor. The methodology of the H-reflex is also shown in Figure 2. A classic review of the Hreflex has been provided by Schieppati (1987). As we will demonstrate in this review, the ability to assess the output characteristics of a given motoneuron pool without including the age-confounded input characteristics of the muscle spindle and musculotendinous structures is very important. Therefore, the H-reflex provides a much clearer picture of the effects of aging on the central components of motor control, while the SSR provides a more global view. When the two are used together, they can provide a more accurate estimate of whether changes are occurring centrally, peripherally, or both. 190 Mynark and Koceja Figure 2 — Measurement of the spinal stretch reflex pathway. (A) Tendon-tap paradigm: by inducing a tap to the appropriate tendon, the resulting EMG activity of the muscle of the target motor-pool can be assessed using surface EMG analysis. (B) H-reflex paradigm: by passing a 1-ms electrical stimulus across the nerve, the afferent fibers from the muscle spindle can be activated, resulting in EMG activity from the target motor pool. Note that the H-reflex paradigm bypasses the muscle spindle apparatus and results in a more synchronous stimulus arriving in the motor pool. (Adapted from Sage, 1997, p. 155) While these components form a very simple reflex arc, the effects of aging on any one component can potentially affect the quality of information passed to all following components. Therefore, in order to better understand the output of the whole reflex arc, it is necessary to evaluate the changes due to aging that have been demonstrated in these components. This review will address these changes by following the spinal stretch reflex from its initiation to its completion. Input Physiology Musculotendinous Structure With aging, there is a general change in the connective tissue structure of the muscles and tendons. Since it is the deformation of these structures that functionally elicits the spinal stretch reflex, any changes to the tissues themselves will affect the sensory input. The primary change to the structural quality of the connective tissue with aging is a general stiffening or loss of compliance (Nielsen, Shalicky, & Viidik, 1998), leading to decreased range of motion (Gajdosik, Vander Linden, & Williams, 1999). One possible explanation for this change is the observed decrease in metabolic activity within the tendon over time (Almekinders & Deol, 1999). Furthermore, aging has been found to significantly decrease tendon glycosaminoglycans and increase Type I collagen concentration (Vailas, Pedrini, Pedrini-Mille, & Holloszy, 1985). These structural changes within the tendon and connective tissue of the muscle stiffen the medium for transmitting the mechanical stimulus that elicits the spinal stretch reflex. The Spinal Stretch Reflex 191 Muscle Spindles Age related changes to the muscle spindles can take two forms: structural and neural. Both of these changes may affect the sensitivity of the spindle. Changes to the structural physiology of the spindle itself can be seen in increases in capsular thickness (Miwa, Miwa, & Kanda, 1995; Swash & Fox, 1972). This thickening is due to an increase in laminar collagen and can impair the muscle spindle’s ability to deform, leading to a decrease in sensitivity to transmitted stretch. Furthermore, decreases in the number of intrafusal muscle fibers (Swash & Fox, 1972) can decrease the speed and accuracy with which the spindle can respond to gamma-motoneuron mediated adjustments in spindle sensitivity (gamma drive). This may increase the time it takes to respond to muscle length changes. Finally, the innervation of the intrafusal fibers by gamma motoneurons can suffer the same degenerative changes as suffered by the extrafusal/alpha motoneuron complex. With regard to the static and dynamic nature of the muscle spindle, Miwa et al. (1995) have demonstrated that while static sensitivity of the rat muscle spindle did not change with age, there was a decrease in the discharge frequency of the muscle spindle. This decrease in discharge frequency may result in a weaker reflex response. In contrast, whereas static sensitivity was found not to be affected by age, dynamic sensitivity was largely decreased with aging (Miwa et al., 1995). This decrease in sensitivity could adversely affect the ability of the spinal stretch reflex to respond to various postural demands. This deficit, combined with the age related decrease in muscular strength in the elderly, may increase the likelihood of falling. The second possible source of age related changes to spindle sensitivity is a result of supraspinally mediated changes in the gamma drive to the spindles themselves. Changes in the “set” of muscle spindles will have a direct effect on the sensitivity and should be considered as a possible contributor to age related output changes. However, there has been no effort to date that has quantified differences in gamma drive between young and elderly populations. Ia Afferent Pathway The Ia afferent pathway forms the first step in transmitting the coded information from the muscle spindles to the central nervous system. Therefore, any age related degeneration of this pathway can potentially diminish the response of the spinal stretch reflex. Even before the Ia afferent exits the spindle capsule, diminished responses can occur by virtue of the degradation of the mechanical connection between the Ia afferent end organ and the spindle bag fibers (Miwa et al., 1995). Several age related changes to the sensory axon have been reported. The greatest impact of aging has been observed in the degeneration of the myelin sheath. The prevalence of demyelination has been noted to increase progressively with age (Ludatscher, Silbermann, Gershon, & Reznick, 1985). This process leads to a decrease in axonal integrity as the interaction between the axon membrane becomes increasingly in greater contact with damaging substances in the extracellular matrix. Decreased axonal integrity effects on the transmission capability of the sensory axon leads to a slowing of the nerve conduction velocity. Nerve conduction velocities have been shown to decrease by over 20% with age (Boxer, Morales, & Chase, 1988). Interestingly, it seems that age related declines occur more quickly in sensory nerves than in their motor counterparts (Lee & Oh, 1994). 192 Mynark and Koceja The transmission of peripheral information along the Ia afferent is not only affected by the structural quality of the pathway itself but also by how the transmission is gated by certain spinal circuits. Foremost among these circuits is the inhibition of the Ia afferent prior to its synapse with the alpha motoneuron. Age related changes to this presynaptic Ia inhibition will have a direct effect on the strength of the signal transmitted from the periphery to the motoneuron. An increase in the tonic levels of Ia inhibition has been demonstrated with aging (Koceja & Mynark, 2000; Morita, Shindo, Yanagawa, et al., 1995). This decreases the effectiveness of information provided by the muscle spindles and leads to a damping of the sensory input. Sensory Processing Motor Neuron While the aging process obviously takes a toll on many components of the spinal stretch reflex, the changes that have the most impact are arguably those that occur to the motoneurons themselves. Since the motoneurons are the final common pathway of all motor output, any changes to the motoneurons can have a dramatic effect on the behavior of the entire system. Several previous reviews have provided an extensive background into the aging of the motor unit and should be referred to for a more extensive analysis of the topic (Larsson & Ansved, 1995; Roos, Rice, & Vandervoort, 1997). Of primary concern in aging is the apparent loss of motoneuron numbers. Many studies have documented these losses in humans. For example, Tomlinson and Irving (1977) observed an initial 8.8% lumbosacral motoneuron loss by age 61 to 70. This deficit increased progressively with a 21.2% loss of motoneurons by age 71 to 80. The observation that motoneuron loss was not apparent until after 60 years of age is supported in a study by Luff (1998). Furthermore, Cruz-Sanchez, Moral, Tolosa, et al. (1998) have demonstrated that this motoneuronal loss occurs equally among spinal segments (C8, T10, and L5) for both men and women. While this review focuses primarily on the spine, it is interesting to note the work of Eisen, Entezari-Taher, and Stewart (1996), who demonstrated that 35% of descending corticomotoneurons were lost or nonfunctioning by age 50 in normal controls. This loss of descending control may provide one explanation for the loss of spinal motoneurons. Without cortical control, these orphaned spinal motoneurons may perish by way of nonuse. A further complication of the effects of aging can occur as a result of the system’s attempt to compensate for the aforementioned loss of motoneurons. As motoneurons are lost, the remaining motoneurons attempt to re-innervate the orphaned muscle fibers (Larsson, 1995). The result of this remodeling is an overall increase in the motor unit innervation ratio (Larsson, Ansved, Edström, et al., 1991) and a larger amplitude of single motor unit output as measured by EMG (Galea, 1996). These changes can decrease the efficiency of the system by reducing the fine-tuning of reflex output. Instead of the ability to produce very specific corrections to changes in muscle length, the reflex arc responds with more gross motor outputs. However, although inefficient, this outcome is preferable to the irreversible de-innervation of significant amounts of muscle tissue. Along with a decrease in population numbers, it has been widely observed that with aging of the motoneuron, there is a concurrent decrease in soma size (Zhang, Goto, Suzuki, & Ke, 1996). This shrinkage is most apparent in the larger motoneu- The Spinal Stretch Reflex 193 rons innervating Type II muscle fibers (Luff, 1998). As the size of the motoneuron decreases, its characteristics change such that it begins to respond more like the motoneurons that innervate Type I muscle fiber. For example, as the soma size decreases, the input resistance of the membrane increases (Chase, Morales, Boxer, & Fung, 1985). This change in membrane characteristics leads to increases in the Ia excitatory potential rise-time and half-width, and a corresponding decrease in the rate of rise even when standardized by the excitatory potential amplitude (Boxer et al., 1988). Interestingly, the final amplitude of the Ia excitatory potential and the resting membrane potential of the motoneuron remain unchanged (Chase et al., 1985). Inputs Since the motoneuron acts primarily as an integrator of the excitatory and inhibitory input presented to it, changes in the output of the motoneuron with age may result not only from the structural properties of the cell itself but also from changes to the input patterns impacting upon the motoneuron. Considering that a typical motoneuron receives input from several thousand sources spread across over 10,000 synapses, it is nearly impossible to completely quantify the input changes over time. However, the following addresses some of the more powerful and best researched sources of direct input to the motoneuron. Unfortunately, there are very few published studies assessing the effects of aging on these inputs. Presynaptic Inhibition. Presynaptic inhibition is defined as the depression of excitatory postsynaptic potentials unaccompanied by concomitant changes in postsynaptic excitability (Davidoff & Hackman, 1984). In other words, the afferent volley transmitted along the Ia pathway may be modulated prior to its arrival at the motoneuron. The concept of presynaptic inhibition as we understand it was introduced by Frank and Fourtes (1957). Afterward, Eccles and his colleagues (Eccles, Kustyuk, & Schmidt, 1962a, 1962b; Eccles, Schmidt, & Willis, 1962c) were instrumental in identifying and defining the effects of presynaptic inhibition. Eccles et al. (1962c) examined monosynaptic reflex spike potentials from the ventral roots of anaesthetized cats, and also monitored afferent volleys as they entered the spinal cord. It was determined that brief stimulation of the group Ia afferent fibers of some muscles produced a prolonged depression of monosynaptic reflex transmission. A presynaptic inhibitory mechanism, as shown in Figure 3, has been postulated to control the transmission efficiency between the Ia afferent fibers and the alpha motoneurons. Currently there are several noninvasive methodologies for assessing presynaptic inhibition of Ia fibers in humans (see reviews by Rudomin & Schmidt, 1999; Stein, 1995). With respect to aging, recent evidence has suggested differences in presynaptic inhibition of soleus Ia fibers between young and elderly persons. Morita et al. (1995) and Koceja and Mynark (2000) reported increased tonic levels of presynaptic inhibition with aging, and Koceja and Mynark (2000) have reported changes in presynaptic inhibition with changes in body position. It is worth noting here that there is some disagreement concerning presynaptic inhibition as an extrinsic factor altering Ia-motoneuron integrity (see Figure 2), as opposed to an intrinsic mechanism of the Ia fiber altering the probability of transmitter release at these terminals. For a review of this topic, see Hultborn and Nielson (1998). Reciprocal Inhibition. As an agonist or prime-mover muscle is contracted during concentric contractions, there is a relaxation of the antagonist muscles. Recip- 194 Mynark and Koceja Ia Afferent Figure 3 — Presynaptic inhibitory neural circuit. An interneuron (A) regulates the release of neurotransmitter from the Ia afferent, thus providing a means for modulating the communication between the Ia afferent and the alpha motoneurons. (Adapted from Latash, 1998, p. 62) rocal inhibition is the process of inhibiting the motoneurons that innervate an antagonist muscle as the agonist muscle is activated. This circuit is mediated by the Ia inhibitory interneuron and allows the joint to flex through a full range of motion. The inhibition exerted by the Ia inhibitory interneuron impacts upon the cell body of the motoneuron, resulting in a postsynaptic inhibitory effect. This is in contrast to presynaptic inhibition, which only affects a specific input to the motoneuron and not the motoneuron itself. Postsynaptic inhibition affects the entire motoneuron, which in turn affects the efficacy of all other inputs to the motoneuron. H-reflex methodology for the assessment of reciprocal inhibition has been developed (see review by Crone & Nielson, 1994) and will prove useful in the measurement of this spinal pathway in aging. However, at present there is little information concerning the role of reciprocal inhibition during movement in elderly persons. Recurrent Inhibition. In 1941 Renshaw discovered that a population of spinal interneurons exerted an inhibitory effect on the alpha motoneuron. Renshaw (1946) further found that this particular population of spinal interneurons was facilitated by the stimulation of the alpha motoneuron. Renshaw concluded that these spinal inhibitory interneurons were mediated by the axon collateral of the alpha motoneuron. These inhibitory interneurons are termed Renshaw cells, and this process of inhibition is termed recurrent inhibition (see Figure 4). In addition to projecting to the motoneurons, Renshaw cells may project to other Renshaw cells and to the Ia inhibitory interneurons that produce reciprocal inhibition of antagonist muscles (Hultborn, Jankowska, & Lindstrom, 1971). The classic hypothesis is that recurrent inhibition serves to limit the firing frequency of motoneurons to prevent overactivation of the muscle. However, various alternative hypotheses have been forwarded. Among these are desynchronization of motor unit activity (Adam, Windhorst, & Inbar, 1978), regulation of dynamic motoneuron sensitivity (Windhorst & Koehler, 1983), prevention of tremor in the spinal stretch reflex loop (Windhorst, Kokkoroyiannis, Laouris, & Meyer-Lohman, 1994), gain regulation of spinal motor output (Hultborn, Lindstrom, & Wigstrom, 1979), and as a circuit to assist co-contraction. The Spinal Stretch Reflex 195 Figure 4 — Recurrent inhibitory neural circuit. The Renshaw cell is interposed between the motoneuron soma and an axon collateral from the soma. The Renshaw cell, an inhibitory interneuron, is responsible for hyper-polarizing the motoneuron soma after a neural motoneuron discharge. (Adapted from Kandel, Schwartz, & Jessell, 1991, p. 585) 196 Mynark and Koceja The exact role of Renshaw cells during voluntary movement and/or aging is not well understood. However, movement related modulations of these cells have been documented utilizing H-reflex techniques developed by Pierrot-Deseilligny and Bussel (1975). For example, it has been shown that Renshaw cell activity is absent during locomotion (Feldman & Orlovsky, 1975; Severin, Orlovsky, & Shik, 1968). Also, using a paired H-reflex technique, Pierrot-Deseilligny and associates have demonstrated that Renshaw cell activity is increased during mild muscle contraction and absent during high force contractions (Hultborn & Pierrot-Deseilligny, 1979a, 1979b; Pierrot-Deseilligny & Bussel, 1975; Pierrot-Deseilligny, Bussel, Held, & Katz, 1976; Pierrot-Deseilligny, Morin, Katz, & Bussel, 1977). However, other work supports the notion of a more active role of Renshaw cells during movement (McCrea, Pratt, & Jordan, 1986; Pratt & Jordan, 1987). Future use of this noninvasive technique for measuring Renshaw activity in humans could be instrumental in identifying any changes that are manifested with aging. Output Motor Axon As might be expected, the effects of aging on the motor axon parallel those of the Ia afferent pathway. Primarily, the decrease in nerve conduction velocity is the most apparent deficit resulting from the aging process. Chase et al. (1985) found a 23.4% decrease in nerve conduction velocity in cat motor axons with age. These changes were not simply due to the loss or shrinkage of larger motoneurons with faster nerve conduction velocities, nor were they due to a decrease in mean axon diameter (Ansved & Larsson, 1990). Instead, these decreases seem to reflect a global shift toward slower nerve conduction throughout the entire motor axon population. Ansved and Larsson (1990) have demonstrated that these deficits are due to the same demyelination and axon degeneration that were discussed with regard to sensory axons, but they have also been hypothesized to occur as a result of changes in axon membrane properties (Chase et al., 1985). While the bulk of the transmission deficiencies can be explained by degeneration of the motor axon, there are some important age related changes at the distal end of the motor axon. Whereas in young people a single preterminal axon innervates the neuromuscular junction, with aging this number increases (Oda, 1984). This leads to a splitting of the descending signal, which may alter signal strength and transmission efficiency. Neuromuscular Junction Final transmission of the descending motor command to the muscle depends on the efficiency of the neuromuscular junction (NMJ). Ludatscher et al. (1985) have provided a great deal of information about the effects of aging on this interface through studies with mice. When comparing young and old mice, they determined that approximately 85% of young NMJs could be classified as normal in size and structure. In contrast, aged mice were found to have only 40% of NMJs within normal limits. Among the changes observed with aging were decreases in the number of synaptic vessicles, decreases in the number of mitochondria, and a vacuolization of axon terminals. Furthermore, a general shrinkage and degeneration of the axon terminals was found. The most common terminal degeneration included Schwann cell degeneration, which was never observed in young NMJs. The Spinal Stretch Reflex 197 In addition to these characteristic changes, Oda (1984) has demonstrated several changes to the macrostructure of the human NMJ. This includes an increase in the length of the NMJ, and a tendency for the NMJ to break up into smaller conglomerates. The motor endplate has been shown to experience a similar breakdown of acetylcholine receptors into smaller groups, which may be related to axonal branching. Furthermore, perijunctional acetylcholine receptor sites begin to appear. Oda (1984) demonstrated that these changes seemed to occur linearly across time and were not the result of a single age related pathology. The work of Ludatscher et al. (1985) and Oda (1984) demonstrated that motor volleys passing through the NMJ may be compromised in the elderly population as the signal itself degrades and the volley is diffused across a wider and less effective motor end plate. Muscle Physiology It has been widely accepted that there is a significant loss of strength with aging. These decreases in strength are complex in nature and involve an interaction of inactivity, nutrition, and genetics in addition to the effects of aging on the neuromuscular system. These interactions make it very difficult to confidently point out the exact contribution of each component in the observed strength deficits. At this point in the spinal stretch reflex, the effects of all the preceding components can have an effect on the muscle response to stretch. Of primary concern is the effect of motoneuron loss on the integrity of muscle fibers. Since, as discussed earlier, the larger motoneurons are the most vulnerable, it is easy to understand the large strength deficits that become manifest with aging. Lexell, Taylor, and Sjostrom (1988) demonstrated in human quadriceps muscle that aging atrophy begins at 25 years of age and is accelerated thereafter. Lexell et al. observed a 10% decrease in muscle fiber cross-sectional area by 50 years of age, which progressed to a 50% decrease by the age of 80 years. As might be expected, most of the area loss was the result of type II muscle fiber shrinkage. The cause of this loss of area is most likely neurological in nature, and in fact extensive neurological changes were previously found to be common with aging (Tomonaga, 1977), while myopathic changes are relatively rare in normal aging (Lexell, Henriksson-Larsen, Winblad, & Sjostrom, 1983). Interestingly, while cross-sectional area decreases are more apparent in type II muscle fiber, Lexell et al. (1988) observed no changes in the proportion of fiber type with age. Functional Significance The basic spinal reflexes have been studied over the last century, but new information continues to emerge concerning the manner in which they adapt the normal pattern of neural activity to varying environmental conditions, and fail to adapt this activity in pathological states (Stein, Yang, Belanger, & Pearson, 1993). However, from the neural complexity of this simple reflex system (see Figure 5), it soon becomes apparent that understanding its input/output relationship remains problematic. It is well accepted that the amplitude of both the SSR and the H-reflex declines with age (Burke, Kamen, & Koceja, 1989; Lin & Sabbahi, 1998), and intuitively agreed that these changes have a detrimental impact on the motor behavior of aged individuals. Since the now classic work on operant conditioning of the spinal stretch reflex in primates (see review by Wolpaw & Carp, 1990), considerable work has emerged 198 Mynark and Koceja Figure 5 — Complexity of the spinal stretch reflex pathway, showing a select few neural circuits. Changes in the excitability of any of these pathways will result in reflex gain changes. Ia IN = Ia inhibitory interneuron; Descending = motor commands originating from the motor cortex; R = Renshaw inhibitory neuron; α = alpha motoneuron; γ = gamma motoneuron. (Adapted from Brooks, 1986, p. 85) concerning the adaptability of the reflex pathways to various perturbations. In Wolpaw’s early work (Wolpaw, 1987; Wolpaw, Braitman, & Seegal, 1983), the spinal stretch reflex could be increased in amplitude (up to 200%) and decreased in amplitude (up to –50%) with appropriate operant conditioning paradigms. Later work confirmed this change for the electrically evoked H-reflex. These amplitude changes were independent of background muscle activity, and presumably motoneuron excitability, which implicated presynaptic inhibition as a potential regulating mechanism of the Ia-alpha motoneuron synapse. More recent work in our laboratory has confirmed this plasticity in the elderly reflex system, and has attempted to link this plasticity with motor behavior. In our original experiment (Koceja et al., 1995), the H-max/M-max ratio in young and elderly persons was examined when supine and weight-bearing. The results indicated that young people depressed the H-max/M-max ratio when standing (–13.7%) whereas the elderly showed no change. However, when examining the elderly, we found considerable variability in their ability to modulate the H-reflex with changes in body position. Within this group, the correlation between reflex modulation (change in reflex amplitude from supine to standing) and standing postural control (e.g., center of pressure excursions during a quiet standing task) was r = 0.54. This indicated that the ability to modulate the reflex system was related to postural steadiness. In a later experiment we attempted to determine whether reflex modulation could be altered with a training paradigm, and whether this resulted in improved postural stability. In this study, elderly persons (age >65 yrs) were able to alter their soleus H-reflex, independent of muscle activity, with a specially designed reflex training paradigm. After a 3-day training period they were able to alter their reflex amplitude by 18.7% and improve their postural stability by 10% (significantly less center-ofpressure excursions on the force platform during quiet standing) (Mynark, 1999). The Spinal Stretch Reflex 199 Furthermore, utilization of noninvasive presynaptic protocols has been instrumental in identifying mechanistic differences in the modulation of the reflex during weight-bearing and non-weight-bearing between young and elderly persons. In our recent study (Koceja & Mynark, 2000), young persons demonstrated an increase in presynaptic inhibition of soleus Ia fibers when standing (+33.8%), whereas elderly persons demonstrated no changes in presynaptic inhibition when changing body positions. These results point to the importance of presynaptic mechanisms in regulating reflex output in different body positions. A more recent study documents an inability of elderly persons to modulate presynaptic inhibition during voluntary contractions (Earles, Vardaxis, & Koceja, in press). In this experiment, presynaptic inhibition of the soleus Ia fibers was measured during tonic voluntary contractions of varying intensities. The results indicated that young persons release presynaptic inhibition to the target motor pool at a faster rate than do elderly persons. Therefore, it is speculated that the release of presynaptic inhibition is a method of increasing motoneuron excitability in young people but not in the elderly. Taken together, these studies indicate the importance of reflex regulation in the control of voluntary movement, and emphasize the importance of reflex testing in uncovering this regulation. With respect to motor behavior, there is some evidence that the modulation of reflex gain is one mechanism that is important for regulating postural control. Reflex gain is defined as the relationship between EMG activity of a target motor pool and reflex amplitude. It has been documented that the gain of the soleus H-reflex differs between young and elderly, and that this mechanism is also correlated to center-ofpressure changes on the force platform during quiet standing (Angulo-Kinzler, Mynar, & Koceja, 1998). Therefore, it seems plausible that age related declines in either the spinal stretch reflex physiology and/or the plasticity of this system play an important role in regulating motor output. Support for this is also found in the pathology literature, in which it is reported that spinal stretch reflex pathways are altered with various disabilities (Nielson, Peterson, & Crone, 1995; Stein et al., 1993). Conclusion In conclusion, it appears that the spinal stretch reflex, once thought to be a simple monosynaptic system, is in fact a quite complex sensory integration unit. Furthermore, reflex methodology can be useful in uncovering alterations in motoneuron excitability during various motor tasks. This review has documented various changes in this system with aging, and perhaps some behavioral consequences of this change. However, whereas considerable information concerning aging has been determined using reflex methodologies, more studies are needed to bridge the gap between motoneuron excitability and motor control. In this regard, several considerations for future studies are presented. It is imperative that studies investigating human aging proceed with strict adherence to the many methodological pitfalls that accompany reflex testing (see review by PierrotDeseilligny & Mazevet, 2000). Further, it is recommended that emphasis be placed not simply on motoneuron excitability as measured with reflex amplitude but also on spinal mechanisms (e.g., presynaptic inhibition and recurrent inhibition) that may act either directly or indirectly to alter motor output. As these methodologies have been discussed in the literature for some time, a more complete understanding of motoneuron excitability can be realized by examining the ancillary spinal pathways that may 200 Mynark and Koceja contribute to motor output utilizing these techniques. Finally, the role of EMG activity (e.g., reflex gain) as it relates to reflex amplitude needs to be examined to differentiate the role of supraspinal pathways versus segmental pathways in the control of movement. With proper methodological considerations in place, important questions concerning motor output and aging can be examined. For example, the exact role of reflex modulation and postural control in elderly persons, the role of task complexity (most notably during weight-bearing) and reflex modulation, and the role of visual and vestibular interactions in reflex modulation, especially with regard to postural control in the elderly, need to be addressed. Only after these questions are answered will we gain a more complete understanding of the link between reflex activity and postural control in the elderly. References Adam, D., Windhorst, U., & Inbar, G.F. (1978). The effects of recurrent inhibition on the cross-correlated firing patterns of motoneurones (and their relation to signal transmission in the spinal cord-muscle channel). Biological Cybernetics, 29, 229-235. Almekinders, L.C., & Deol, G. (1999). The effects of aging, antiinflammatory drugs, and ultrasound on the in vitro response of tendon tissue. American Journal of Sports Medicine, 27, 417-421. Angulo-Kinzler, R.M., Mynark, R.G., & Koceja, D.M. (1998). Soleus H-reflex gain in elderly and young adults: Modulation due to body position. Journal of Gerontology, 53, M120-M125. Ansved, T., & Larsson, L. (1990). Quantitative and qualitative morphological properties of the soleus motor nerve and the L5 ventral root in young and old rats. Relation to the number of soleus muscle fibers. Journal of the Neurological Sciences, 96, 269-282. Barr, M.L., & Kiernan, J.A. (1983). The human nervous system (4th ed.). Philadelphia: Harper & Rowe. Boxer, P.A., Morales, F.R., & Chase, M.H. (1988). Alterations of group Ia-motoneuron monosynaptic EPSPs in aged cats. Experimental Neurology, 100, 583-595. Brooks, V.B. (1986). The neural basis of motor control. New York: Oxford University Press. Burke, J.R., Kamen, G., & Koceja, D.M. (1989). Long-latency enhancement of quadriceps excitability from stimulation of skin afferents in young and old adults. Journal of Gerontology, 44, M158-M163. Chase, M.H., Morales, F.R., Boxer, P.A., & Fung, S.J. (1985). Aging of motoneurons and synaptic processes in the cat. Experimental Neurology, 90, 471-478. Crone, C., & Nielson, J. (1994). Central control of disynaptic reciprocal inhibition in humans. Acta Physiologica Scandinavica, 152, 351-363. Cruz-Sanchez, F.F., Moral, A., Tolosa, E., de Belleroche, J., & Rossi, M.L. (1998). Evaluation of neuronal loss, astrocytosis and abnormalities of cytoskeletal components of large motor neurons in the human anterior horn in aging. Journal of Neural Transmission (Budapest), 105, 689-701. Davidoff, R.A., & Hackman J.C. (1984). Spinal inhibition. In R.A. Davidoff (Ed.), Handbook of the spinal cord (Vols. 2 & 3, pp. 385-460). New York: Marcel Dekker. Earles, D.R., Vardaxis, V., & Koceja, D.M. (In press). Regulation of motor output between young and elderly subjects. Clinical Neurophysiology. Eccles, J.C., Kustyuk, P.G., & Schmidt, R.F. (1962a). Central pathways responsible for depolarization of primary afferent fibres. Journal of Physiology, 161, 237-257. The Spinal Stretch Reflex 201 Eccles, J.C., Kustyuk, P.G., & Schmidt, R.F. (1962b). Presynaptic inhibition of the central actions of flexor reflex afferent. Journal of Physiology, 161, 258-281. Eccles, J.C., Schmidt, R.F., & Willis W.D. (1962c). Presynaptic inhibition of the spinal monosynaptic reflex pathway. Journal of Physiology, 161, 282-297. Eisen, A., Entezari-Taher, M., & Stewart, H. (1996). Cortical projections to spinal motoneurons: Changes with aging and amyotrophic lateral sclerosis. Neurology, 46, 1396-1404. Feldman, A.G., & Orlovsky, G.N. (1975). Activity of interneurons mediating reciprocal Ia inhibition during locomotion. Brain Research, 84, 181-194. Frank, K., & Fourtes, M.G.F. (1957). Presynaptic and postsynaptic inhibition of monosynaptic reflexes. Federation Proceedings, 16, 39-40. Gajdosik, R.L., Vander Linden, D.W., & Williams, A.K. (1999). Influence of age on length and passive elastic stiffness characteristics of the calf muscle-tendon unit of women. Physical Therapy, 79, 827-838. Galea, V. (1996). Changes in motor unit estimates with aging. Journal of Clinical Neurophysiology, 13, 253-260. Hultborn, H., Jankowska, E., & Lindstrom, S. (1971). Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurons. Journal of Physiology, 215, 591-612. Hultborn, H., Lindstrom, S., & Wigstrom, H. (1979). On the function of recurrent inhibition in the spinal cord. Experimental Brain Research, 37, 399-403. Hultborn, H., & Nielson, J.B. (1998). Modulation of transmitter release from Ia afferents by their preceding activity—A “postactivation depression.” In P. Rudomin, R. Romo, & L.M. Mendell (Eds.), Presynaptic inhibition and neural control (pp. 178-191). New York: Oxford University Press. Hultborn, H., & Pierrot-Deseilligny, E. (1979a). Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. Journal of Physiology, 297, 229-251. Hultborn, H., & Pierrot-Deseilligny, E. (1979b). Input–output relations in the pathway of recurrent inhibition to motoneurones in the cat. Journal of Physiology, 297, 267-287. Kandel, E.R., Schwartz, J.H., & Jessell, T.M. (1991). Principles of neural science. New York: Elsevier. Koceja, D.M., & Mynark, R.G. (2000). Comparison of heteronymous Ia facilitation from supine to standing in young and elderly subjects. International Journal of Neuroscience, 103, 1-17. Koceja, D.M., Trimble, M.H., & Markus, C.A. (1995). Postural modulation of the soleus Hreflex in young and old subjects. Electroencephalography and Clinical Neurophysiology, 97, 387-393. Larsson, L. (1995). Motor units: Remodeling in aged animals. Journal of Gerontology, 50A, 91-95. Larsson, L., & Ansved, T. (1995). Effects of ageing on the motor unit. Progress in Neurobiology, 45, 397-458. Larsson, L., Ansved, T., Edström, L., Gorza, L., & Schiaffino, S. (1991). Effects of age on physiological, immunocytochemical and biochemical properties of fast-twitch single motor units in the rat. Journal of Physiology, 443, 257-275. Latash, M.L. (1998). Neurophysiological basis of movement. Champaign, IL: Human Kinetics. Lee, K.W., & Oh, S.J. (1994). Early appearance of aging phenomenon in the interdigital nerves of the foot. Muscle & Nerve, 17, 58-63. 202 Mynark and Koceja Lexell, J., Henriksson-Larsen, K., Winblad, B., & Sjostrom, M. (1983). Distribution of different fiber types in human skeletal muscles: Effects of aging studied in whole muscle cross sections. Muscle & Nerve, 6, 588-595. Lexell, J., Taylor, C.C., & Sjostrom, M. (1988). What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the Neurological Sciences, 84, 275-294. Lin, F.M., & Sabbahi, M. (1998). The aging effects on the EMG and mechanical responses of the human wrist flexor stretch reflexes. Electromyography & Clinical Neurophysiology, 38, 323-331. Ludatscher, R.M., Silbermann, M., Gershon, D., & Reznick, A. (1985). Evidence of Schwann cell degeneration in the aging mouse motor end-plate region. Experimental Gerontology, 20, 81-91. Luff, A.R. (1998). Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Annals of the New York Academy of Sciences, 854, 92-101. McCrea, D.A., Pratt, C.A., & Jordan, L.M. (1980). Renshaw cell activity and recurrent effects on motoneurons during fictive locomotion. Journal of Neurophysiology, 44, 475-488. Miwa, T., Miwa, Y., & Kanda, K. (1995). Dynamic and static sensitivities of muscle spindle primary endings in aged rats to ramp stretch. Neuroscience Letters, 201, 179-182. Morita, H., Shindo, M., Yanagawa, S., Yoshida, T., Momoi, H., & Yanagisawa, N. (1995). Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Experimental Brain Research, 104, 167-170. Mynark, R.G. (1999). The effects of balance training on the segmental reflex system of elderly subjects. Unpublished doctoral dissertation, Indiana University. Nielsen, H.M., Skalicky, M., & Viidik, A. (1998). Influence of physical exercise on aging rats. III. Life-long exercise modifies the aging changes of the mechanical properties of limb muscle tendons. Mechanisms of Ageing and Development, 100, 243-260. Nielson, J., Peterson, N., & Crone, C. (1995). Changes in transmission across synapses of Ia afferents in spastic patients. Brain, 118, 995-1004. Oda, K. (1984). Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. Journal of the Neurological Sciences, 66, 327-338. Pierrot-Deseilligny, E., & Bussel, B. (1975). Evidence for recurrent inhibition by motoneurons in human subjects. Brain Research, 88, 105-108. Pierrot-Deseilligny, E., Bussel, B., Held, J.P., & Katz, R. (1976). Excitability of human motoneurones after discharge in a conditioning reflex. Electroencephalography & Clinical Neurophysiology, 40, 279-287. Pierrot-Deseilligny, E., Morin, C., Katz, R., & Bussel, B. (1977). Influence of voluntary movement and posture on recurrent inhibition in human subjects. Brain Research, 124, 427-436. Pierrot-Deseilligny, E., & Mazevet, D. (2000). The monosynaptic reflex: A tool to investigate motor control in humans. Interest and limits. Nuerophysiologie Clinique, 30, 67-80. Pratt, C.A., & Jordan, L.M. (1987). Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. Journal of Neurophysiology, 57, 56-71. Renshaw, B. (1941). Influence of discharge of motoneurons upon excitation of neighboring motoneurons. Journal of Neurophysiology, 4, 167-183. Renshaw, B. (1946). Central effects of centripetal impulses in axons of spinal ventral roots. Journal of Neurophysiology, 9, 191-204. Roos, M.R., Rice, C.L., & Vandervoort, A.A. (1997). Age-related changes in motor unit function. Muscle & Nerve, 20, 679-690. The Spinal Stretch Reflex 203 Rudomin, P., & Schmidt, R.F. (1999). Presynaptic inhibition in the vertebrate spinal cord revisited. Experimental Brain Research, 129, 1-37. Sage, G.H. (1977). Introduction to motor behavior: A neuropsychological approach (2nd ed.). Reading, MA: Addison-Wesley. Schieppati, M. (1987). The Hoffmann reflex: A means of assessing spinal reflex excitability and its descending control in man. Progress in Neurobiology, 28, 345-76. Severin, F.V., Orlovsky, G.N.O., & Shik, M.L. (1968). Reciprocal influences on work of single motoneurons during controlled locomotion. Bulletin of Experimental Biology and Medicine, 66, 5-9. Stein, R.B. (1995). Presynaptic inhibition in humans. Progress in Neurobiology, 47, 533-544. Stein, R.B., Yang, J.F., Belanger, M., & Pearson, K.G. (1993). Modification of reflexes in normal and abnormal movements. Progress in Brain Research, 97, 189-196. Swash, M., & Fox, K.P. (1972). The effect of age on human skeletal muscle. Studies of the morphology and innervation of muscle spindles. Journal of the Neurological Sciences, 16, 417-432. Tomlinson, B.E., & Irving, D. (1977). The numbers of limb motor neurons in the human lumbosacral cord throughout life. Journal of the Neurological Sciences, 34, 213-219. Tomonaga, M. (1977). Histochemical and ultrastructural changes in senile human skeletal muscle. Journal of the American Geriatrics Society, 25, 125-131. Vailas, A.C., Pedrini, V.A., Pedrini-Mille, A., & Holloszy, J.O. (1985). Patellar tendon matrix changes associated with aging and voluntary exercise. Journal of Applied Physiology, 58, 1572-1576. Wolpaw, J.R. (1987). Operant conditioning of primate spinal reflexes: The H-reflex. Journal of Neurophysiology, 57, 443-459. Wolpaw, J.R., Braitman, D.J., & Seegal, R.F. (1983). Adaptive plasticity in primate spinal stretch reflex: Initial development. Journal of Neurophysiology, 50, 1296-1311. Wolpaw, J.R., & Carp, J.S. (1990). Memory traces in spinal cord. Trends in Neurosciences, 13, 137-142. Windhorst, U., & Koehler, W. (1983). Dynamic behavior of alpha-motoneuron sub-pools subjected to in homogenous Renshaw cell inhibition. Biological Cybernetics, 46, 217-228. Windhorst, U., Kokkoroyiannis, T., Laouris, Y., & Meyer-Lohman, J. (1994). Signal transmission from motor axons to group Ia muscle spindle afferents: Frequency responses and second-order non-linearities. Neuroscience, 59, 149-163. Zhang, C., Goto, N., Suzuki, M., & Ke, M. (1996). Age-related reductions in number and size of anterior horn cells at C6 level of the human spinal cord. Okajimas Folia Anatomica Japonica, 73, 171-177. Acknowledgments This work was supported in part by a grant from the National Institutes of Health (R29 AHG/OD 13660-05) to D.M. Koceja.