* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download gamete interaction in flowering plants
Cell nucleus wikipedia , lookup
Cell encapsulation wikipedia , lookup
Signal transduction wikipedia , lookup
Cell membrane wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Programmed cell death wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
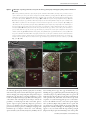
Cell–Cell Communication in Plant Reproduction Let’s get physical: gamete interaction in flowering plants Stefanie Sprunck1 Cell Biology and Plant Biochemistry, University of Regensburg, Universitätsstrasse 31, 93053 Regensburg, Germany Abstract Fertilization comprises a series of precisely orchestrated steps that culminate in the fusion of male and female gametes. The most intimate steps during fertilization encompass gamete recognition, adhesion and fusion. In animals, some binding-effector proteins and enzymes have been identified that act on the cell surfaces of the gametes to regulate gamete compatibility and fertilization success. In contrast, exploring plant gamete interaction during double fertilization, a characteristic trait of flowering plants, has been hampered for a long time because of the protected location of the female gametes and technical limitations. Over the last couple of years, however, the use of advanced methodologies, new imaging tools and new mutants has provided deeper insights into double fertilization, at both the cellular and the molecular level, especially for the model plant Arabidopsis thaliana. Most likely, one consequence of inventing double fertilization may be the co-evolution of special molecular mechanisms to govern each successful sperm delivery and efficient gamete recognition and fusion. In vivo imaging of double fertilization and the recent discovery of numerous female-gametophyte-specific expressed genes encoding small secreted proteins, some of whom were found to be essential for the fertilization process, support this hypothesis. Nevertheless, recent findings indicate that at least the membrane-merger step in plant gamete interaction may rely on an ancient and widely used gamete fusion system. Introduction Sexual reproduction is a fundamental biological process that depends on fertilization, the union of two gametes produced by meiosis, leading to the formation of an individual genetically distinct from its parents. In the majority of higher eukaryotes, including plants, these two gametes differ in size and/or form and are contributed by different parents. By definition, the smaller gamete is considered as male (sperm cell), whereas the larger gamete is regarded as female (egg cell). For successful fertilization, the gametes need to accomplish fundamental tasks: (i) the sperm cell has to be in the immediate vicinity of the egg cell, (ii) the gametes have to assure species-restricted gamete recognition and fusion, and (iii) the egg cell has to prevent the fusion of more than one sperm cell (block of polyspermy). The progressive stages of gamete recognition and fusion are often collectively termed as gamete interaction. However, depending on the living environment of a sexually reproducing organism (i.e. water or land) and the requirements for either internal or external fertilization, distinct fertilization mechanisms have evolved to control gamete attraction, recognition, adhesion and fusion [1–3]. In animals and lower plants, including mosses and ferns, most male gametes (sperm or spermatozoa) are motile, being propelled by a long flagellum along a Key words: Arabidopsis thaliana, double fertilization, egg cell, gamete recognition and fusion, plasmogamy, sperm cell. Abbreviations used: CLSM, confocal laser-scanning microscopy; CRP, cysteine-rich protein; FG, female gametophyte; GCS1, GENERATIVE CELL-SPECIFIC 1; HAP2, HAPLESS 2; MG, male gametophyte; mt, mating type; ZmES, Zea mays embryo sac. 1 email [email protected] Biochem. Soc. Trans. (2010) 38, 635–640; doi:10.1042/BST0380635 gradient of chemoattractants, synthesized and secreted by the egg or by somatic cells, such as follicle cells, associated with the egg [3]. In contrast, the sperm cells of flowering plants (angiosperms) differ from sperm of non-seed plants and of most animals in their lack of flagella. A key innovation that allowed angiosperms to carry out sexual reproduction on land, without the need for water, is a form of internal fertilization in which the non-motile sperms are delivered to the female gametes via a pollen tube. Moreover, fertilization in angiosperms is unique among all known organisms in that not one, but two, female reproductive cells are fertilized in a process called double fertilization. A complex mechanism involving two male gametes (sperm cells) and two female gametes (egg cell and central cell) results in two distinct fertilization products, embryo and endosperm, that are both required to achieve successful seed development [4,5]. Events preceding gamete interaction: pollination and sperm cell delivery into the FG (female gametophyte) Plant gametes are, in contrast with animal gametes, not direct products of meiosis, but differentiate within multicellular haploid generations, the MG (male gametophyte) and the FG respectively. In angiosperms, the gametophytic generations are reduced to a few cells that are located within the diploid sporophytic tissues of the flower. The FG (or embryo sac) is enclosed by the ovule that is located within the ovary. The seven-celled FG of the model plant Arabidopsis thaliana C The C 2010 Biochemical Society Authors Journal compilation 635 636 Biochemical Society Transactions (2010) Volume 38, part 2 Figure 1 Structures of the A. thaliana FG and MG (A) The seven-celled FG is enclosed by the outer integuments (oi) and inner integuments (ii) of the ovule, providing a small opening (micropyle; mp) as entry for the pollen tube. The ovule is attached by the funiculus (fun) to the inner surface of the ovary. The synergid nuclei (sn) are positioned towards the filiform apparatus (f) that forms at the micropylar pole of the synergids. The egg cell nucleus (ecn) is positioned at the chalazal side of the egg, close to the central cell nucleus (ccn). Three antipodals (ap) are located at the chalazal pole (chz) of the FG. Broken lines surrounding shaded areas indicate the cell vacuoles. The big central cell vacuole (ccv) fills a large part of the FG. (B) Tricellular MG. The larger vegetative cell (vc) encloses two small sperm cells (sc1, sc2) that are physically connected to each other and conjointly surrounded by the plasma membrane of the vegetative cell [pm(v)]. The vegetative cell nucleus (vn) is less compact and irregularly shaped. The sperm cell nuclei are indicated by black circles, the sperm cell plasma membranes [pm(s)] are coloured grey. comprises two female gametic cells, the egg cell and the central cell, which are flanked by accessory cells. Two synergid cells neighbour the egg cell at the micropylar end of the FG, while three antipodals are adjacent to the central cell at the chalazal end of the FG (Figure 1A) [6]. The pollen that develops within the anther represents the MG. Arabidopsis pollen is tricellular, consisting of one larger vegetative cell encompassing the two sperm cells (Figure 1B). For double fertilization to succeed, the two non-motile sperm cells must be transported through the pistil into the FG, which strictly depends on the directional growth of the pollen tube formed by the vegetative cell of the MG. The initiation of pollen tube growth requires adhesion of the pollen grain to a receptive female stigma and hydration, providing first checkpoints for a species-restricted interaction between the MG and the female reproductive tract [7–9]. Once germinated, the tip-growing pollen tube invades the stigma tissue and navigates across the female reproductive tract towards the ovule, assisted by complex communication between the tube cell and surrounding sporophytic tissues [8–10]. Synergid cells secrete small and species-specific proteins such as LURE1/2 and ZmEA1 (Zea mays EGG APPARATUS 1), which are involved in the last phase of pollen tube attraction, guiding tube growth through the micropyle into the FG [11–13]. The first molecular players FERONIA, LORELEI and ZmES (Z. mays EMBRYO SAC) 1–4 found to be necessary for pollen tube growth arrest and burst indicate that species-specific cell recognition and signalling mechanisms also exist between the receptive synergid and a compatible pollen tube, which may C The C 2010 Biochemical Society Authors Journal compilation trigger synergid cell death and subsequent sperm cell delivery inside this synergid cell [14–17]. The released pollen tube content mingles with the cytoplasm of the degenerating synergid and spreads, as the synergid membrane disintegrates, into a narrow space between the egg and central cell plasma membranes [18,19]. Typically, only one pollen tube penetrates each ovule, limiting FG entry to one sperm cell pair. Rapid termination of pollen tube attractant(s) synthesis and/or secretion or degradation after successful fertilization may explain why the FG loses its ability to attract further pollen tubes [20]. Late pre-zygotic events within the FG In contrast with the molecular events involved in sperm cell delivery into the FG, almost nothing is known about later processes, covering the phase from sperm cell delivery until gamete fusion (plasmogamy). Attempts to elucidate these issues at the cellular and molecular levels are hampered by the fact that angiosperm gamete interactions are shortlived events, taking place deeply embedded in the maternal tissues of the ovule and ovary. Previous knowledge was mainly based on cytological observations of fixed tissues or after in vitro fertilization of isolated male and female gametes [20,21]. From these studies, it is known, for example, that the degenerating synergid exhibits a dramatic F-actin (filamentous actin) reorganization and a significant accumulation of calcium, which was suggested to contribute to sperm cell transport to the fusion site and to the process of gamete fusion respectively [19,20]. However, details about Cell–Cell Communication in Plant Reproduction Figure 2 CLSM analysis of growing pollen tubes and sperm cell delivery, plasmogamy and karyogamy during double fertilization in A. thaliana (A) In vitro-germinated pollen tube expressing the sperm cell marker HTR10-mRFP1, showing strong red fluorescence in sperm cell nuclei (scn). The sperm cells are transported conjointly within the growing pollen tube. (B) In vitro-germinated pollen tubes expressing the marker ARO1-GFP in the cytoplasm of the vegetative cell reveal the physical connection of the two sperm cells (asterisk). (C–H) Fluorescence images (D, F and H) and respective merged bright-field images (C, E and G) of FGs expressing the egg cell marker ARO1-GFP, analysed 5–9 h after hand-pollination using the sperm cell marker line HTR10-mRFP1 as pollen donor. The micropyle is oriented towards the upper left corner in each picture. (C and D) Sperm cell delivery into the degenerating synergid cell (dsy). (E and F) A sperm cell pair stays temporarily at the chalazal pole of the degenerating synergid, in the narrow gap between the egg cell and the central cell which depicts the area of gamete interaction. (G and H) Plasmogamy between the first sperm cell and the egg cell has occurred. The sperm cell nucleus 1 (sn1) is visible inside the egg cell, near the egg cell nucleus (en), whereas the second sperm cell (sn2) has not yet fused with the central cell. (J and K) Visualization of karyogamy, after pollinating the egg cell marker line EC1pro :NLS-(3×)GFP with the sperm cell marker line HTR10-mRFP1. Karyogamy between the second sperm cell nucleus and the central cell nucleus (sn2/cn) is almost complete, indicated by the decondensation of the sperm cell chromatin (broken circles). In contrast, the nucleus of the first sperm cell (sn1) is attached to the egg cell nucleus (en), but has not yet fused. Scale bars, 10 μm (A, C–J); 5 μm (B and K). the following plasmogamy and karyogamy have been fairly unclear. Recently, advanced molecular and genetic approaches and new imaging tools using living material provided deeper insights into the last phase of double fertilization, at least for the model plant Arabidopsis [4]. Male and female gamete cell isolation and transcriptome-level analyses offered the possibility of identifying both male and female gametespecific expressed genes [19,22–28]. Respective promoters now serve as valuable tools to drive expression of fluorescent proteins in Arabidopsis gametes, enabling live imaging of the fertilization process. Figure 2 shows the events following sperm cell delivery into the FG, analysed by CLSM (confocal laser-scanning microscopy). Two egg cell marker lines, one labelling the egg cytoplasm [19] and one line labelling the egg cell nucleus [29], were pollinated with pollen of a sperm cell marker line [23]. Sperm cells are produced after pollen mitosis of the generative cell, and former electron microscopic work on pollen of several flowering plant species revealed that a traverse cell-wall-like structure connects the sperm cell pair after cytokinesis (Figure 1B) [5]. Thus the two sperm cells are transported conjointly within the growing pollen tube (Figures 2A and 2B). Strikingly, the discharged sperm cells appear to stay associated within the degenerating synergid (Figures 2C–2F), despite the fulminating release of the pollen C The C 2010 Biochemical Society Authors Journal compilation 637 638 Biochemical Society Transactions (2010) Volume 38, part 2 tube contents when the tube tip bursts. Back-to-back, both sperm cells arrive at the chalazal pole of the degenerating synergid and stays temporarily in the region between the chalazal end of the egg cell and the micropylar end of the central cell (Figures 2E and 2F). The first plasmogamy appears to invariably occur between the egg cell and one of the sperm cells, while the fusion of the second sperm with the central cell is somewhat delayed (Figures 2G and 2H). Nevertheless, karyogamy between this sperm cell nucleus and the central cell nucleus takes place immediately after plasmogamy, indicated by a rapid decondensation of the male chromatin within the central cell nucleus. In contrast, the nucleus of the first sperm cell stays attached to the egg cell nucleus, but does not fuse immediately (Figures 2J and 2K). It is known that male and female gametes must synchronize their cell cycles for successful fertilization, and Arabidopsis sperm cells are likely to be positioned in G2 -phase when they meet their female counterparts [30]. Although it remains to be determined conclusively, initial data suggest that the egg and central cell reside in G1 /S and G2 /M transition respectively [4]. Thus the observed delay of karyogamy between the egg and sperm nucleus may reflect a phase in which the cell cycle states of egg and sperm cell need to become synchronized. Angiosperm gamete interactions may require unique molecular mechanisms Unlike any other taxa, flowering plants have to face particular problems caused by the unique feature of double fertilization: successful seed development is dependent on fertilization of both the egg and central cell. As typically only one pair of sperm cells is delivered into the FG, the gamete recognition and fusion system needs to be as efficient as possible. It seems plausible that specific molecular mechanisms co-evolved to ensure that one sperm fuses with each female gamete in a timely manner. However, the molecular players of angiosperm gamete interaction are largely uncharacterized, and many questions remain to be answered. It is, for example, not yet clear whether the two male gametes randomly choose their female interaction partner, or whether there is preferential fusion with a particular female gamete. Also unclear is how polyspermy is prevented, and whether the delivered sperm cells become activated before they fuse. Available Arabidopsis mutants were utilized to address some of these questions. Recent data on the retinoblastoma-related 1 (rbr1) mutant, producing supernumerary but functional eggs, revealed that the isomorphic sperm cells of Arabidopsis are both functionally equivalent for fertilizing the egg cell [29], arguing against preferential fusion in this species. Fertilization experiments with different mutants producing single sperm-like cells, however, yielded contradictory results [31,32], but may need closer examination. Evidence for an in vivo polyspermy block on the egg cell, but not on the central cell, was given by fertilization experiments using the polyspermic tetraspore (tes) mutant [33], indicating that the exclusive polyspermy block on the egg may be not only to avoid C The C 2010 Biochemical Society Authors Journal compilation multiple fertilization of the egg, but also to ensure that each female gamete receives one of the two sperm cells [34]. Moreover, the physical association between the two sperm cells arising during mitosis of the generative cell may be an important mechanistic element of double fertilization, as it may be important for both a concerted transport of the two sperm cells within the growing pollen tube and synchronous delivery to the site of gamete interaction. The spatial partitioning of the two sperm cells may also help to avoid spontaneous sperm–sperm fusion when the cells are released into the calcium-rich micro-environment of the degenerating synergid [20]. In consequence, the precisely timed separation of the sperm cells may be one prerequisite for successful double fertilization. In Arabidopsis, this appears not to take place until the sperm cells have reached their fusion site (Figures 2E and 2F). Gamete adhesion and fusion Membrane fusion events generally require molecules that tether and dock membranes and bring them into close proximity. Furthermore, molecules are required that locally disturb the lipid bilayers in order to reduce the energy barriers for fusion [35]. Still, gamete fusion is a specialized form of membrane fusion that is, like other cell–cell fusion events, substantially different from intracellular membrane fusion [36]. Studies on fertilization in vertebrate and invertebrate species, as well as on mating in the unicellular eukaryotic model organisms Chlamydomonas reinhardtii and Saccharomyces cerevisiae provided important insights into the process of gamete fusion [2,37–40]. Although the details of fertilization are highly variable for the different taxa, gamete fusion exhibits some common principles. After gametes of opposite sex or mating types come into close proximity, the subsequent events generally comprise (i) pre-fusion attachment, where adhesive interactions are formed between the plasma membranes of the two gametes, and (ii) the coalescence of the two juxtaposed lipid bilayers (membrane merger). Molecules that act on the surface of the gametes, e.g. as cell adhesion molecules mediating physical gamete interactions, as ligand–receptor pairs involved in signalling, or as fusogenic proteins mediating membrane fusion, are regarded as key components of these well-co-ordinated processes. However, despite gamete fusion being such a fundamental and ancient process, surprisingly few candidate proteins have been identified that play essential roles in gamete adhesion and fusion. Major players in mammalian sperm–egg interaction are the ubiquitously expressed tetraspanin family members CD9 and CD81 which possess four transmembrane domains and act on the egg surface, and the sperm-specific protein IZUMO, an Ig superfamily single-transmembranedomain protein. Putative additional players in mammalian gamete interaction are members of the large family of disintegrin-domain-containing ADAM (a disintegrin and metalloproteinase) proteins on sperm, integrins and yet unidentified GPI (glycosylphosphatidylinositol)-anchored Cell–Cell Communication in Plant Reproduction proteins on the egg plasma membrane, as well as family members of the CRISPs (cysteine-rich secretory proteins) associated with the sperm surface [40]. In the unicellular biflagellated haploid green alga Chlamydomonas, mt (mating type) recognition and initial adhesion of the flagella of mt(+) and mt(−) gametes is achieved by mt-specific agglutinins, fibrous proteins that belong to the family of HRGPs (hydroxyproline-rich glycoproteins) residing on the surface of the flagellar membrane. The membrane fusion event of Chlamydomonas gametes is dependent on at least two consecutively acting proteins [37,41]. FUS1, a transmembrane protein containing five Ig-like repeats in its extracellular domain, is located at the apex of the fertilization tube of activated mt(+) gametes. Although FUS1, which is not found in other species, is essential for prefusion attachment between the mating structures of activated mt(+) and mt(−) gametes, the membrane merger step is dependent on the single-transmembrane protein GCS1 (GENERATIVE CELL-SPECIFIC 1)/HAP2 (HAPLESS 2) [41], being mainly expressed in mt(−) gametes [24]. Interestingly, GCS1/HAP2 of Chlamydomonas represents a homologue of a sperm-cell-specific membrane protein in flowering plants that was termed GCS1 [24] or HAP2 [42] respectively, the first identified key player in Arabidopsis gamete interaction. Although not yet shown for Arabidopsis gametes, GCS1/HAP2 is probably involved in the membrane merger of flowering plant gametes, similar to its Chlamydomonas homologue [41]. As GCS1/HAP2 is not specific for flowering plants, but can be found in a wide range of organisms, including green and red algae, slime moulds, ciliates, choanoflagellates, cnidarians and parasites such as Plasmodium falciparum, it may represent an ancient and highly conserved component of a gamete fusion machinery. In contrast, the molecules involved in gamete recognition and the pre-fusion adhesion processes appear to be subjected to more rapid evolution, probably to establish species-specific fertilization barriers [2]. Species-specific gamete interaction in flowering plants? It may be argued that angiosperm gametes do not need species-specific recognition mechanisms at the level of gamete interaction, as pre-zygotic barriers efficiently operate during pollen germination, pollen tube growth and pollen tube discharge [7–9,14,16]. However, elaborate cell–cell interactions can be postulated based on the vast number of FG-specific expressed genes encoding small and potentially secreted polymorphic proteins such as various subgroups of the CRP (cysteine-rich protein) superfamily [11,25,43– 45]. For instance, the two CRPs LURE1 and LURE2 are specifically secreted by Torenia fournieri synergids and act as chemoattractants on Torenia pollen tubes [11], whereas ZmES4 is secreted from maize synergid cells to induce pollen tube burst [16]. Accordingly, gamete-specific expressed CRPs may fulfil as-yet-unknown but important functions during double fertilization, e.g. as signalling molecules involved in intercellular communication. One example is a small subfamily of egg-cell-specific CRPs of Arabidopsis that is specifically secreted upon sperm cell arrival. After knocking down the whole gene family, the Arabidopsis gametes are unable to fuse, indicating an essential role for these CRPs during gamete interaction (S. Sprunck, S. Rademacher and T. Dresselhaus, unpublished work). Although the functional redundancy of the various polymorphic proteins generated by the FG cells hamper their functional analysis, their specific expression pattern and first examples of candidate gene down-regulation suggest that they play key roles in the complex process of double fertilization, including gamete interaction(s). Conclusions In flowering plants, we still know almost nothing about the late events of double fertilization, as most attention had been directed until now towards the understanding of the mechanisms involved in sperm cell delivery towards and into the FG. However, gamete recognition, adhesion and fusion are key for reproductive success. Unravelling underlying molecular mechanisms will help us to solve long-standing questions about how flowering plant gametes achieve the two gamete fusion events during double fertilization in such an efficient and timely manner. It will be exciting to identify additional players of flowering plant gamete interaction and to study their evolutionary history to find out: (i) to what extent the successive steps of gamete interaction are conserved between eukaryotic organisms, (ii) whether these processes represent additional hybridization barriers, and (iii) whether the knowledge generated can be used to overcome such putative levels of pre-zygotic fertilization barriers. Progress is likely to be made in the near future, as plant gametes including those of the model systems A. thaliana and rice are now accessible for transcriptomic-based approaches ([46], L. Šoljić, T. Dresselhaus and S. Sprunck, unpublished work, and M. Sen, Y. Zhang, X. Gou and S.D. Russell, personal communication). Acknowledgements I thanks Thomas Dresselhaus for critical reading of the manuscript and Fred Berger for providing the sperm cell marker line HTR10mRFP. Funding The work in my laboratory is supported by the German Research Council (DFG) [grant number SP 686/1]. References 1 Márton, M.L. and Dresselhaus, T. (2008) A comparison of early molecular fertilization mechanisms in animals and flowering plants. Sex. Plant Reprod. 21, 37–52 2 Vieira, A. and Miller, D.J. (2006) Gamete interaction: Is it species-specific? Mol. Reprod. Dev. 73, 1422–1429 C The C 2010 Biochemical Society Authors Journal compilation 639 640 Biochemical Society Transactions (2010) Volume 38, part 2 3 Kaupp, U.B., Kashikar, N.D. and Weyand, I. (2008) Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 70, 93–117 4 Berger, F., Hamamura, Y., Ingouff, M. and Higashiyama, T. (2008) Double fertilization: caught in the act. Trends Plant Sci. 13, 437–443 5 Raghavan, V. (2006) Some reflections on double fertilization, from its discovery to the present. New Phytol. 159, 565–583 6 Yadegari, R. and Drews, G.N. (2004) Female gametophyte development. Plant Cell 16 (Suppl.), S133–S141 7 Hiscock, S. and Allen, A.M. (2008) Diverse cell signalling pathways regulate pollen–stigma interactions: the search for consensus. New Phytol. 179, 286–317 8 Rea, A.C. and Nasrallah, J.B. (2008) Self-incompatibility systems: barriers to self-fertilization in flowering plants. Int. J. Dev. Biol. 52, 627–636 9 Swanson, R., Edlund, A.F. and Preuss, D. (2004) Species specificity in pollen–pistil interactions. Annu. Rev. Genet. 38, 793–818 10 Lausser, A., Kliwer, I., Srilunchang, K.O. and Dresselhaus, T. (2009) Sporophytic control of pollen tube growth and guidance in maize. J. Exp. Bot. 61, 673–682 11 Okuda, S., Tsutsui, H., Shiina, K., Sprunck, S., Takeuchi, H., Yui, R., Kasahara, R.D., Hamamura, Y., Mizukami, A., Susaki, D. et al. (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458, 357–361 12 Márton, M.L., Cordts, S., Broadhvest, J. and Dresselhaus, T. (2005) Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307, 573–576 13 Márton, M.L. and Dresselhaus, T. (2010) Female gametophyte controlled pollen tube guidance. Biochem. Soc. Trans. 38, 627–630 14 Escobar-Restrepo, J.M., Huck, N., Kessler, S., Gagliardini, V., Gheyselinck, J., Yang, W.C. and Grossniklaus, U. (2007) The FERONIA receptor-like kinase mediates male–female interactions during pollen tube reception. Science 317, 656–660 15 Capron, A., Gourgues, M., Neiva, L.S., Faure, J.E., Berger, F., Pagnussat, G., Krishnan, A., Alvarez-Mejia, C., Vielle-Calzada, J.P., Lee, Y.R. et al. (2008) Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell 20, 3038–3049 16 Dresselhaus, T. and Márton, M.L. (2009) Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Curr. Opin. Plant Biol. 12, 773–780 17 Sandaklie-Nikolova, L., Palanivelu, R., King, E.J., Copenhaver, G.P. and Drews, G.N. (2007) Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol. 144, 1753–1762 18 Faure, J.E., Rotman, N., Fortuné, P. and Dumas, C. (2002) Fertilization in Arabidopsis thaliana wild type: developmental stages and time course. Plant J. 30, 481–488 19 Gebert, M., Dresselhaus, T. and Sprunck, S. (2008) F-actin organization and pollen tube tip growth in Arabidopsis are dependent on the gametophyte-specific Armadillo repeat protein ARO1. Plant Cell 20, 2798–2814 20 Weterings, K. and Russell, S.D. (2004) Experimental analysis of the fertilization process. Plant Cell 16 (Suppl.), S107–S118 21 Wang, Y.Y., Kuang, A., Russell, S.D. and Tian, H.Q. (2006) In vitro fertilization as a tool for investigating sexual reproduction of angiosperms. Sex. Plant Reprod. 19, 103–115 22 Dresselhaus, T. (2006) Cell–cell communication during double fertilization. Curr. Opin. Plant Biol. 9, 41–47 23 Ingouff, M., Hamamura, Y., Gourgues, M., Higashiyama, T. and Berger, F. (2007) Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr. Biol. 17, 1032–1037 24 Mori, T., Kuroiwa, H., Higashiyama, T. and Kuroiwa, T. (2006) GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat. Cell Biol. 8, 64–71 25 Sprunck, S., Baumann, U., Edwards, K., Langridge, P. and Dresselhaus, T. (2005) The transcript composition of egg cells changes significantly following fertilization in wheat (Triticum aestivum L.). Plant J. 41, 660–672 C The C 2010 Biochemical Society Authors Journal compilation 26 Ueda, K., Kinoshita, Y., Xu, Z.J., Ide, N., Ono, M., Akahori, Y., Tanaka, I. and Inoue, M. (2000) Unusual core histones specifically expressed in male gametic cells of Lilium longiflorum. Chromosoma 108, 491–500 27 Xu, H., Swoboda, I., Bhalla, P.L. and Singh, M.B. (1999) Male gametic cell-specific gene expression in flowering plants. Proc. Natl. Acad. Sci. U.S.A. 96, 2554–2558 28 Steffen, J.G., Kang, I.H., Macfarlane, J. and Drews, G.N. (2007) Identification of genes expressed in the Arabidopsis female gametophyte. Plant J. 51, 281–292 29 Ingouff, M., Sakata, T., Li, J., Sprunck, S., Dresselhaus, T. and Berger, F. (2009) The two male gametes share equal ability to fertilize the egg cell in Arabidopsis thaliana. Curr. Biol. 19, R19–R20 30 Friedman, W.E. (1999) Expression of the cell cycle in sperm of Arabidopsis: implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes. Development 126, 1065–1075 31 Nowack, M.K., Grini, P.E., Jakoby, M.J., Lafos, M., Koncz, C. and Schnittger, A. (2006) A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat. Genet. 38, 63–67 32 Chen, Z., Tan, J.L., Ingouff, M., Sundaresan, V. and Berger, F. (2008) Chromatin assembly factor 1 regulates the cell cycle but not cell fate during male gametogenesis in Arabidopsis thaliana. Development 135, 65–73 33 Scott, R.J., Armstrong, S.J., Doughty, J. and Spielman, M. (2008) Double fertilization in Arabidopsis thaliana involves a polyspermy block on the egg but not the central cell. Mol. Plant 1, 611–619 34 Spielman, M. and Scott, R.J. (2008) Polyspermy barriers in plants: from preventing to promoting fertilization. Sex. Plant Reprod. 21, 53–65 35 Martens, S. and McMahon, H.T. (2008) Mechanisms of membrane fusion: disparate players and common principles. Nat. Rev. Mol. Cell Biol. 9, 543–556 36 Chen, E.H., Grote, E., Mohler, W. and Vignery, A. (2007) Cell–cell fusion. FEBS Lett. 581, 2181–2193 37 Goodenough, U., Lin, H. and Lee, J.H. (2007) Sex determination in Chlamydomonas. Semin. Cell Dev. Biol. 18, 350–361 38 Singson, A., Hang, J.S. and Parry, J.M. (2008) Genes required for the common miracle of fertilization in Caenorhabditis elegans. Int. J. Dev. Biol. 52, 647–656 39 Wilson, N.F. (2008) Gametic cell adhesion and fusion in the unicellular alga Chlamydomonas. Methods Mol. Biol. 475, 39–51 40 Vjugina, U. and Evans, J.P. (2008) New insights into the molecular basis of mammalian sperm–egg membrane interactions. Front. Biosci. 13, 462–476. 41 Liu, Y., Tewari, R., Ning, J., Blagborough, A.M., Garbom, S., Pei, J., Grishin, N.V., Steele, R.E., Sinden, R.E., Snell, W.J. and Billker, O. (2008) The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 22, 1051–1068 42 von Besser, K., Frank, A.C., Johnson, M.A. and Preuss, D. (2006) Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133, 4761–4769 43 Cordts, S., Bantin, J., Wittich, P.E., Kranz, E., Lörz, H. and Dresselhaus, T. (2001) ZmES genes encode peptides with structural homology to defensins and are specifically expressed in the female gametophyte of maize. Plant J. 25, 103–114 44 Jones-Rhoades, M.W., Borevitz, J.O. and Preuss, D. (2007) Genome-wide expression profiling of the Arabidopsis female gametophyte identifies families of small, secreted proteins. PLoS Genet. 3, 1848–1861 45 Punwani, J.A. and Drews, G.N. (2008) Development and function of the synergid cell. Sex. Plant Reprod. 21, 7–15 46 Borges, F., Gomes, G., Gardner, R., Moreno, N., McCormick, S., Feijó, J. A. and Bector, J. D. (2008) Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol. 148, 1168–1181 Received 10 September 2009 doi:10.1042/BST0380635