* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 7.5 Proteins – summary of mark schemes
Genetic code wikipedia , lookup
Gene expression wikipedia , lookup
Lipid signaling wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Expression vector wikipedia , lookup
Magnesium transporter wikipedia , lookup
Paracrine signalling wikipedia , lookup
Point mutation wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Interactome wikipedia , lookup
Signal transduction wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Protein purification wikipedia , lookup
Homology modeling wikipedia , lookup
Western blot wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Biochemistry wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Metalloprotein wikipedia , lookup
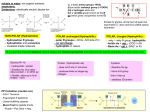
7.5 Proteins – summary of mark schemes 7.5.1 Explain the four levels of protein structure, indicating the significance of each level. Mark Scheme A. B. C. primary structure / level: order / sequence of amino acids; linked by peptide bonds; determines the type / function of protein / 2º and 3º structures; D. E. secondary structure / level: regular folding / beta-pleated sheets / spiralling / alpha-helices; held through hydrogen bonding; F. tertiary structure / level: 3-dimensional conformation of a polypeptide / protein; G. held with ionic bonds, hydrogen bonds, disulfide bonds / bridges and hydrophobic bonds; (must give at least two bonds) H. determines overall shape / a named example eg: active sites on enzymes; I. J. K. L. 7.5.2 quaternary structure linking together of polypeptides to form a single protein; using the same bonding as for tertiary structure; linking of a non-polypeptide structure / prosthetic group; named example of quaternary structure eg hemoglobin (has four polypeptides); Outline the difference between fibrous and globular proteins, with reference to two examples of each protein type. Mark Scheme Fibrous vs globular A. repetitive amino-acid sequences B. long and narrow / long strands C. support / structural functions D. (mostly) insoluble in water vs. vs. irregular amino acid sequences; vs. rounded / spherical / ball shaped; vs. metabolic / other functions; (mostly) soluble in water; Fibrous examples E. collagen; F. myosin; G. keratin; H. fibroin / elastin / silk protein in insects and spiders; Globular examples. I. catalyses / other named enzyme; J. hemoglobin / myoglobin; K. insulin / other peptide hormone; L. immunoglobulin / other globular protein; 7.5.4 State four functions of proteins, giving a named example of each. Mark Scheme A. B. C. D. E. F. G. H. I. J. K. L. storage – zeatin (in corn seeds) / casein (in milk); transport – hemoglobin / lipoproteins (in blood); hormones – insulin / growth hormone / TSH / FSH / LH; receptors – hormone receptor / neurotransmitter receptor / receptor in chemoreceptor cell; movement – actin / myosin; defense – antibodies / immunoglobin; enzymes – catalase / RuBP carboxylase; structure – collagen / keratin / tubulin / fibroin; electron carriers – cytochromes; pigments – opsin active transport – sodium pumps / calcium pumps; facilitated diffusion – sodium channels / aquaporins;