* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chem E2b - Organic Chemistry II What is Organic Chemistry?
2-Norbornyl cation wikipedia , lookup
Elias James Corey wikipedia , lookup
Marcus theory wikipedia , lookup
Aromaticity wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Discodermolide wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Aromatization wikipedia , lookup
George S. Hammond wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Bottromycin wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Aldol reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Petasis reaction wikipedia , lookup
Chem E2b - Organic Chemistry II
Read syllabus before asking any questions…please.
3 midterms (100 pts each)
6-7 quizzes or take-home test questions
Final exam
Lab grade
There are NO make-up exams for the midterms!!!
There are NO make-up quizzes!!!
What is Organic Chemistry?
The chemistry of molecules
associated with living organisms
The Chemistry of C, H, N, O, S, & PContaining molecules
Natural
Products
Loads of FUN!!
Unnatural
Bioactive
Molecules,
eg Drugs
Polymers,
Materials
1
Consumer
Products:
From Food to
to the Package that
Contains it!
What Molecules Are Important? All of them…
H3C O
CH3
O2N
H3C
Me
NO2
O-Na+
MeO
O
NO2
O
naproxen sodium
(alleveTM)
tri-nitrotoluene
(TNT)
testosterone
NH2
Cl
O
O
O
OH
HO
Cl
OH
N
N
Cl
OH
OH
NH
HO
N
O
sucralose
(SplendaTM)
O
HO
N
NH2
HO
tryptophan
OH
adenine
short-hand drawings:
CH3CH2CH3
propane
H H
H
C
C
C
H H H H
H
H3C
CH3
E2a Review: Things You Should Already Know for this Class
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
Hybridization
Molecular Orbitals: orbital types and interactions, diagrams
Conformational analysis: drawing chair conformations and Newman projections
Functional group names and nomenclature
Stability of carbocation and anion intermediates
Resonance
Curved arrow mechanism, identifying electrophiles and nucleophiles
Acidity and Basicity
Kinetics and thermodynamics: reaction coordinate diagrams
Relative Reactivity
Stereochemistry
Reagents and Reactions of: alkenes, dienes, alkynes, alkyl halides and alcohols,
substitution and elimination reactions
2
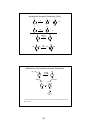
Review - Molecular Orbitals
Bonding in ethene:
Review - Acidity
Brønsted–Lowry Acids and Bases: acid donates a proton, base accepts a proton
• Strong reacts to give weak
• Weaker base = stronger
conjugate acid
• Stable bases (anions) are
weak bases
Lewis Acids and Bases
• Lewis acid: non-proton-donating acid;
will accept two electrons
• Lewis base: electron pair donors
3
Review - Acidity and Anion Stability
Factors that influence anion stability (more stable conjugate anion is a better acid):
1) Size of atom - applies only to comparison within columns of periodic table
2) Electronegativity - applies to comparison in rows, as well as carbon hybridization
3) Resonance - more resonance is more stable
4) Electron-withdrawing groups stabilize (inductive effect), electron-donating groups
destabilize
5) Aromaticity - an anion is more stable if it is aromatic
CH3CH2OH
most
acidic
pKa = 15.5
Strongest
conjugate base
Weakest
conjugate base
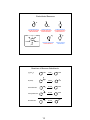
Review: Carbocation Stability
• Carbocations are important intermediates in electrophilic additions to alkenes, and in
SN1and E1 reaction mechanisms.
1)
2)
3)
4)
hyperconjugation: more substituted carbocation is more stable
Resonance/conjugation: carbocation with more resonance is more stable
Electron-donating groups stabilize, electron-withdrawing groups destabilize
Aromaticity: an aromatic carbocation is very stable
H
+
H
+
CH3
H3C
N
>
H3C
H
+
CH3
O
>
H3C
CH3
H
+
CH3
>
CH3
H3C
O
O
more electron-donating
substituent
less electron-donating
substituent
4
Review - Resonance
Localized vs. delocalized electrons:
Examples of resonance contributors:
Phenol is more acidic than cyclohexanol because the phenoxide ion is stabilized by resonance:
(conjugate base of phenol)
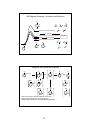
Review - Conformational Analysis
0°
Conformations of n-Butane:
(Newman projections)
A staggered conformer
is more stable than an
eclipsed conformer
H3C CH3
H
H
H
CH3
H
H
H
HCH3
H
H
CH3
H
H
H
eclipsed
CH3
gauche
A
B
H
CH3
H
H
CH3
H CH3
H
H
H3C
H
H
D
H
H
H
H
gauche
anti
C
CH3
H3C
E
F
Chair conformations:
Steric strain of 1,3-diaxial interactions
makes axial conformer less stable
5
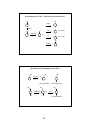
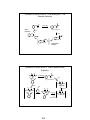
Review: Thermodynamics and Kinetics
Reaction coordinate diagrams:
∆G°
=
∆H° – T∆S°
∆G° = Gibbs standard free energy change
Enthalpy (∆H°) = the heat given off or
absorbed during a reaction
Entropy (∆S°) = a measure of freedom of
motion (usually ∆S° is small compared to
∆H° and ∆G° ~ ∆H°)
∆G‡ = (∆G of transition state) – (∆G of reactants)
∆G‡ = energy of activation
Two “Rules of 1.4”:
1) Increasing ∆G‡ by 1.4 kcal/mol
decreases the rate by a factor of 10
2) changing ∆G˚ by 1.4 kcal/mol
changes the product ratio by a factor
of 10
The rate-limiting step controls the overall rate of the reaction
The highest hill on the reaction coordination diagram is the rate-limiting step
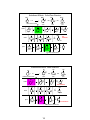
Review: Stereochemistry
Cl
Cl
CH3
CH3
Enantiomers
Cl
Cl
Diastereomers
Diastereomers
Cl
Cl
CH3
Enantiomers
CH3
Cl
Cl
Cl
CH3
H3C
lowest priority
group oriented behind
1
Cl
Enantiomers
Br
Cl
Diastereomers
CH3
Diastereomers
Cl
MESO
CH3
H3C
Cl
Cl
6
3
'
1-2-3
counterclockwise = S
Cl
CH3
H
H3CH2C
Cl
H3C
2
CH3
H3C
Functional Group Review
H
CH3
R
R
alkane
F
R
N
R
Cl
R
Br
R
alkyl chloride
I
R
alkyl bromide
O
epoxide
benzene
diene
alkyne
alkene
alkyl fluoride
R
R
R
SH
R
alkyl iodide
CN
R
nitrile
(cyano group)
thiol
Me
R
R
O
OH
R
H
amine
O
R
alcohol
R
R
ether
R
S
O
O
sulfonate ester
(OTs, tosylate)
R
aromatic compounds
(substituted benzenes)
carbonyl compounds
O
R
O
N
H
amide
O
R
R
O
ester
O
O
O
O
O
R
R
OH
carboxylic
acid
R
H
aldehyde
R
R
ketone
R
Cl
acid
chloride
R
O
R
anhydride
Review: Polar Organic Reactions (anions and cations)
• Reactions involving cations: electrophilic addition to an alkene, SN1 and E1, etc
• Reactions involving anions: anything with a nucleophile (SN2, E2, etc)
• Curved arrow mechanism: from an electron-rich center to an electron-poor center
(Robinhood rule)
Movement of a
pair of electrons
Movement of one electron
“fish-hook arrow”
7
Reactions of Benzene
Z
+
Y Z
R
Y
R
Z-
R
Y
(a)
H
H
H
Y Z
+
Y
H
addition
(path a)
Z-
(b)
substitution
(path b)
H
Z
Y
H
benzene +Y-Z
(nonaromatic)
H
Y
+ Hbenzene (- H+) + (Y+)
(aromatic)
Electrophilic Substitution is Thermodynamically Favored
First step is rate determining
8
Sidenote: Synthesis of Aspirin from Crude Oil
O
OH
OH
O
CO2/base
[O]
O
OH
Me
Me
O
O
O
O
OH
Me
acetyl salicylic acid
(Aspirin)
oil well
Willow Trees:
Relieves Fever,
Upsets Stomach
Summary of Electrophilic Additions
FeX3/X2
Halogenation
HNO3/H2SO4
Nitration
H2SO4/∆
Sulfation
X
NO2
SO3
O
Friedel-Crafts
Acylation
RCOCl/AlCl3
Friedel-Crafts
Alkylation
RCl/AlCl3
9
R
R
Halogenation of Benzene
•
•
•
In general, benzene is less reactive than most alkenes.
Alkenes generally react with neutral electrophiles, e.g. Br2, Hg(OAc)2, etc.
Benzene generally reacts with cationic electrophiles, which are more reactive due to the
positive charge
Br
R
Br
Br
R
Br
H
Br
Br
X
Br
FeBr3
H
Br
+ Br Br Fe Br
+
H
Br
Br
Br Fe Br
Br
Br
Nitration of Benzene
O
H
O
N+
O
O
O-
H2SO4
HSO4-
H
O+
HNO3
N+
O-
+N
+
H
O
H
O
H
H
O
N
+
O
+
O
N+
H
O
N+
-
O
10
HSO4-
O-
+
H2SO4
Friedel-Crafts Acylation
O
R
Cl
Cl
Cl
Al
O
Cl
Cl
Al
Cl+ - Cl
R
+
O
O
Cl
+ AlCl4-
+
R
R
an acyl chloride
an acylium ion
+
O
O
H
H
O
R
R
R
AlCl4-
AlCl3
Friedel-Crafts Alkylation
R
R
Cl
Cl
Cl
Al
R
Cl
R
H
Cl
Cl
Al
Cl
Cl
+
R
an alkyl chloride
+
R
+ AlCl4-
a carbocation
H
R
R
H
R
+
R
H
R
R
AlCl4-
H+ AlCl4-
11
Carbocation Rearrangements Occur During Friedel-Crafts
Alkylations
R
H
Cl
R
Cl
Cl
Al
R
Cl
+
R
+ AlCl4-
1,2 alkyl shift
1,2 hydride shift
ring expansion
Me
Cl
Me
Me
Me
Me
O%
H
100%
+
H
Me
Me Me
Me
Me
Me
AlCl3
Me
+
Me
Me
Me
Friedel-Crafts Acylation/Reduction: Net Alkylation
H H
R
Cl
AlCl3
+
H
H
R
X
H2; Pd/C
O
O
R
•
•
Cl
AlCl3
+
R
Because of the problems associated with rearrangements, etc, primary alkyl groups are best
introduced by a Friedel-Crafts Acylation, followed by reduction to replace the carbonyl group
with two H’s
The use of H2/Pd only works with carbonyl groups with benzene rings on one or both sides.
12
Disubstituted Benzenes
Br
Br
Br
Br
Br
1,2-dibromobenzene
ortho-dibromobenzene
o-dibromobenzene
1,3-dibromobenzene
meta-dibromobenzene
m-dibromobenzene
Br
1,4-dibromobenzene
para-dibromobenzene
p-dibromobenzene
NO2
ortho
Z
meta
NO2
ortho
Br
meta
para
Br
1-bromo-3-nitrobenzene
meta-nitrobenzene
Cl
2-bromo-4-chloro1-nitrobenzene
Reactions of Benzene Substituents
Br
NaOH
Me
KOt-Bu
SN2 (& SN1)
OH
Br
E2 (& SN1)
H2; Pd/C
Me
H
H2; Pd/C
OH
O-
H2; Pd/C
Alkene Reduction:
O
Carbonyl Reduction:
O
N+
Nitro Reduction:
13
NH2
Effect of Substituents on Reactivity
R
R
R
H
E
+
E+
E
-H+
R
H
E
+
R
R
E
•
•
Electrophilic Substitution reactions proceed via carbocation intermediates.
Carbocation formation is the rate limiting step, so the stability of the carbocation that is
formed determines the speed of the reaction
Substituent Effects on Reactivity
R = Electron Withdrawing (Slower)
R=H
R = Electron Donating (FASTER)
R
+
H
E
R
•
•
Electron donating groups help stabilize positive charge, so they make benzene rings more
reactive
Electron withdrawing substituents destabilize positive charge, so they make benzene rings
less reactive
14
Inductive Effects on Benzene Reactivity
electron donating
group
electron withdrawing
group
Z
H
Y
>
>
LESS REACTIVE
(Electrophilic Substitution)
MORE REACTIVE
(Electrophilic Substitution)
•
•
•
•
•
•
•
•
As with acidity, inductive effects are generally WEAKER than resonance effects
Z = NR2, OR: Strongly Activating (resonance)
Z = NHCO2R, OCO2R: Moderately Activating (inductive)
Z = R (Alkyl, vinyl): Weakly Activating (inductive)
Y = F, Cl, Br, I: Weakly Deactivating (inductive withdrawal/resonance donation)
Y = CO2R: Moderately Deactivating (resonance)
Y = CN, SO2R, NO2: Strongly Deactivating (resonance)
Y = NH3+: Strongly Deactivating (inductive, positively charged)
Effects of Substituents on Orientation
1) All activators are o,p-directors
Me
Me
Me
Me
Br
FeBr3/Br2
Br
Br
p-bromotoluene
o-bromotoluene
m-bromotoluene
NOT OBSERVED
2) Weak deactivators (halogens) are o,p-directors.
Br
Br
Br
Br
Br
FeBr3/Br2
Br
o-dibromobenzene
Br
p-dibromobenzene
m-dibromobenzene
NOT OBSERVED
3) All moderate and strong de-activators are m-directors
O
Me
O
Me
O
O
Me
Me
NO2
HNO3/H2SO4
NO2
m-nitroacetophenone
15
o-nitroacetophenone
NOT OBSERVED
NO2
p-nitroacetophenone
NOT OBSERVED
Substituent Effects: Ortho/Para Directors
MeO
+
MeO
MeO
MeO
H
+
H
H
MeO
NOT
OBSERVED
H
E
E
H
MeO +
MeO
MeO
-H+
+
para:
+
+
H
H
E
MeO
-H+
+
H
E
-H+
H
E
+
MeO
MeO
E
para
MeO
H
MeO
-OR-
H
E
+
+
H
E
H
E
+
MeO
H
E
+
MeO
H
meta
+
H
E
meta:
-OR-
ortho
MeO
+
H
E
+
E+
stable resonance form
ortho:
MeO
MeO
H
E
H
H
E
H
H
E
H
E
E
stable resonance form
Substituent Effects: Meta Directors
+
+
E+
NO2
-OR-
H
E
+
-OR-
+
H
meta
-H+
H
E
+
E
para
NO2
NO2
H
E
+
NO2
H
NO2
H
E
+
H
E
ortho
unstable resonance form
ortho:
NO2
NO2
NO2
E
NOT OBSERVED
NO2
meta:
NO2
H
E
+
+
H
NO2
H
E
H
NO2
NO2
-H+
+
H
E
E
H
NO2
NO2
NO2
-H
+
para:
+
+
H
H
E
+
H
H
E
H
H
E
E
unstable resonance form
16
NOT OBSERVED
Hill Diagram Summary: Activation and Direction
R
O
H
+
N+
R
H
R
+N H
R
O
S R
O
OO
N
R = Deactivating/m-directing
R
o,p - m frontier
R = weakly deactivating/o,p-directing
activation frontier
R=H
F, Cl, Br, I
R = Activating/o,p-directing
R
+
R
H
N
R
R
O
R
O
H
E
R
+
H
E
O
N
H
OR
R
Halogens are Ortho/Para Directors
Cl
Cl
Cl
E+
+
Cl
H
E
-H+
-OR-
+
H
Cl
E
-OR-
H
E
E
MeO
+
+ Cl
H
E
NO2
H
E
H
E
+
MeO +
Cl +
NO2
+
H
H
E
H
H
E
Cl behaves like MeO for directing substitution (resonance)
Cl behaves slightly like NO2 for activation (induction)
As with acidity, resonance is more important than induction!
17
H
H
E
Sample Problem: Planning A Synthesis with Aromatic
Substitution
SO3H
Br
Sample Problem: Planning A Synthesis with Aromatic Substitution
O
Me
NO2
18
Arenediazonium Salts: Selective Monosubstitution
CN
NO2
CuCN
X
H2; Pd/C
CuX
N2+
NH2
NaNO2/HCl
(X = Cl or Br)
I
Cl-
("HONO")
KI
(X = Cl or Br)
OH
HCl/H2O
Synthesis with Arenediazonium Salts
Me
Me
Me
Cl
FeCl3/Cl2
Cl
o-chloroethylbenzene
Me
p-chloroethylbenzene
Me
Me
NaNO2/HCl
Cl-
CuCl
("HONO")
NH2
N2+
Cl
p-chloroethylbenzene
19
Nucleophilic Aromatic Substitution (SNAr)
R
R
R
E
E+
+ H+
E
X
Nu
Nu+ X-
Cl
OH
NaOH/∆
pH 14
"Nu-"
NO2
NO2
Cl
O2N
NO2
H2O
pH 7
OH
O2N
NO2
NO2
NO2
Mechanism of Nucleophilic Aromatic Substitution
HO-
X
OH
NaOH/∆
pH 14
NO2
NO2
addition
elimination
HO X
HO X
-
NO2
NO2
other
resonance
forms
•
X needs to be Small (F and Cl are best), and the benzene ring needs to be highly activated (at
least one NO2).
20
Benzyne
Cl
NH2
NaNH2
SNAr?
Cl
H2N
H
-
NH2
*
H
NaNH2
*
AND
H
elimination
NH3
-
H2N
*
H2N
NH2
- *
NH3
-NH2
Benzyne
Intermediate
H2N
*
-
Structure of Benzyne
R
R
Alkyne
Benzyne
21
*
Heteroaromatics
5-membered heterocycles
N
S
O
H
pyrrole
furan
thiophene
6-membered heterocycles
N
N
N
pyridine
isoquinoline
quinoline
6,5-fused heterocycles
N
indole
O
H
S
benzofuran
benzothiophene
All of these heterocycles are aromatic (recall rules of aromaticity & Hückels Rule)
They all participate in Electrophilic Aromatic Substitution (EAS) Reactions
EAS: 5-Membered Heterocycles
O O
H3C O CH3
3
4
EAS:
2
5
O
CH3
N
N1
+
H3C
OH
O
H
2-acetylpyrrole
H
4
3
2
5
EAS occurs
preferentially
at the C2-position
Br2
O1
Br
O
2-bromofuran
4
3
HNO3
2
5
H2SO4
S
1
S
NO2
2-nitrothiophene
Consider Mechanism:
EAS at C2
H
20-allylic carbocation
E+
N
N
20-allylic carbocation
E
E
H
H
H
EAS at C3
N
N
H
H
20-alkyl carbocation
22
E
N
H
H
H
+
E
E
N
H
H
Relative Reactivities of 5-Membered Heterocycles in EAS
>
>
>
O
N
H
S
Pyrrole, furan, and thiophene are all more reactive than benzene as heteroatom lp
can better stabilize the carbocation intermediate
Relative reactivities are reflected in L.A. needed to mediate Friedel-Crafts acylations
O
O
+
AlCl3: Strong L.A.
AlCl3
H3C
O
SnCl4: Weaker L.A.
+
S
H3C
SnCl4
H2O
Cl
O
+
BF3: Weak L.A.
O
CH3
H2O
Cl
O
BF3
H3C
H2O
Cl
CH3
S
CH3
O
O
O
NO L.A.
+
N
H
O
H3C
O
no cat.
CH3
required
CH3
N
H
less reactive
acylating agent
O
Reactions of Six-Membered Heterocycles
+
SN2:
H3C I
N-methylpyridinium iodide
N
N
I
CH3
+
N-Oxidation:
+ H2O
HO OH
N OH
N
N
OH
O
Pyridine-N-Oxide
+ Br2
EAS:
N
Br
FeBr3
Preferential substitution @C3, why ??
300 °C
N
30%
High Temps required due to the fact that
electron withdrawing N-atom destablizes
carbocation intermediates.
B
Mechanism:
Y
+
N
Y+
slow
H
N
23
Y
fast
+
N
HB+
Electrophilic and Nucleophilic Substitutions on Pyridine
What about Nucleophilic Aromatic Substitutions ?
4
3
5
2
6
N
1
OCH3
NaNH2
∆
N
NH2
SNAr takes place ONLY at C2 and C4 positions
OMe
Br
Why ???
N
NaOMe
∆
N
Regiochemistry of SNAr
Regiochemistry of Nu Addition:
2-Different LG’s: If LG’s are different, Nu will preferentially substitute at LG which is weaker base
(better LG)
better LG
Br- is weaker
base than CH3O-
Br
N
NH2
OCH3
NaNH2
∆
CH3
N
N
OCH3
CH3
Cl
24
NaOMe
∆
N
OMe
Other Reactions of Pyridine
NBS
Benzylic Bromination
∆, ROOR
R
N
R
N
Br
Reactions of Pyridine Diazonium Salts
H2O
NaNO2 / HCl
N
NH2
0 °C
N
N
Cl
N
N
OH
enolic form
NH2
N
NaNO2 / HCl
N
N Cl-
OH
N
O
H
keto form (more stable)
α-pyridone
O
H2O
0 °C
N
N
enolic form
N
H
keto form (more stable)
γ-pyridone
Summary Slide
• Aromatic compounds undergo electrophilic aromatic
substitution (EAS), (mechanistically related to alkene
additions) in which aromaticity is restored in the product
• Electron donating substituents activate benzene toward EAS,
and direct the electrophiles to the ortho and para positions
• Electron withdrawing substituents deactivate benzene toward
EAS, and direct electrophiles to the meta positions
• Halogens are deactivating (inductive effect) and ortho/para
directing (resonance)
• Nucleophiles can add to arenediazonium salts, strongly
activated aryl halides (SNAr), or benzyne intermediates
• 5-Membered heteroaromatics participate in EAS at C2position.
• 6-Membered heteroaromatics participate in EAS (C3-position)
and SNAr reactions (C2 and C4 positions)
25
Neutral Organic Reactions - Radicals
heterolytic cleavage
H
H
H
C
C
H
H
H
H
H
H
+
C
C
formation of polar,
charged species
H
H
H
methyl anion
methyl cation
homolytic cleavage
H
H
H
C
H
C
H
H
H
H
H
+
C
C
formation of non-polar,
neutral species
H
H
H
methyl radical
methyl radical
Structure of radical is somewhere between a cation and anion,
half-filled p-orbital is a SOMO (Singly Occupied Molecular Orbital)
both are
electron-deficient
Thermodynamics
• Bond breaking is endothermic (∆H˚ is positive)
• Bond formation is exothermic (∆H˚ is negative)
H
H
H
H
H
C
H
C
H
+
C
H
C
H
H
∆H˚ = 90 kcal/mol
H
H
Energy of activation (∆G‡) = ∆H˚ when bonds are broken homolytically, but no bonds
are formed, IF no solvation is involved (in the gas phase)
formation of bond:
cleavage of bond:
σ∗
sp3
H H
C
H
H H
C
H
H
C
HH
sp3
H
C
H
sp3 H
sp3
energy
H
H
H C C
H
H
H
σ
stabilization energy
= 90 kcal/mol
reaction progress
∆H˚ is negative
26
90 kcal/mol required
to break bond
∆H˚ is positive
Bond Dissociation Energy (BDE)
X + Y
∆H˚ = ∆G≠ = BDE
energy
Bond Dissociation Energy
(BDE) = energy required to
break a bond homolytically
X Y
reaction progress
Bond
Cl
Br
I
RO
Cl
Br
I
I
Br
Cl
Bond Dissociation
Energy (kcal/mol)
Cl
Br
I
OR
H
H
H
CH3
CH3
CH3
Bond Dissociation
Energy (kcal/mol)
Bond
58
46
36
38
103
87
71
84
70
57
H
H3C
H3C
H2C
H3C
H3C
H2N
CH3O
HO
104
105
88
67 (π bond only)
92
85
107
104
119
H
H
CH3
CH2
OH
NH2
H
H
H
See Bruice Table 3.1, page 129
Two Factors Can Decrease the BDE (make the bond easier to
break)
1) Make bond less stable (raise the energy of the reagent)
H2
C
H2C
H3C
CH2
H2
C
CH3
∆H˚ =
65 kcal/mol
∆H˚ =
85 kcal/mol
CH2
CH2
H2C
energy
H3C
CH2
+
CH3
∆H˚ =
65 kcal/mol
CH2
H2 C
CH2
∆H˚ =
85 kcal/mol
CH3CH2CH3
cyclopropane has strain energy that raises the energy
of the C–C bond, therefore making it easier to break
reaction progress
2) Make radical more stable (lower the energy of the transition state)
BDE (kcal/mol)
-from cleavage
of a C–H bond,
with R = CH3
85
105
85
96
27
99
101
104
Stability of Radicals: Just Like Carbocation Trends
1) Hyperconjugation: more substituted radicals are more stable
2) Conjugation/Resonance: more resonance, more stable radical
H
C
most
resonance
CH2
>
>
CH2
>
H3C
no
resonance
CH2
3) Hybridization: more s character of the SOMO, less stable radical
sp3-hybridized,
least s character
H3C
CH2
>
H
H
>
>
R C
H
C
sp-hybridized,
most s character
Hyperconjugation in Carbocations and Radicals
Carbocation
stabilization:
antibonding MO
A σ C–H bond donates
into the empty p-orbital to
stabilize the carbocation
bonding MO
σ C–H = donor (HOMO)
empty p-orbital = acceptor (LUMO)
Cationic, 2-electron system
Radical
stabilization:
A σ C–H bond donates
into the SOMO (singly
occupied molecular
orbital) to stabilize the
radical
half-filled
p orbital
H
H
H
C
C
antibonding MO
H
H
σ C–H = donor (HOMO)
SOMO = acceptor
bonding MO
Radical, neutral, 3-electron system
Hyperconjugation is less stabilizing for a radical because the one electron in the antibonding
MO has a destabilizing effect. (recall He2+ with a similar 3-electron system)
28
Radical Reactions with Alkenes
reaction proceeds through a
carbocation intermediate,
rearrangements are possible
reaction proceeds through a
radical intermediate,
NO rearrangements are
possible
hv or ∆
Formation of bromine radical:
∆H˚ = 38 kcal/mol
Initiation steps
to create radicals
This is NOT a general reaction. The peroxide effect does NOT work with any other HX.
Mechanism: More Stable Radical Forms Faster
carbocation mechanism:
+
H3C
CH3
H3C
H Br
δ+ δ-
+
Br-
Br
H+ adds to primary carbon to form a secondary carbocation,
which is more stable than a primary carbocation
radical mechanism:
H3C
+
.Br
CH3
H3C
secondary
alkyl bromide
H-abstraction and
formation of new radical
H3C
Br
Br adds to primary carbon to form a secondary radical,
which is more stable than a primary radical
29
H Br
H3C
Br
primary
alkyl bromide
+ .Br
Synthesis Using Primary Alkyl Bromides
A primary alkyl bromide is a good substrate for an SN2 reaction:
O
HBr, ROOR
H3C
hv
NaO
Br
H3C
O
CH3
O
H3C
CH3
nucleophilic
substitution
Recall the previous general method that we used to convert an alkene to a primary SN2
substrate with a good leaving group:
H3C
1. BH3
2. NaOH, H2O2
H3C
OH
1. TsCl/pyridine
2. Nucleophile
hydroboration
and oxidation
H3C
Nu
formation of good leaving group
followed by nucleophilic substitution
Examples of nucleophiles = NaCN, NaOH, NaOMe, NaSMe, NaOAc, NaCl, NaBr
Radical Mechanism Sample Problem
HBr
Provide the mechanism for this reaction.
ROOR, hv
∆H˚ = 38 kcal/mol
Problem-solving strategy:
1. Draw a step that breaks the weak bond in the initiator
2. Draw a reaction of the initiator with one of the starting material (SM)
3. Draw a reaction of SM radical with another SM (repeat if needed)
4. Draw a termination step
30
Br
Radical Reactions with Alkanes
Not practical or useful with F2 or I2
Major product because
secondary radical is
more stable
Remember, rearrangements are not possible with radicals!!
Mechanism of Radical Reactions with Alkanes
H-abstraction
goes back to starting material
very minor by-product
desired product!!
The rate-determining step of the overall reaction is hydrogen abstraction
(Same mechanism for monobromination - practice at home…)
31
Again, More Stable Radical Forms Faster
H-abstraction at benzylic
and allylic positions is easier
because benzyl and allyl
radicals are stabilized by
resonance
NBS as a Milder Brominating Reagent for Allylic Bromination
Advantage: the low
concentration of Br2 and
HBr present cannot be
added to the double bond
NBS provides a good source of HBr and Br2 in low concentration
O
H3C
Br
hv or ∆
+
N Br
O
RO- OR
(peroxide)
+
H3C
Br
H3C
Unsymmetrical alkenes will form two different products because the allyl radical intermediate
has two resonance contributors that are not equivalent
H
H3C
+
.Br
H3C
32
H3C
CH2
+
HBr
Phenolic Compounds as Anti-Oxidants
• Radical reactions occur in the body, usually initiated by metal ions in enzymes.
Unwanted radicals cause damage to cells, leading to disease.
• Phenolic compounds, such as BHT, BHA and Vitamins A & E are “anti-oxidants” that act
as radical scavengers (inhibitors). They are typically used as food preservatives.
OH
CH 3
CH 3
CH 3
CH 3 OH
H 3C
H 3C
CH 3
CH 3
CH 3
CH3
HO
CH3
H3C
OCH 3
O
CH3
CH 3
CH3
CH3
CH3
CH3
vitamin E
(α-tocopherol)
BHA
BHT
(butalyted hydroxyanisole) (butalyted hydroxytoluene)
Radical scavenger mechanism:
H
R
unstable
free radical
Reactive!!
O
CH3
CH3
CH3
+
O
OCH3
radical
scavenger
CH3
CH3
CH3
+
R H
OCH3
very stable radical (resonance)
Unreactive!!!
Phenolic compounds
donate an H to the
free radical to form
two stable unreactive
species
Natural “anti-oxidants” that serve as radical scavengers in vitro can be found in chocolate,
green tea, wine, grape juice, fruits and vegetables, etc. Recent studies have shown that
dark chocolate has 2x as many phenolic compounds as milk chocolate, 2x as many as green
tea or wine, and 20x as many as in tomatoes.
Stereochemistry of Radical reactions
CH3
H3C
Br
Br2, hv
Racemic mixture of products
(a pair of enantiomers)
CH3
H3C
• Both enantiomers are formed because the radical
intermediate is planar, like a carbocation
• If there is already an asymmetric center present in the
molecule, then diastereomers will be formed.
Cl
H3C
CH3
Cl
Br2, hv
Cl
CH3
H3C
Br
33
+
CH3
H3C
Br
a pair of
diastereomers
Summary of Radical Reactions
Know practical information of which radical reactions work (see below)
Know thermodynamics and effects of bond and radical stability on BDEs
Know radical stability to identify which product will form and be able to explain why a
radical is stable - be able to draw resonance structures!!
Know stereochemistry of radical reactions
•
•
•
•
Br
Br2
Br2, ∆
CH3
CH3
Br
hv or ∆
Br
NBS, ∆
HBr
H3C
CH3
H3C
secondary
alkyl bromide
HBr
ROOR
H3C
1.
2.
3.
4.
ROOR
Br
Br
allyl bromide
primary alkyl bromide
Addition of H-Br to alkenes with peroxides
Halogenation (Cl2 or Br2) of alkanes
Substitution of benzylic hydrogens with Cl2 or Br2
Substitution of allylic hydrogens with NBS
Radicals: Sample Problems
1) Based on the BDEs given, explain why an acyl peroxide forms radicals more easily than an
alkyl peroxide? (include structures in your answer)
RO OR
alkyl peroxide
CH3
CH3
O
O
∆H˚ = 38 kcal/mol
O
O
∆H˚ = 29 kcal/mol
acyl peroxide
2) How many products are possible for the reaction of 3-methyl-1-cyclohexene with NBS?
Draw the structures of these products. (your answer does not need to include enantiomers)
CH3
O
+
N Br
O
hv or ∆
RO- OR
(peroxide)
3) In order to demonstrate why BHA is such a good radical scavenger, draw the 5 major
resonance structures for the radical formed upon the reaction of BHA with a free radical.
OH
OCH3
CH3
CH3
CH3
BHA
34
Summary of Reactive Intermediates
Name
Structure
Stability
Properties
R
carbocation
3˚ > 2˚ > 1˚ > Me
C
R
R
R
radical
3˚ > 2˚ > 1˚ > Me
C
R
R
C
R
Nucleophilic, electron-rich,
strong base, lone pair (HOMO)
is a good donor
all very reactive
Neutral divalent carbon, empty porbital is a good acceptor and
lone pair is a good donor, so it is
both electophilic AND nucleophilic
R
R
carbene
electron-deficient, singly occupied
MO (SOMO) is reactive, can be
either electrophilic or nucleophilic
Me > 1˚ > 2˚ > 3˚
R
carbanion
Electrophilic, electron-deficient,
strong acid, empty p-orbital
(LUMO) is a good acceptor
C
R
Carbene Formation
A carbene is a neutral species containing a divalent carbon
Br
Br
CH3
H3C
CH3
C
Br
empty 2pz orbital
(carbocation)
KO
Br
Br
C
Br
(or KOH)
H
103˚
C
Br
dibromocarbene
filled sp2 hybrid orbital
(lone pair/carbanion)
treatment with
a strong base
Mechanism of formation:
Br
Br
Deprotonation
with strong base
Br
Br
Br
C
α-elimination
Br
C
C
Br
H
Br
dibromocarbene
OH
35
Formation of Carbenes from a Diazo-Compound
empty 2pz orbital
(carbocation)
CH2N2 = diazomethane (a yellow gas)
H
C N N
H
hv or ∆
H
C
H
+
103˚
N2
H
C
H
carbene and N2 gas
filled sp2 hybrid orbital
(lone pair/carbanion)
resonance structures of diazomethane:
H
H
C N N
H
C N N
H
C N N
H
H
carbon has
anion character
carbon has
cation character
Examples of other diazo-compounds: (brightly colored, but dangerous!!)
O
H3C
N2
N2
H3C
4,4-dimethyldiazocyclohexa2,5-diene (a purple liquid)
H3 C
diazocyclopentadiene
(an iridescent orange liquid)
O
N2
methyl diazoacetate
(a yellow liquid)
Carbenes react with Alkenes to form Cyclopropanes
H
CH2N2
CH2
hv or ∆
H3C
H
H
CH3
N2
hv or ∆
H
H3C
H
Stereochemistry of the
alkene is retained in the
cyclopropane product
H
CH3
H
CHBr3, KOH
Br
C
H
Br
One-step stereospecific syn addition mechanism
36
Carbenes: Molecular Orbital Interactions with Alkenes
Consider what orbitals are available to interact for bonding:
H alkene π∗ = LUMO
H
(acceptor)
H
H
H
H alkene π = HOMO
H
empty 2pz orbital = LUMO
(acceptor)
H
C
H
filled sp2 hybrid
orbital = HOMO (donor)
(donor)
H
Two stabilizing (bond forming) interactions are possible:
Stabilizing Interaction #1:
Stabilizing Interaction #2:
empty p orbital of
carbene (acceptor)
H
H
C
H
H
H
H
H
H
empty p
orbital
alkene π
C
lone pair of
carbene (donor)
H
H
H
H
alkene π
*
sp2
carbene
alkene π* (acceptor)
alkene π (donor)
There are two destabilizing interactions that DO NOT occur:
1) interaction between alkene π and filled sp2 of carbene (because both are donors)
2) interaction between alkene π* and empty p orbital of carbene (because both are acceptors)
Carbenes: Sample Problems
1) Draw at least 5 of the major resonance structures for
4,4-dimethyldiazocyclohexa-2,5-diene (a purple liquid):
H3C
CH3
N2
2) Fill in the products or reagents for these reactions:
H3C
H3C
H3C
H3C
O
H3CO
N2
hv
37
H
Cl
Cl
H
Determining the Structures of Organic Molecules
How do you identify the products you synthesize?
OH
H3C
1) BH3
2) NaOH, H2O2
H2O, H2SO4
H3C
CH3
H3C
OH
How can you tell which reagents give which products?
How can you tell if only one major product is formed?
CH3
CH3
CH3
NO2
HNO3
Does the nitro group add once
or twice?
-OR-
H2SO4
Is addition selective for the
ortho and para positions?
NO2
NO2
Chromatography - Good for separations,
but not for identification….
Chromatogram:
What is the structure
of this compound?
2.05
min
4.37
min
All we can tell here is
that the compound is
more polar
3.22
min
Time
Gas chromatography (GC) and Liquid chromatography (LC) only give information
about polarity (based on retention time), not the structure
(Recall, last semester in lab, you performed silica gel column chromatography to purify a
solid compound and you ran gas chromatography to check your SN2 reaction)
38
4 Techniques to Identify Structures
of Organic Compounds (Analytical Chemistry)
1.
UV/vis spectroscopy - information about conjugated π-systems
2.
Mass spectroscopy - identify molecular mass of the compound and some
structural features (functional groups)
3.
IR spectroscopy - tells you important functional groups present
4.
NMR spectroscopy - tells you functional groups, connectivity of atoms
(framework), some stereochemistry, etc.
http://www.spectroscopynow.com
Spectroscopy and the Electromagnetic Spectrum
• Spectroscopy is the study of the interaction between matter and electromagnetic radiation
high frequency = short wavelength = high energy
• A visible spectrum is obtained if visible light is absorbed
• An ultraviolet (UV) spectrum is obtained if UV light is absorbed
• An infrared (IR) spectrum is obtained if infrared light is absorbed
• An nuclear magnetic resonance (NMR) spectrum is obtained if radiowaves
are absorbed
39
UV/vis Spectroscopy: Background Info
LUMO (lowest unoccupied
molecular orbital)
HOMO (highest occupied
molecular orbital)
LUMO
Electronic Transitions
hv
HOMO
• When a molecule absorbs light, an electron is promoted to a higher energy MO (from the
HOMO to the LUMO), and the molecule is in an “excited state”
• Although several electronic transitions exist between the MOs, only two transitions are low
enough in energy to occur with UV and Visible light.
electronic transition
with the lowest energy
Only organic compounds with π-electrons can produce UV/Vis spectra!!
40
Effects of Conjugation
LUMO
π∗
LUMO
LUMO
HOMO
HOMO
HOMO
π orbitals
of ethene
CH2
H2C
π orbitals of
1,3-butadiene
165
217
H2C CH2
λmax (nm)
π
CH2
H2C
π orbitals of
1,3,5-hexatriene
256
See table 8.3
Bruice p. 325
Conjugation raises the energy of the HOMO and lowers the energy of the LUMO
As conjugation increases, the HOMO-LUMO gap decreases
More conjugation = less energy required for electronic transition = longer wavelength
UV/Vis with Conjugated Carbonyl Compounds
Two peaks are observed in the
spectrum because two electronic
transitions can occur.
The HOMO-LUMO
gap decreases with
conjugation
41
Functional group effects
Two structural features will show an increase in the wavelength of the chromophore:
1) Increased conjugation
2) A substituent with a lone pair attached to the chromophore (an auxochrome)
An auxochrome is a substituent in a chromphore that alters the λmax and
the intensity of the absorption
CH3
H3C
N
N
N
amine-substituted azobenzene - a yellow dye (approx. 400nm)
Sidenote #1: Polyenes and Vision or
“do carrots help you see better?”
• Vitamin A is a source of 11-cis-retinal
• Opsin, a vision protein, binds 11-cis-retinal to form the Rhodopsin complex (rods)
• When Rhodopson absorbs light, 11-cis-retinal isomerizes to 11-trans-retinal, causing it to be
released from opsin. Upon release, a nerve impulse is generated and perceived by our brain
as light in black or white vision
• Same mechanism exists with iodopsin, another vision protein, to give us color vision (cones)
• 1 carrot = 2000 mg of retinal equiv. (sweet potato and mango have 1200 and 800 mg each)
cis-alkene
H2N
opsin
lysine side-chain
H
Rhodopsin
(500 nm)
O
H
N
11-cis-retinal
H
opsin
hv
11-trans-retinal
+
H
release
N
H
opsin
trans-alkene
nerve impulse
= vision
42
opsin
UV/Vis Spectroscopy: Sample Problems
1) Match the following UV absorption maxima with the corresponding compounds:
353 nm, 313 nm, 256 nm, 227nm, 180nm
CH3
H3C
CH3
CH3
H3C
H3C
CH3
2) How can you use UV/Vis spectroscopy to identify if this reaction has consumed the
starting reagents and produced the desired cyclohexene structure shown?
O
O
CH3
+
∆
CH3
Mass Spectrometry
70 eV
R X
R X
electron beam
dislodges electron
fragmentation
+
R
cation fragment
observed in MS
molecular ion
radical cation
observed in MS
X
radical fragment
NOT observed in MS
A molecular ion (radical
cation) is recorded in a
mass spectrum.
High-Resolution Mass Spectrometry will
give you the exact molecular formula - useful
data for identifying an unknown structure.
43
Fragmentation
70 eV
CH3CH2CH2CH2CH3
(electron beam
pentane
MW = 72
Only cations are recorded in
the mass spectrum
m/z = mass to charge ratio of the fragment
The base peak at m/z = 43 is the most abundant cation, which is not usually the
same as the molecular ion
Fragmentations Give the Most Stable Cations
Fragmentation of the molecular ion occurs one of two different ways to give the
most stable cations:
a) A C–X bond is cleaved heterolytically, where all electrons go to the more
electronegative atom (usually X)
b) A C–X bond is cleaved homolytically, at the α-position to give a stabilized cation
across the C–X bond
Examples of homolytic cleavage to give stable cations:
70 eV
H2C CH CH2
R
H2C
CH CH2
+
H2C
R
R
m/z = 41
70 eV
R Z CH2 CH3
R Z CH2 CH3
+
R Z CH2
Z = N, O, S
R can also be H
70 eV
R
R
C O
R
C O
R
R C
O
acylium ion
44
+
R
CH3
Fragmentations at Functional Groups
• The weakest bond is the C–Cl bond
heterolytic cleavage
• Both heterolytic and homolytic cleavage
of the C–Cl bond occur.
Cl isotope has 1/3
the abundance
α-cleavage
homolytic cleavage
positive charge shared by C and Cl atoms
Fragmentations of
an Ether Group
45
Fragmentations of an
Alcohol Group
γ-abstraction
Formation of new radical cation by
formation of small neutral molecule
(such as H2O, ROH, NH3, H2, ethene, etc)
Fragmentations Occur to Give Stable Cations
acylium ions stabilized by resonance
γ-abstraction
Formation of new radical cation
and a small neutral molecule
46
Summary
•
•
•
•
1)
2)
3)
4)
Fragmentations occur to give cations recorded in the mass spectrum; only
positively charged fragments are recorded.
The base peak is the peak with the greatest intensity, due to its having the
greatest abundance
Weak bonds break in preference to strong bonds
Bonds that break to form more stable fragments break in preference to
those that form less stable fragments
Alkanes, alkenes and aromatics: cleave to give the most stable
carbocations
Alcohol: loss of water or α-cleavage to give stabilized cation
Ethers: loss of an alkyl group or α-cleavage to give stabilized cation
Ketones and aldehydes: loss of alkyl group to give stabilized acylium ion or
McLafferty rearrangement (γ-abstraction)
Be able to propose or identify favorable fragmentations for 2 or 3 of the largest peaks
in the spectrum. You DO NOT need to account for all of the peaks in a spectrum.
Mass Spectroscopy Sample Problems
CH3
1) Predict the cation structure and base peaks for toluene
2) Account for the peaks at m/z 87, 111 and 126 in the mass spectrum of
2,6-dimethyl-4-heptanol.
CH3 OH CH3
H3C
CH3
47
Introduction to Infrared Spectroscopy
The covalent bonds in molecules are constantly vibrating
Stretching vibrations
Bending vibrations
It takes more energy to stretch
a bond than to bend a bond
Each stretching and bending vibration of a bond occurs with a characteristic
frequency that gives a specific absorption band (peak) in the IR spectrum
An Infrared Spectrum
High frequency = short wavelengths (inversely proportional)
Wavelength (µm)
Wavenumber (cm-1)
Functional group region
(4000-1400 cm-1)
Fingerprint region
(1400-600 cm-1)
Peaks are similar for
each functional group
Pattern is unique for
each compound
High frequency = large wavenumbers (directly proportional) - high energy
48
What Determines the Intensity of an IR peak?
Greater change in dipole moment = more intense absorption
A symmetrical bond will have no dipole moment and will therefore be infrared
inactive (have no absorption band)
Cl
H
H
H
H
Cl
H3C C C CH3
unsymmetrical
unsymmetrical
symmetrical
symmetrical
symmetrical
Remember, you are looking only at each bond, not the entire molecule
What Determines the Position of an IR peak?
1) Smaller atomic mass = larger wavenumbers (higher frequency)
C H
3000 cm-1
C D
2200 cm-1
C O
1100 cm-1
C Cl
700 cm-1
increasing wavenumber
2) Stronger bonds = larger wavenumbers (higher frequency)
a) Higher bond order = stronger
C N
2200 cm-1
double bond C N
-1
triple bond
single bond C N
b) Hybridization:
more s character = stronger
sp
1600 cm
2
sp
-1
3
1100 cm
sp
C C H
3300 cm-1
C C H
3050 cm-1
C C H
2900 cm-1
c) Other factors: electron delocalization, the electronic effect of neighboring
substituents, and hydrogen bonding
Any effect that makes a bond stiffer and harder to stretch will increase the wavenumber
49
Carbonyl Compounds: Resonance and Inductive effects
stronger, less
flexible C=O
bond
O
O
O
>
>
1788 cm-1
C=O stretch
1718 cm-1
1691 cm-1
(resonance)
(ring strain)
stronger C=O,
more double
bond character
C=O stretch
(approx.)
R
O
O
O
O
R
weaker, more
flexible C=O
bond
O
>
>
>
R
H
R
R
R
N
R
weaker C=O,
less double bond
character
H
ester
aldehyde
ketone
amide
1740 cm-1
(inductive
withdrawl)
1730 cm-1
1720 cm-1
1660cm-1
(resonance)
Stronger C=O because
No resonance to
weaken C=O bond
Resonance
weakens
C=O bond
Alcohol and Acid Characteristic Peaks
After a carbonyl peak, the broad O-H peak is the most characteristic peak to look for
O-H of alcohol
O-H of acid
An O-H bond of an alcohol or acid are both
very broad and intense.
The O-H of an acid is even more broad,
usually covering the C-H peaks
Hydrogen-bonding effect:
3500-3200 cm-1
3300-2500 cm-1
An O–H bond is weaker and easier to stretch when it is hydrogen-bonded
Reality check: water contamination in your sample can make it look like there
is an OH present when there is not.
50
Alcohol group
Broad
OH peak
Alcohol group +
carbonyl group
Broad
OH peak
Carboxylic
acid group
Super broad
OH peak
In addition to position,
identify type of carbonyl
by looking at secondary
peaks…
Ketone:
no secondary peaks
Aldehyde: C-H stretch
at 2720 and 2820 cm-1
Amide: N-H stretch in
3500-3300 cm-1 range,
and/or C-N stretch in
1200-1000 cm-1 range
(similar for an ester with a C-O stretch
and an acid with an O-H stretch)
51
Do not confuse an alkene or alkyne with a carbonyl - the intensities are
much weaker than a carbonyl peak
Note the differences in
the sp, sp2 and sp3 C-H
stretches (both the
intensities and the
positions).
sp2 C-H stretch
at 3080 cm-1
Also note the intensity
and position difference
of the CC double and
triple bond.
C=C stretch
at 1650 cm-1
sp3 C-H stretch
at 2850-2950 cm-1
Even when the intensity
is diminished, the shift of
the sp3 C-H is distinct
from the C-H of an
aldehyde because it is
higher frequency and no
carbonyl peak is present.
CC triple bond
stretch at 1650 cm-1
O-H
stretch
3 C-H
sp3sp
C-H
stretch
stretch
-1 -1
at 2800-2950
at 2850-2950
cmcm
sp C-H stretch
at 3300 cm-1
Be able to identify compounds with benzene rings (sp2 vs. sp3 carbons)
52
How to develop a “6th sense” for analysis of IR spectra:
Problem Solving Strategy
1.
2.
3.
4.
5.
6.
7.
8.
Look in 1800-1600 cm-1 range for strong sharp peak indicating a carbonyl,
consider its relative position.
Look for secondary peaks to distinguish between carbonyl compounds.
For example, a broad OH peak (3300-2500 cm-1) to indicate a carboxylic
acid.
Look in 3650-3200 cm-1 range for strong broad peak indicating alcohol or
amine.
Look for C-O and C-N peaks in 1250-1000 cm-1 range, indicating an ether
or tertiary amine.
Look in 2800-3100 cm-1 range for sp2 vs. sp3, indicating alkene or benzene
ring, look for C=C bond (1680-1600 cm-1) or triple bond (2100-2160 cm-1)
Do NOT confuse C=C bond or triple bond with a carbonyl - the carbonyl
has a strong intensity and the others have medium or small intensity
Consider symmetry that would account for “missing” absorption bands
Look in 1800-2800 cm-1 region - usually desolate, but has very
characteristic peaks for CN and CC triple bonds
Look in Appendix VI in your Bruice textbook to help with practice problems
IR Spectroscopy Sample Problems - Practice!!
Common Question Types:
1.
2.
3.
4.
5.
6.
Given an IR spectra for an unknown molecule, identify three functional
groups present in the molecule
Rank and/or explain which bond has a lower frequency (lower
wavenumber) for a series of bonds given
For several carbonyl compounds given, be able to identify which carbonyl
group exhibits the highest wavenumber.
Identify which compound has a vibration that is IR inactive
Know how to distinguish between a pair of compounds using IR data.
Use IR data, along with mass spectrometry and NMR data, to propose a
structure for an unknown organic molecule (in some cases, you will be
given a molecular formula)
Rank the relative frequencies of the following C=O bonds, where 1 = larger wavenumber
and 3 = smaller wavenumber
O
H3C
O
CH3
Cl
53
H3C
O
CH3
F
H3C
CH3
CH3
Introduction to NMR Spectroscopy
NMR = Nuclear Magnetic Resonance
MRI = Magnetic Resonance Imaging
Nobel Prize (Physics) in 1954
Nobel Prize (Chemistry) in 1991
Nobel Prize (Chemistry) in 2002
Nobel Prize (Physiology and Medicine) in 2003 for MRI
NMR spectroscopy provides 4 pieces of data to identify the about
the carbon-hydrogen framework of an organic molecule:
1)
2)
3)
Number of peaks: tells the number of different types of protons (or carbons)
Relative area of the peaks: tells the relative types of different protons
Position of the peaks: tells the chemical environment of the proton, ie. the
neighboring functional groups
Peak splitting pattern: tells the number of protons on adjacent atoms
4)
NMR Theory
A nucleus must have a nuclear spin of +1/2 or -1/2 to be NMR active
Examples include: 1H, 13C, 19F, 29Si, 15N, and 31P
Any spinning charged particle generates a magnetic field; therefore, think of a
nucleus as a mini-magnet that can be affected by an applied magnetic field (Bo)
-
-
energy
+
aligned against the field:
higher energy β nuclei
-
-
+
-
+
+
+
-
+
In the absence of an
applied magnetic field Bo
-
+
+
+
-
+
-
-
-
+
+
+
+
-
-
-
aligned with the field:
lower energy α nuclei
(slightly favored)
In the presence of an
applied magnetic field Bo
+ pole
Bo
- pole
54
NMR Theory
-
-
+
+
+ +
-
+
+
-
+
+
+
-
-
+
Energy
supplied
+
-
Energy
released
+
-
-
-
-
+
+
+
+
+
+
+
Lower energy
α nuclei
-
+
Higher energy
β nuclei
+ pole
Absorption of energy
can “flip” the nuclear
spin - converts low
energy to high energy
Bo
- pole
Spin “relaxes” to equilibrium
state through the magnetic
field and energy is released,
which can be detected as a
signal
Signals in 1H NMR spectra - How many?
• Each set of chemically equivalent protons in a compound gives rise to a different
signal/peak in the 1H NMR spectrum - Look for symmetry!
propyl group
isopropyl group
ethyl group
+ methyl group
methyl group
tert-butyl group
+ methyl group
“vinyl” protons
“aryl or aromatic” protons
55
+
energy
Radiowaves supply enough energy to “flip the spin” of a nucleus
NMR Time-scale
IR spectroscopy is like a fast camera - you get an instantaneous picture of all the
vibrations of a molecule (10-13 s)
NMR spectroscopy is like a slow camera - you get a blurry picture that is timeaveraged for the molecule (10-3 s)
Cyclohexane example:
chair-chair
interconversion
H
H
(ring flip)
(12.1 kcal/mol energy barrier)
H
H
1 peak @ 25 ˚C (fast ring flip)
2 peaks @ –90 ˚C (slow ring flip)
the rate of chair–chair conversion
is temperature dependent
What Determines the Position of an NMR signal?
Less electron
density = less
shielded
more electron
density = more
shielded
56
NMR - Chemical Shifts
• The chemical shift is a measure of how far the peak/signal is from the reference signal (TMS)
• The common scale for chemical shifts = δ
Position of TMS =
Internal reference
signal (0 ppm)
CH3
H3C Si CH3
CH3
TMS = tetramethylsilane
distance downfield from TMS (Hz)
δ=
operating frequency of the spectrometer (MHz)
NMR - Chemical Shifts
1) Presence of an electronegative atom: more electronegative = less shielded
Electron withdrawing effects cause a proton to be deshielded and the NMR signals
appear at higher frequency (further downfield, at larger δ values)
2) Presence of an adjacent π-bond: C=C and C=O are also electron-withdrawing
CH3
CH3
2.6 ppm
O
CH3
CH3
2.4 ppm
2.2 ppm
CH3
2.1 ppm
H3C
CH3
1.2 ppm
3) Hydrogens directly attached to a π-bond: sp2 carbon of alkene has high s character that is
electron-withdrawing, deshielding the hydrogen
7.5 ppm
H
R
H
R
R
57
5.0 ppm
but isn’t this a rather big effect
for just an sp2 carbon center?
Is there another effect?
Diamagnetic Anisotropy - Effects of π-electrons
The π electrons are less tightly held by the nuclei than are σ electrons; they
are more free to move in response to a magnetic field. This creates an
induced magnetic field
Benzene
(7-8 ppm)
Alkene
(5-6 ppm)
Alkyne
(2.5 ppm)
Bo - Binduced
Bo + Binduced
Protons are shielded
(at lower frequency)
Protons are deshielded
(at higher frequency)
The induced magnetic field (Bo + Binduced) creates a unique environment for
hydrogens that are bonded directly to carbons that form π bonds
NMR - Chemical Shifts
Protons in more
electron poor
environment
R C C H
2.4 ppm
Protons in more
electron dense
environment
What about protons attached directly to a triple-bonded carbon?
Proton on an alkyne experiences [Bo - Binduced], therefore, proton is shielded
58
Chemical Shift Sample Problem
1)
2)
How many signals would you expect to see in the 1H NMR spectrum for each
of the following compounds?
For each molecule, indicate which protons are least shielded, ie. will give the
NMR signal with the highest chemical shift value (furthest downfield).
F
C
H2
H2
C
C
H2
Br
H3C
O
C
H2
H2
C
C
H2
H
N
O
CH3
Hb
H3C
CH3
Ha
3) The hydrogens attached directly to the carbon of an aldehyde are very distinct because
they occur at especially high chemical shift values, usually greater than 9 ppm. Explain
why this hydrogen is so deshielded?
NMR - Integration
• The area under each peak is proportional to the number of protons that give rise to
that signal
• The height of each integration step is proportional to the area under a specific signal
• The integration tells us the relative number of protons that give rise to each signal,
not the absolute number
59
NMR - Splitting of the Signals
• The splitting of signals, caused by spin–spin coupling, occurs when different kinds
of protons are close to one another
• An 1H NMR signal is split into N + 1 peaks, where N is the number of equivalent
protons bonded to adjacent carbons
• The number of peaks in a signal is called the multiplicity of the signal
Splitting is observed if the protons are separated by three σ-bonds OR three σ-bonds
and a π-bond (alkene or alkyne)
What is the Theory Behind Splitting Patterns?
Splitting for a doublet (N = 1):
1:1 ratio
Splitting for a quartet (N = 3):
1:3:3:1 ratio
The ways in which the
magnetic fields of three
protons can be aligned
60
NMR - Splitting of the Signals
An 1H NMR signal is split into N + 1 peaks,
where N is the number of equivalent protons
bonded to adjacent carbons
Singlet, N = 0
Doublet, N = 1
Triplet, N = 2
Quartet, N = 3
Quintet, N = 4
Sextet, N = 5, etc
Quartet
(N = 3)
Triplet
(N = 2)
Doublet
(N = 1)
Quintet
(N = 4)
NMR - Coupling Constants (J)
The coupling constant (J) is the distance between two adjacent peaks of a split
NMR signal in hertz
Coupled protons have the
same coupling constant
quartet
doublet
The magnitude of the coupling constants is determined by the angle between the
two C-H bonds with the coupled protons – known as the Karplus relationship.
Why are coupling constants important?
1) They give you information about which protons are coupled to each other
2) They can help determine some stereochemical elements
61
NMR - Coupling Constants with Alkenes
Coupling constants for alkenes can be used to identify the cis and trans
geometry of an alkene - trans coupling constant is always larger!!
In general….
You can always tell alkene stereochemistry by using NMR
You can almost always tell regioisomers apart by using NMR
You can usually tell diastereomers apart by using NMR
You can NEVER tell enantiomers apart by using NMR
NMR
Protons attached to benzene or an alkene are at relatively high frequency
because of diamagnetic anisotropy
The signals for the Hc, Hd, and He protons overlap
Notice benzene ring does not have “ideal” splitting pattern - all signals are
similar and overlap - multiplets
ethyl splitting
pattern
62
NMR - Samples
The signals for the Ha, Hb, and Hc
protons do not overlap
Notice the difference in frequency for each proton signal - Look at resonance structures of
nitrobenzene to assign each hydrogen.
NMR - Protons Bonded to Oxygen and Nitrogen
Hydrogen-bonding and proton exchange cause O-H and N-H signals to be broad
without acid
No proton exchange,
splitting is observed
(ethyl pattern with
additional splitting)
with acid
proton exchange occurs,
splitting is NOT observed
(isolated ethyl
pattern with NO
additional splitting)
What happens if you add a drop of D2O to your NMR sample? The D exchanges for H
and the peak disappears
(this is a great experiment to test for an N-H or O-H peak)
63
Carbon (13C) NMR Spectroscopy
• The number of signals reflects the number of different kinds of carbons in a compound
• Integration and signal splitting are typically not used
• The overall intensity of a 13C (an isotope of 12C) signal is about 6400 times less than the
intensity of an 1H signal
• The chemical shift ranges over 220 ppm
• The reference compound is TMS
Same resonance and electron withdrawing effects determine chemical shift :
O
O
Electron deficient carbon of a carbonyl group is
shifted downfield to high frequency (165 – 220 ppm)
H
110 - 170 ppm
H
100 - 150 ppm
H3 C
Z
40 - 80 ppm
Diamagnetic anisotropy accounts for the
chemical shifts of aryl and vinyl carbons at
higher frequencies (downfield)
Electron-withdrawing effects of an electronegative
atom will cause smaller downfield shifts
(Z = OH, OR, NH2, NHR, Cl, Br, etc.)
Carbon NMR Spectroscopy
Proton-coupled 13C spectra – splitting is observed (uncommon)
Proton-decoupled 13C spectra – NO splitting is observed (very common)
Example of a proton-decoupled 13C spectra:
Each chemically equivalent carbon atom is a singlet
TMS
64
1H
1H
and 13C NMR Shift Comparison
NMR shifts:
δ (ppm)
13C
NMR shifts:
δ (ppm)
From Organic Chemistry Textbook by Wade
Types of NMR Questions
1)
2)
3)
4)
5)
How many signals will be in an 1H NMR spectra for a given molecule (how
many protons are chemically equivalent)?
How many signals will be in an 13C NMR spectra for a given molecule (how
many carbons are chemically equivalent)?
Relative chemical shift values, for example, identify which proton will give a
signal with the highest chemical shift value (farthest downfield) for a given
molecule.
Assign a spectra - which proton accounts for which peak, and what is the
splitting pattern
Identify an unknown compound using NMR and IR data (see examples in
the practice problems handed out in class)
Do lots of practice problems!!!
65
NMR Sample Problems
1) Label the splitting pattern that will be observed in the 1H NMR spectrum for each of the
indicated hydrogens.
H2
C
C
H2
CH3
3) An unknown compound C4H8Br2, gave the following 1H NMR data. What is the compound?
Singlet at 1.97 ppm (6H integration)
Singlet at 3.89 ppm (2H integration)
NMR Sample Problems
How would you use NMR data to determine which is the major product in each
of the following reactions?
CH3
X
H3C
X
X2, hv
1)
-OR-
X = Br or Cl
H3C
CH2
CH3
Cl
KOH, EtOH
2)
-OR-
CH3
H3C
CH3
Cl
HCl
-OR-
3)
66
Cl
“One C=O bond to Rule them All”
O
Carbonyl structure
and reactivity:
nucleophilic
O
C
electrophilic
Substitutent (Z) on
carbonyl determines
reactivity:
O
O
O
R
Cl
acid
chloride
>
R
H
>
R
aldehyde
R
R
>
R
O
anhydride
ketone
Most
electrophilic
carbon
Most inductive
electron-withdrawing
O
ester
or acid
R
O
O
O
O
O
>
R
>
R
N
R
>
H
amide
R
O
carboxylate
ion
Most
nucleophilic
oxygen
increasing reactivity
with nucleophiles
Most resonance
Introduction to Carbonyl Compounds
H3C
CH3
H3C
1. O3
H3C
2. H2O2
H
CH3
+
O
H3C
H
H2O, H2SO4
H3C C C CH3
1. disiamylborane
Ketone or aldehyde synthesis
using ozonolysis
O
Ketone synthesis from
an internal alkyne
O
H3C
CH3
2. H2O2, NaOH
O
H2O, H2SO4
HgSO4
H3C C C H
1. disiamylborane
2. H2O2, NaOH
H3C
Ketone or aldehyde synthesis
from a terminal alkyne
O
H3C
H
O
O
H3C
CH3
Cl
+
AlCl3
CH3
67
Synthesis of an aromatic ketone
using Friedel-Crafts acylation
with an acid chloride
Introduction to Carbonyl Compounds
O
R
O
H
R
aldehyde
R
R
O
R
OH
carboxylic
acid
ketone
O
O
O
R
N
R
R
H
amide
ester
O
O
O
R
R
Cl
acid or acyl
chloride
R
O
R
C
nitrile
anhydride
carbonyl oxygen
O
O
R
O
R'
O
O
Cyclic ester = lactone
Cyclic amide = lactam
NH
lactone
lactam
carboxyl oxygen
Systematic nomenclature is rarely used for carbonyl compounds, but common names are
somewhat consistent for a series of carbonyl compounds:
O
O
H3C
H
H3C
acetaldehyde
O
OH
H3C
acetic acid
Cl
H3C
acetyl chloride
O
O
O
CH3
O
H3C
acetic anhydride
NH2
acetamide
H3C
C
N
acetonitrile
Carbonyl Structure and Bonding
C=O bond (1.23 Å) is shorter and
stronger than a C=C bond (1.33 Å)
C O
δ+ δ-
Large dipole moment
O
O
O
C
C
high energy structure,
do not ever draw this!!!
minor
contributor
major
contributor
Carbon is an electrophile (has Lewis acid character)
Oxygen is a nucleophile (has Lewis base character)
R
Cl
acid
chloride
O
O
O
>
R
H
aldehyde
Most
electrophilic
carbon
>
R
R
ketone
R
O
anhydride
R
>
O
ester
or acid
R
increasing reactivity
with nucleophiles
68
O
O
O
O
O
>
R
>
R
N
H
amide
R
>
N
R
O
carboxylate
ion
Most
nucleophilic
oxygen
Molecular Orbitals of C=O Double Bond
Compare the molecular orbitals of
the C=C and C=O double bond:
alkene π*
(LUMO)
C=O π*
(LUMO)
The C=O bond is unsymmetrical!!
alkene π
(HOMO)
C=O π
(HOMO)
energy
π* C O
C 2p
O 2p
π C O
The 2p orbital of oxygen is lower
energy (because oxygen is more
electronegative) and contributes
more to the π molecular orbital.
The higher energy 2p orbital of
carbon contributes more to the π∗
molecular orbital.
Resonance vs. Inductive Effects
O
R
Cl
Most inductive electron
withdrawing effects
Resonance structure shows
that carbonyl oxygen is more
Lewis basic than the carboxyl
oxygen (OCH3 or OH)
Most resonance donation
Most Lewis basic oxygen
High double bond character in amide (as seen in resonance structures)
causes trigonal planar geometry of nitrogen, indicating sp2-hybridization
Recall relative IR stretch frequencies based on resonance and inductive effects…
69
General Comments About Acid-Catalyzed Reactions of
Carbonyl Compounds
O
R
H+
O
X
R
Protonation of the carbonyl oxygen makes any carbonyl
a better electrophile (more susceptible to nucleophilic
attack) because the C=O LUMO is lowered
H
X
aldehydes
ketones
esters
amides
Complexation of a carbonyl oxygen (a lewis base) with a Lewis acid will also make the
carbonyl a better electrophile by lowering the LUMO
BF3
O
R
X
O
R
BF3
Lewis acids = BF3, AlCl3, ZnCl2, FeBr3
X
The proton and Lewis acid both have an electron-withdrawing effect. Any electronwithdrawing effect will lower the LUMO and increase the electrophilicity of the C=O.
Physical Properties: High Boiling Points
intermolecular hydrogen-bonds and dipole-dipole interactions
Hydrogen-bonding
accounts for the super
broad OH peak in the IR
spectra of an acid
Amides have the
highest boiling points
N-H of an amide is also
broad in the IR spectra,
but not as much
70
Reactivity of Carbonyl Compounds
Recall order of reactivity of carbonyls with nucleophiles:
R
O
O
O
Cl
acid
chloride
>
R
H
>
aldehyde
R
R
ketone
R
R
O
O
O
O
O
>
>
anhydride
O
ester
or acid
R
R
>
O
N
R
H
amide
R
>
R
O
carboxylate
ion
Increasing reactivity
with a nucleophile
addition
Nucleophilic Acyl
Substitution:
Y = good leaving
groups
elimination
carbonyl
product
Z = oxygen, nitrogen, hydride and carbon nucleophiles
addition
Nucleophilic Addition:
Z = oxygen, nitrogen,
hydride and carbon
nucleophiles
protonation
a tetrahedral
intermediate
tetrahedral
product
Nucleophilic Acyl Substitution Reactions
General mechanism:
addition
elimination
Y:- must be a
good leaving
group
RDS is elimination
RDS is addition
(a) the nucleophile (Z:–) is a weaker base (k–1 >> k2)
(b) the nucleophile (Z:–) is a stronger base (k2 >> k–1)
(c) the nucleophile (Z:–) and the leaving group have similar basicities
71
Nucleophilic Acyl Substitution
For nucleophilic acyl substitution, a carbonyl compound must have a good leaving group
(a weak base) that can be replaced by a nucleophile
in nucleophilic acyl substitution reactions:
O
Cl
<
O
R
<
OR
~
OH
<
weakest base
best leaving group
A weaker base is
easier to eliminate
NH2
strongest base
poor leaving group
See Bruice Table 17.1 on page 672
O
R
O
H
R
R'
(R’ = alkyl or aryl group)
Ketones and aldehydes DO NOT undergo nucleophilic acyl
substitution because there is not a good leaving group that
can eliminate.
(However, remember that they are great electrophiles and we will
discuss other addition reactions with them soon.)
MO diagram of Nuclelophilic Acyl Substitution
acceptor
Donor
(on nucleophile)
Examples of nucleophiles (Lewis bases) for acyl substitution reaction:
H 2O
CH3OH
OH
NH2CH3
H3C
72
H
N
O
CH3
O
CH3
Reactions of Acid Chlorides
esters
acids
O
H3C
O
O
Cl
+
O
H3C
CH3
O
O
CH3
anhydrides
amides
Reactions of Acid Chlorides with Amines
Not a good
nucleophile
• Requires 2 equivalents of amine because HCl is formed and amine will be
protonated and no longer be a good nucleophile for the reaction
• Only primary and secondary amines can be used to synthesize an amide
O
H3C
+
Cl
H3C
N
CH3
No reaction to give amide products
CH3
(2 equivalents)
But a tertiary amine still has a lone pair to serve as a donor, why doesn’t it react ?
73
Tertiary Amines Still Promote the Reaction
O
+
H3C
Cl
H3C
+
NH2
H3C
(1 equiv)
H
O
N
N
H
CH3
N
+
Cl-
(1 equiv)
A tertiary amine will still add to a carbonyl, but since NO
deprotonation can occur, it will always be the best leaving group
O
H3C
Cl
Cl-
+
H3C
Cl
H3C
NH2
(1 equiv)
O
O
N
+
H3C
N
H
CH3
+
O
(1 equiv)
H
N
H
CH3
H
Cl-
N
+
+
H3C
N
CH3
Cl-
H3C
O
N
H3C
NH2
O+
N
H
OH
N
CH3
Cl-
H3C
N
H2
CH3
Sample Problem: Mechanism
Provide a mechanism for the following conversion of an acid chloride to an ester:
O
+
H3C
Problem solving
strategy:
O
CH3OH
Cl
H3C
When nucleophile is neutral:
Step 1 = addition
Step 2 = deprotonation
Step 3 = elimination
OCH3
When nucleophile is an anion:
Step 1 = addition
Step 3 = elimination
Must draw three steps and show tetrahedral intermediate!!
74
Reactions of Anhydrides
ester + acid
acid (2 equivalents)
amide
Anhydrides are still reactive enough to synthesize esters, amides and acids;
however, they are actually more stable than an acid chloride making them
more practical to use.
Reactions of Esters
1) Amidolysis
2) Hydrolysis
Reaction is reversible and both
acid and ester will be present
at equilibrium
3)Trans-esterification
Reaction is reversible and
both esters will be present
at equilibrium
75
Acid-catalyzed Hydrolysis of an Ester
No negatively charged species
Several of proton-transfer steps
Need to drive equilibrium by adding excess
water or removing the alcohol product
Acid catalyzed Hydrolysis of an Ester
Esters with tertiary alkyl groups undergo faster hydroylsis by SN1-related mechanism
CH3 O
H3C
H3C
O
H
R
H
H3C
+O
H3C
O
R
H3C
H2O
Provides both an
acid and an alcohol
product
CH3
H3C
H3C
OH
CH3
SN1
OH
+
H3C + CH3
O
tertiary
carbocation
CH3
H3C
H3C
B:
O H
H
Once the carbonyl oxygen is protonated, the carboxylic acid is an
excellent leaving group for an SN1 reaction
76
R
Hydroxide Ion-Promoted Hydrolysis of an Ester:
Saponification
Hydroxide ion increases the rate of formation of the tetrahedral intermediate
This reaction is
NOT reversible
Under basic conditions, the carboxylic acid will be deprotonated. The
carboxylate ion is NOT electrophilic and the reverse addition will not occur
Hydrolysis of an Amide Requires Harsh Conditions
Amides are very stable and hydrolysis conditions require acid or base, with heat
• Requires both heat and acid to hydrolyze an amide
• Nitrogen must be protonated to become a good leaving group
H3C
O H
NHCH2CH3
O
+
H3C
HO
HCl, H2O
N
O
H
HNCH2CH3
H+
H2NCH2CH3
OH
O
H2N
O
H+
OH
H3N
OH
β-lactam
A cyclic amide (a lactam) will be easier to hydrolyze if there is ring strain
77
Sidenote: Importance of the Amide Bond in Biological
Systems
amide
bond
R1
O
N
H
H
N
N
H
R2
O
R3
O
H
H
N
O
R4
N
H
R5
O
N
H
R4
H
H
N
O
N
R3
O
R5
N
O
H
R2
O
N
amino acids linked by amide bonds: Linear Structure
N
H
O
Amide bonds are difficult
to hydrolyze, making
them perfect for proteins.
H
N
O
R1
O
alpha helix
with hydrogen-bonds
Of course, there are
enzymes that can
hydrolyze the amide bond
quite readily!!
protein structure
Reactions of Carboxylic Acids
1) Acid-base reactions (recall pKa’s)
O
H3C
OH
+
H
N
O
CH3
CH3
H3C
+
O
H2N
CH3
CH3
No amides will
form because
the amine will
be deprotonated
2) Activated by converting to an acid chloride
Converts carboxylic acid to a
very electrophilic (activated)
carbonyl to use in nucleophilic
acyl substitition reactions
Mechanism:
78
Reactions of Carboxylic Acids cont’d
3) Lactonization with Br2 or I2
OH
O
I
I2
O +
CH2Cl2
H I
O
I
I
OH
O
I+
O
I
I-
O+
H
I-
The carbonyl oxygen is more nucleophilic than the carboxyl OH and will add to to
the halonium intermediate (electrophile)
Sidenote: Activated Carboxylic Acid Derivatives for Biosynthesis
Similar to anhydrides,
great leaving groups
for synthesis:
ATP is a great energy storage device: thermodynamically reactive and kinetically unreactive
Good sterics, good
leaving group, not
as much energy
OK sterics, good
leaving group,
gives more energy
AMP
Reactions are determined by sterics, leaving group ability and “energy requirements”
of the biochemical pathway. Enzymes play a big role!
79
Reactions of Nitriles
H3C
Br
NaCN
H3C
S N2
O
HCl/H2O
CN
H3C
∆
butanenitrile
(propyl cyanide)
OH
Butanoic acid
(butyric acid)
Acid-catalyzed hydrolysis to a carboxylic acid is even more difficult than an amide hydrolysis
Reduction of a nitrile to give an amine:
H2, Pd/C
CN
H3C
H3C
NH2
(2 equiv of H2 is required for reaction, but since H2 is added in the gas form, excess is always added)
Sample Problems
1) Rank the reactivity of the following esters towards hydrolysis, where 1 = most reactive
and 3 = least reactive.
Hint: Since the carbonyl is the
electrophile, the rate is
increased by anything electronwithdrawing and decreased by
anything electron-donating
O
CH3
Cl
O
O
2) Rank the relative energy level of the
LUMO for the following C=C and C=O
bonds, where 1 = lowest LUMO (most
electrophilic) and 3 = highest LUMO
CH3
CH3
O
O
R
O
OCH3
R
H
OCH3
3) Show three ways to prepare an amide from N,N-dimethylamine
80
OCH3
O
O
CH2
H3C
CH3
Lidocaine Synthesis: Nucleophilic Acyl Substitution vs. SN2
Nucleophilic
acyl subsitution
O
O
R NH2
R
Cl
Cl
(excess
amine)
H
N
R
N
H
SN2
If only one equivalent of amine is used, nucleophilic acyl substitution will be faster than
an SN2 because acyl halides are better electrophiles (more reactive) than alkyl halides
CH3
Step 1:
O
+
NH2
CH3
NaOAc
Cl
Cl
Cl
N
H
CH3
1 equivalent amine
Step 2:
CH3
O
CH3
O
Cl
CH3
NaI, Et2NH
CH3
O
N
N
H
CH3
N
H
CH3
Lidocaine
CH3
Synthesis of Aspirin
An experiment from Chem E2a lab: Acyl transfer with acetic anhydride
OH
H3C
OH
O
O
O
O
CH3
CH3
O
O
+
H3PO4
H3C
OH
O
O
Salicyclic acid
Aspirin™
(acetylsalicylic acid)
• Active ingredient in wart
removal medicine (40 %)
• Active ingredient in some acne
treatment medicine (1.5%)
How to get rid of excess acetic anhydride in a reaction?
hydrolysis gives acetic acid:
O
H3C
H2O
O
O
O
H3C
CH3
Acetic anhydride
O
H
+
O
H
Acetic acid
81
O
CH3
O
H
Sidenote: Aspirin Mechanism of Action
The reverse acyl transfer from Chem E2a lab:
Cyclooxygenase
O
CH3
Ser
O
OH
Cyclooxygenase
OH
OH
OH
enzyme-mediated
O
O
salicylic acid
Aspirin™
+
Ser
H3C
O
O
Aspirin is a nonsteroidal anti-inflamatory drug (NSAID) that inhibits the
biosynthesis of prostaglandins (which cause inflammation) by PGH2 synthase.
Specifically, Aspirin inactivates the cyclooxygenase (Cox) activity of PGH2
synthase by an irreversible covalent modification mechanism: acylation of the
Ser530 amino acid, a strategic location which blocks entry of the enzyme
substrate into the binding site
Ibuprofen is an NSAID that also inactivates cyclooxygenase activity of PGH2
synthase, but inactivation with Ibuprofen occurs by competitive substrate
binding (a non-covalent, reversible mechanism)
β-Lactam Antibiotics: Medicine and Molecular Orbitals
Penicillin mechanism of action: Inhibits Transpeptidase to prevent cross-linking of small
peptide chains (petidoglycan strands) in the cell wall of gram+ bacteria.
But how is penicillin reactive if amides are so unreactive to hydrolysis?
N
O
O
N
H
O
C
N
H
H
β-lactam
Planar structure allows good
overlap between lone pair on N (in
p-orbital) and the C=O π bond to
give good resonance stabilization
3-D picture = planar
H
N
O
CH 3
H
S
N
O
penicillin G
CH 3
CH 3
O
H S
O
C
N
CH 3
CO 2 H
OH
3-D picture = bent
82
Ring constraints of fused β-lactam
structure force lone pair to be almost
perpendicular to the C=O π bond
and resonance stabilization is lost
Sample Problems
1)
2)
3)
4)
General
question
types:
Provide reagents or products in a reaction scheme (or short syntheses)
Know mechanisms and relative reactivity trends for Nucleophilic Acyl Substitution
Remember IR and NMR data relating to the C=O bond
Be able to draw resonance structures and understand how they influence reactivity
1) The β-lactam of penicillan G is fairly labile in your stomach (acidic conditions). Provide
a curved-arrow mechanism showing how it is rendered inactive in your stomach.
H
R
S
N
O
CH3
CH3
CO2H
penicillin G
β-Lactam Antibiotics: Medicine and Sterics
Bacteria become resistant to penicillan G by producing an enzyme (penicillanase) that
catalyzes the hydrolysis of the amide bond. However, simple structural modifications of
penicillan G can make a drug that is effective against penicillan G-resistant bacteria.
H
N
O
CH3
H
N
H
S
N
CH3 O
CH3
O
O
penicillin G
CH3
H
S
N
CH3
O
OH
O
More bulky
aryl group
CH3
OH
methicillin
Methicillan is effective in treating patients infected with penicillan G-resistant bacteria
because the bulky aryl group prevents it from binding in the active site of the penicillinase.
It does not, however, prevent it from inhibiting the transpeptidase target.
83
Sample Problem
2) Provide a synthesis of methyl butyrate from bromopropane:
O
Br
H3C
H3C
starting material
OCH3
desired product
Reactivity of Aldehydes and Ketones
Recall order of reactivity of carbonyls with nucleophiles:
R
O
O
O
Cl
acid
chloride
>
R
H
aldehyde
>
R
R
>
R
R
O
>
anhydride
ketone
O
O
O
O
O
ester
or acid
R
R
>
R
O
N
H
amide
R
>
R
Increasing reactivity
with a nucleophile
O
O
>
H
H
formaldehyde
O
>
R
H
an aldehyde
R
R
a ketone
An aldehyde is more electrophilic because an H atom is electron-withdrawing
compared to an alkyl group: more electron-withdrawing effect = greater
positive charge on carbon (less stable)
84
O
carboxylate
ion
Two Factors: Electronic and Sterics
Very
reactive H
O
O
>
H
H3C
H
>
H3C
O
O
O
>
CH3
H3C
CH3
CH3
Reactivity based on stability
and sterics: stability more
important than sterics
>
H3C
CH3
Less
reactive
CH3 CH3
Reactivity determined by sterics:
more sterics = less reactive
Electronic effect (stability):
A hydrogen is more electron-withdrawing than an alkyl group.
EWG = Greater positive charge = more electrophilic = Lower LUMO
(Remember, Electron-withdrawing groups always lower the LUMO)
Sterics has two effects:
1) The carbonyl carbon of an aldehyde is more accessible to the nucleophile
2) Ketones have greater steric crowding in their transition states, so they
have less stable transition states
Aldehydes and Ketones - Nomenclature
Aldehydes - remove “e” from hydrocarbon name and add “al”
Ketones - remove “e” from hydrocarbon name and add “one”
85
Nucleophilic Addition Reactions
Recall Nucleophilic Acyl Substitution:
addition
elimination
carbonyl
product
Nucleophilic Addition:
addition
protonation
a tetrahedral
intermediate
tetrahedral
product
(Both general reactions can also be acid-catalyzed)
Addition of Carbon Nucleophiles: Grignard Reagents
Grignard reagents react with aldehydes, ketones, and carboxylic acid derivatives
to form new C–C bonds
Recall: Synthesis of a
Grignard reagent from
an alkyl bromide:
Very polar
C–Mg bond!
Grignard reactions with aldehydes give secondary alcohols:
Grignard reactions with ketones give tertiary alcohols:
86
Grignard Reactions with an Ester - Double Addition
MgBr
O
Methyl
benzoate
OH
1)
2.5 equivalents
OCH3
Triphenylmethanol
2) H2O
PhMgBr
(1st equiv)
Nucleophilic
acyl substitution
H2O
PhMgBr
(2nd equiv)
O
OMgBr
Nucleophilic
addition
Addition of the first equivalent of Grignard reagent will form a ketone
Addition of the second equivalent of Grignard reagent will form an alcohol
Grignard Addition to CO2: Synthesis of a Carboxylic Acid
O
Mg, Et2O
Br
MgBr
1. CO2
OH
2. HCl, H2O
bromobenzene
benzoic acid
Useful synthetic methods to recall:
1. BH3
H3C
H3C
H3C
2. H2O2, NaOH
OH
Br
Br
OH
H3C
PBr3
NaCN
Radical
bromination
CH3
O
HCl, H2O
CN
H3C
(or NBS)
Alkyl bromide formation
Br
H3C
Br2, hv
Hydroboration/oxidation
Two methods
to synthesize
a carboxylic
acid
H3C
CH3
Br2
FeBr3
OH
Br
Electrophilic aromatic
substitution: bromination
87
SN2 and
Nitrile
hydrolysis
Synthesis Sample Problem
1) How would you synthesize the following compound from any carbonyl compound and
any alkyl bromide? You may use any inorganic reagents.
OH
H3C
CH3
2) Provide a synthesis of this carboxylic acid from benzene:
H3C
CO2H
CH3
Other Carbon Nucleophiles: Acetylide anion
Recall: Previous reactions of acetylide anion with electrophiles:
H
H3C
2)
H
H3C
1) NaNH2 (or nBuLi)
H3C
CH3
H3C
Br
1) NaNH2 (or nBuLi)
2)
OH
H3C
O
88
SN2 with
alkyl halides
Epoxide
opening
Hydride Ion as a Nucleophile
General mechanism: reduction of ketone to secondary alcohol by addition of a
hydride ion, followed by protonation
Tetrahedral products
CH3
What are sources of hydride ion “H:–”?
NaBH4 = sodium borohydride - four “H:–” equivalents
Dibal-H = diisobutylaluminum hydride - one “H:–” equivalent
LiAlH4 = lithium aluminum hydride (LAH) - four “H:–” equivalents
CH3
CH3
Al
H3C
H
Dibal-H
Relative ease of reduction:
Most
reactive
O
O
O
>
R
H
O
>
R
R
R
OR
R
O
>
>
NR2
R
Least
reactive
O
NaBH4
LiAlH4 - most reactive
NaBH4 Reductions
H
Na
H B H
H
• Only works with aldehydes and ketones
• Each molecule of NaBH4 provides 4 hydride equivalents (therefore, you only need
one molecule of NaBH4 for every four molecules of carbonyl compound)
Aldehydes give
primary alcohols
Ketones give
secondary alcohols
Because NaBH4 is less reactive, it can be very selective. You can selectively reduce
a ketone and the ester will remain untouched.
Only the ketone will be reduced!!
89
H
LiAlH4 reductions
Li
H Al
H
H
1) LiAlH4 is a stronger reducing agent than NaBH4
2) LiAlH4 is used to reduce compounds that are nonreactive toward NaBH4
(basically, LiAlH4 will reduce any carbonyl compound)
3) Double hydride addition will occur to give the completely reduced product
Amine substitution
depends on what
amide substitution
started as
An ester is reduced to a primary alcohol (double hydride addition)
A carboxylic acid is reduced to a primary alcohol (double hydride addition)
An amide is reduced to an amine (double hydride addition)
LiAlH4 Double Addition Mechanism with an Ester
elimination
addition
addition
protonation
The first hydride addition is a nucleophilic acyl substitution and a carbonyl
product is obtained. The second hydride addition, a nucleophilic addition
reaction, follows immediately because the aldehyde is even more reactive.
90
Dibal-H Reductions
Dibal-H allows the addition of only one equivalent of hydride to an ester
because it is less reactive than LiAlH4
LiAlH4 reduction gives alcohol (double hydride addition):
Dibal-H reduction gives aldehyde (single hydride addition):
CH3
Dibal-H
H3C
CH3
Why is Dibal-H less reactive than LiAlH4?
1) As a neutral species, it is less nucleophilic than
the anionic aluminum species
2) It is more sterically hindered
CH3
Al
H
Reduction Sample Problems
Provide products for each of the following reactions:
1. NaBH4
O
2. HCl, H2O
H
1. NaBH4
2. H2O
2. HCl, H2O
1. LiAlH4
O
H
1. Dibal-H
1. LiAlH4
CH3
O
2. HCl, H2O
O
OCH3
O
1. LiAlH4
O
H3CO
2. HCl, H2O
N
CH3
2. HCl, H2O
CH3
O
O
91
1. LiAlH4
2. HCl, H2O
Alcohols Can be Oxidized to Carbonyl Compounds
[O]
Oxidation
Reduction: Oxygen
content decreases
and/or hydrogen
content increases
R
OH
Reduction
[H]
Oxidation: Oxygen
content increases
and/or hydrogen
content decreases
O
R
H
Oxidation with chromic
acid reagents:
Secondary alcohols can
be oxidized to ketones
Aldehydes and ketones
can be oxidized to
carboxylic acids
Oxidation Mechanism with Chromic Acid
What are the different Chromium reagents?
All three are variations of the same reagent, (chromic acid):
H2CrO4 = Chromic acid
O
CrO3/H2SO4 = H2CrO4
HO Cr OH
Na2Cr2O7/H2SO4 = H2CrO4
Mechanism:
Proceeds by substitution
and an E2 elimination
92
O
PCC Oxidation of Alcohol to an Aldehyde
Chromic acid will oxidize a primary alcohol all the way to a carboxylic acid:
H2CrO4
OH
H3C
H+
O
H3C
H2O
H2O
H
Primary
alcohol
OH
H3C
H2CrO4
OH
O
H3C
H
aldehyde
OH
hydrate
Carboxylic acid
The oxidation of a primary alcohol can be stopped at the aldehyde if
pyridinium chlorochromate (PCC) is used as the oxidizing agent
CrO3
+
HCl
H
N
N
+
CrO3ClŠ
PCC
The oxidation with PCC stops at the aldehyde because PCC can be used in a non-aqueous solvent
to avoid formation of the hydrate. The hydrate is what oxidizes further to the carboxylic acid
Swern Oxidation
Swern is also a useful method to oxidize a primary alcohol to an aldehyde because
over-oxidation can be avoided….. And less toxic than chromium reagents
Mechanism:
Me
O
O
Cl
Cl
Me
O
oxalyl
chloride
S
R
OH
Me
Cl
Cl
Me
S
Me
SN2
Cl
Cl
O
O
Cl
O
O
DMSO
Cl
O S Me
Cl
O
S
Me
Me
Me
Me
O
S
R
Me
O
Me
S
R
H
NEt3
triethylamine
Me
dimethylchlorosulfonium
ion
R
H
S
O
Me
S
H
H
O
R
CH2
E2
+
Proceeds by substitution
and an E2 elimination
Note: also converts secondary alcohols to Ketones
93
H
aldehyde
Me
S
Me
dimethylsulfide
Dimethylsulfide is:
1) A great leaving group
2) Super Stinky!!!
Sidenote: Analysis of Blood Alcohol Content
H3C
OH
H2SO4
+ Na2Cr2O7
H3C
O
+ H2Cr2O3
OH
The ethanol from your lungs (in equilibrium with your blood) will be oxidized by
chromic acid. The chromate ion produced can either be detected by the a
simple color change that indicates if any alcohol is present, or a quantitative
Breathalyzer™ can be used for a more accurate reading of the alcohol content.
However, a direct blood test is the most accurate measurement.
H2SO4
Na2Cr2O7
reduced chromate ion
ROH
Red-orange
color
green color
Synthesis Sample Problems
1) Provide a synthesis of Ibuprofen from the starting matieral provided:
H3C
H3C
CH3
CH3
O
OH
CH3
Starting material
Ibuprofen (AdvilTM)
2) Provide a synthesis of the ketone below, starting with any alcohol that is 4 carbons or
less, and using any inorganic reagents.
CH3 O
H3C
CH3
CH3
Desired product
94
Reactions of Aldehydes and Ketones with Nitrogen
Nucleophiles: Imines from 1° amines
H
H
R
O
H2N
aldehyde
R
H2O
+
R
N
R
"Imines"
aldimine
1° amine
-ORR
R
R
O
H2N
ketone
R
H2O
+
R
N
"Schiff
Bases"
R
ketimine
1° amine
Bonding in an imine:
CH3
CH3
C
N
CH3
sp2-hybridized carbon
sp2-hybridized nitrogen
Reactions of Aldehydes and Ketones with Nitrogen
Nucleophiles: Enamines from 2° Amines
R
R
HN
O
R
R
H2 O
R
aldehyde
or ketone
+
R
N
R
2° amine
enamine
recall...
R
R
R
R
R
O
"keto" tautomer
OH
"enol" tautomer
95
Mechanism of Imine Formation
addition
protonation
deprotonation
protonation
deprotonation
elimination
Mechanism of Enamine Formation
96
Carbonyl Group Removal via Imine (hydrazone) Formation:
The Wolf-Kischner Reduction
Me
Works with
any ketone
H2N
O
NH2
Me
ketone
Me
NaOH
hydrazine
N NH2
H
∆
hydrazone
H
alkane
Mechanism to form hydrazone is EXACTLY as with imines
Reduction mechanism:
This ketone reduction is more general than the H2 with Pd/C
reduction conditions that ONLY work with benzylic ketones
Reactions of Aldehydes and Ketones with Oxygen
Nucleophiles: Gem-Diols (Hydrates) from Water
O
R
H2O
R
ketone or
aldehyde
Water
HO
OH
R
R
gem-diol or
"hydrate"
Recall...
R
R
OH
OsO4/H2O2
R
R
OH
vic-diol
gem, geminal => gemini, "twins"...attached to same carbon
vic, vicinal => vicinity, "neighbors"...attached to neighboring carbons
97
Carbonyl Reactivity Predicts the Amount of Hydrate
Present
most stable,
least reactive
least stable,
most reactive
most stable
Acetals and Ketals
O
R
H
aldehyde
R
H+
OH
H2O
alcohol
R
ketone
R
RO
OR
R
H
acetal
O
R
+
H+
OH
H2O
alcohol
+
RO
OR
R
R
ketal
Examples of “H+”acids = HCl, H2SO4, p-TsOH
SO3H
p-TsOH =
H3C
98
Mechanism is Similar to Hydrate Formation, conditions are
Similar to Imine Formation (dehydrating)
This reaction does not happen unless you get rid of the water!
The Dean-Stark Apparatus
H
O
5g
condenser
cold water
HO
MeO
Dean-Stark
Trap
O
pTsOH
water
level
O
OH
benzene
(reflux)
O
reaction flask
6.4g
MeO
99
+ H2O (0.54 mL)
O
Applications of Ketals in Synthesis
OH
O
Me
LiAlH4
OH
O
Me
O
??
OMe
BOTH
carbonyl groups
reduced
Me
LESS REACTIVE
carbonyl group
reduced?
NaBH4
OH
O
Me
OMe
MORE REACTIVE
carbonyl group
reduced
Ketals are Useful as “Protecting Groups”
O
Me
HO
O
O
OMe
OH
O
Me
??
pTsOH
D/S trap
benzene
Me
OH
H2O,
HCl
LiAlH4
O O
O
Me
OMe
more reactive
carbonyl group
protected
more reactive
carbonyl group
de-protected
O
OH
LESS reactive
carbonyl group
reduced
100
OH
Applications of Protecting Groups with Alcohols
Re-call from E2a: TMS ethers are useful protecting groups for alcohols
OMe
Me
Me
OH
OMe
MeI/NaH
(excess)
OMe
Me
Me
BOTH
Alcohols converted
to methyl ethers
OH
MeI/NaH
??
Me
Me
OH
LESS REACTIVE
alcohol converted
to methyl ether
OH
Me
Me
OMe
MORE REACTIVE
alcohol converted
to methyl ether
Protecting Groups Are Important for Organic Synthesis
Protecting groups are often necessary, but remember, each protecting group
adds 2 steps to your synthesis, so they should be minimized or avoided.
101
Sidenote: Acetals in Aesthetic Chemistry: The Nanoputians…
Addition of Sulfur Nucleophiles to Aldehydes and Ketones:
Formation of Thioketals
+ H2O
Thioketals Offer a Milder Alternative to the Wolf-Kischner Reduction
102
The Wittig Reaction: Conversion of Aldehydes and Ketones
to Alkenes
O
X Y
+X Yylide resonance
contributor
Ph
Ph P
Ph
Me
S
OMe
Me
S+
Me
DMSO
H
CH3
Ylide Formation and the Mechanism of the Wittig
Reaction
103
Wittig Reaction and Alkene Stereochemistry
stabilized ylide
O
+
Ph3P
-
UNstabilized ylide
+
Ph3P
OMe
O
-
H
OMe
OMe
R
O
R
Me
-
O
O
+
Ph3P
+
Ph3P
OMe
E-alkene predominates
H
Me
H
H
+
Ph3P
Z-alkene predominates
Me
-
R
H
Sample Problem
Propose a synthesis of the following product from the indicated starting material
Ph
OH
Ph
104
OH
Nucleophilic Addition to α,β-Unsaturated Aldehydes and
Ketones
Mechanism of Conjugate Addition
105
Nucleophiles That are Weak Bases Will Generally Form
Conjugate Addition Products
O
Nu
OH
Carbonyl C=O bond broken
Nu H
O
O
R
R SH
H Br
Br
S
O
Carbonyl C=O
left intact
R
NH
R
O
R
H CN
O
N
N
R
C
Grignard Reagents Prefer 1,2-addition (direct),
Cuprates Prefer 1,4-addition (conjugate)
O
R
O
HO
1) R2CuLi
1) RMgBr
2) H3O+
2) H3O+
106
R
Summary
• Amine nucleophiles add to aldehydes and ketones to form imines and
enamines
• Alcohols and thiols (diols and dithiols) add to aldehydes and ketones to
form acetals and thioacetals
• Acetals are useful as protecting groups so that chemistry can take place
on less reactive carbonyl groups
• Thioacetals are useful for two-step reduction of carbonyls to alkanes as
a mild alternative to the Wolf-Kischner reduction
• Unsaturated carbonyl compounds can undergo direct (1,2) or conjugate
(1,4) addition
• 1,4 addition is preferred for weak nucleophiles, 1,2 addition is
preferred by very strong (eg Grignard) nucleophiles. Cuprates allow
1,4 addition of carbon nucleophiles to predominate
Carbonyl Compounds Part III: Reactions at the αCarbon
So far, carbonyl groups have only served as electrophiles at the carbon
bound to oxygen, or the terminal carbon of an unsaturated carbonyl.
We will now see that the α-carbon of a carbonyl compound can act as a
nucleophile.
alcohol: pKa = 16
carboxylic acid: pKa = 5
O
O
H3C
O
H
H3C
O-
+ H+
H3C
O
H
H3C
O
H3C
H
H
+ H+
ketone: pKa = 20!!
alkane: pKa = 50
H
O-
H3C
-
H
+ H+
H
O
H
H3C
H
107
H
H3C
H
H
+ H+
Electron-Withdrawing Groups Stabilize α-Anions
For a more
extensive table see:
http://daecr1.harvard.edu/pdf/evans_pKa_table.pdf
The Effect of Stabilization is Additive: More Groups, More
Stability
108
Enols and Enolates are Nucleophiles
Enols are like very reactive alkenes...
OH
+
H3C
O
Br
Br
H Br
Br
H3C
Enolates are carbon nucleophiles
O
+
H3C
O
R
X
O
H3C
H3C
O
+
H
R
O
R
H3C
OH
R
Keto-Enol Tautomerization
109
+
X
Enol-Keto Interconversion is Catalyzed by Base or Acid
Halogenation of Ketones: Acid Catalysis
Only 1 α-hydrogen is replaced by a halogen
110
Halogenation of Ketones: Base Promotion
Stoichiometric Formation of an Enolate With Lithium
Diisopropylamide (LDA)
Preparation of LDA from diisopropylamine and n-butyllithium
Me
Me
Me
N
H
Me
Me
+
H3C
Li
DIA
Me
Me
N
Li
LDA
111
Me
+
H3C
CH3
Alkylation of Ketones, Esters, and Nitriles
Note Similarity to…
R
n-BuLi
R
H
Li
H3C
Br
R
CH3
Sample Problem: Ketone Alkylation
Propose a synthesis of the following branched ketone from a linear ketone starting
material:
O
H3C
CH3
CH3
112
Enolates of Unsymetrical Ketones: Regiochemistry
Problem with α-Branched Ketones
Thermodynamic and Kinetic Enolates
'kinetic'
enolate
CH3 CH3
H3C
N
O
CH3
H
H
'thermodynamic'
enolate
OLi
LDA
CH3
OLi
CH3
H
Li
LDA
1.1 equiv LDA:
major
(Low Temperature)
minor
0.95 equiv LDA:
minor
(Higher Temperature)
Mechanism of Equilibration:
CH3
+
major
CH3I
O
H
H
H
OLi
CH3
+
O
CH3
H3C
CH3I
O
CH3
CH3
CH3
OLi
O
CH3
+
CH3
The kinetic enolate is formed more quickly because of reduced steric hindrance, and when
there is no excess ketone around, it cannot equilibrate. When there is excess ketone, the
kinetic enolate can deprotonate the ketone and form more of the thermodynamic enolate.
113
Hydrazones Give Alkylation Products That Would Result
from the Kinetic Enolate
+Li
Bu-
Ketone Alkylation Can Be Problematic: Polyalkylation,
Regiochemistry, and O-Alkylation are Possible
Enamines are monoalkylated, and are a good alternative when over-alkylation
is a problem, especially in the case of methylation.
114
Carbonyl Alkylation: Stereochemistry and the Evans
Asymmetric Alkylation
New Stereocenter Formed (product is racemic)
products cannot easily be separated!
O
O
MeO
O
N
O
LDA
CH3I
O
Li
H
CH3
MeO
H
Ph
O
Ph
O
O
O
CH3
N
O
H
H
Ph
Ph
i-Pr
i-Pr
major
minor
(diastereomeric proiducts can be easily separated)
Br
O
CH3
H
CH3
i-Pr
H3C
single enantiomer:
chiral auxilliary
derived from valine
+
Ph
O
N
O
CH3
MeO
PhCH2Br
O
CH3
O
LDA
CH3
CH3
N
Sm(OTf)3/MeOH
O
O
Me
Cl
N
O
Et3N
O
H
CH3
+ MeO
H
i-Pr
Ph
single enantiomer
Synthesis of Evans Auxilliaries
O
CH3 O
H3C
CH3
OH
LAH
H3C
NH2
OH
NH2
•
•
OR:
Cl
O
N
O
O
Cl
Cl
H
i-Pr
Cl
O
O
Cl
"triphosgene" (0.33 equiv)
O
Me
Ph
OH
NH2
norephedrine
•
Cl
Cl
occurs in nature as one
enantiomer.
Synthesis of the other enantiomer
of chiral ester would require the
enantiomeric chiral auxilliary...
Cl
phosgene
(1 equiv)
+ Et3N
O
Ph
N
Me
Chiral auxilliaries are useful for making enantiomerically pure products by a variety of
reactions, not just alkylation.
Since the two products are diastereomers, purification can be used to increase the purity
of the product, and thus the final enantiomeric purity once the auxilliary is cleaved.
The auxilliary can be recovered by purification and used in subsequent reactions.
115
H
The Aldol Addition
•
•
It was first observed as a dimerization
of aldehydes to produce a molecule that
was named “aldol” because it was a βhydroxy aldehyde
These two examples represent
homoaldol reactions, or reactions
where both the electrophile and
nucleophile are derived from the same
molecule.
The Cross Aldol Addition: The Nucleophile and
Electrophile Emanate from Two Different Carbonyl
Compounds
A-A
A-B
B-A
B-B
116
The Cross Aldol Reaction
•
•
•
•
While the dimerization of aldehydes and ketones through the aldol reaction is not terribly
useful, the cross aldol reaction is very useful.
The cross aldol reaction involves an electrophile and nucleophile derived from different
carbonyl compounds.
In the cross aldol reaction, the enolate nucleophile usually comes from an ester or a ketone,
and the electrophile is usually an aldehyde.
Because the carbonyl that supplies the nucleophile and the electrophile are both acidic, the
nucleophile must be generated in the absence of the electrophile.
Intramolecular Aldol Addition: 5- and 6-Membered Rings
are Favored.
117
Stereochemistry of the Aldol Reaction: The Acetate and
Propionate Aldol Addition Reactions
O
CH3O
OLi
LDA
CH3
O
RCHO
CH3O
OH
CH3O
R
one new stereocenter created
("acetate" aldol product)
OLi
O
CH3O
O
CH3O
MeO
CH3
E(O)-enolate
LDA
R
CH3
anti-aldol product
RCHO
OR
CH3
OH
OLi
O
OH
CH3
CH3O
MeO
Z(O)-enolate
R
CH3
syn-aldol product
two new stereocenters created
("propionate" aldol product)
Polyketide Natural Products are Synthesized by
Successive Aldol Additions
O
H3C
H3C
CH3
OH
H3C
OH
Me
O
O
O
CH3
OH
OH
O
OH
OH
CH3
HO
OH
H3C H3C H3C OH CH3 CH3 CH3
OH
CH3
7 "propionate" units make up the skeleton of erythronolide
Erythronolide B
O
O
RO
H
Me
Me
O
O
OH
CH3
RO
CH3
CH3
H
CH3
118
O
OP
OH
OP
CH3
RO
CH3 CH3
Update: The Aldol Reaction is Nothing More than a
Special Case of Carbonyl Addition…
"Carbonyl Addition"
O
R
Nu
Nu =
H
R
Li
Nu
R
The Aldol Reaction
OLi
Nu =
O
R
OH
R
H
R
O
R
Sample Problem
119
H-
RMgBr
OH
The Aldol Condensation: Elimination of Aldol Products to
α,β-Unsaturated Carbonyl Compounds
Condensation reactions involve the loss of water or an alcohol so that the molecular
weight of the product is less than the sum of the two starting materials.
Aldol Addition vs Condensation
O
R
O
LDA
OH
R
RCHO
Me
aldol addition
∆
•
•
•
HEAT
HCl/H2O
O
R
NaOH/H2O
∆
Me
aldol condensation
NaOH/RHCO
∆
Use of LDA to form an enolate, followed by addition of an aldehyde will produce the aldol
addition product
If the aldol addition product is then treated with either aqueous acid OR base, the
condensation product will be formed
If the aldol components are combined with base, it is likely that the condensation product will
predominate
120
The Aldol Condensation Produces “Wittig-like”
Products…when is it useful?
O
O
O
O
Ph
Ph
Ph
Ph
O
Ph
Ph
Ph
Ph3P
PPh3
Ph
O
O
Ph
Ph
Ph
Wittig
Ph
Aldol
The aldol condensation is one step, not two (like the Wittig), so in cases where
control of alkene geometry is not important, aldol condensation is OK
In many cases, such as cyclizations, only one product is possible from aldol
condensation, so it is the way to go…
O
Ph
Ph
KOH
O
Ph
O
K
Ph
+
O
Ph
Ph
Ph
H
Ph
O
O
O
Ph
Ph
O
Ph
Ph
K
Ph
O
Ph
Ph
O
Ph
O
H
Ph
Ph
H
EtOH
H
Ph
O
O
K
Ph
EtOK
O
O
Ph
The Michael Reaction: Conjugate Addition of an
Enolate
When an enolate nucleophile reacts with a conjugated carbonyl compound,
it is called the Michael Reaction.
This reaction works well with very stable enolates, ie those derived from βcarbonyl compounds
121
Ph
The Michael Reaction: Conjugate Addition of an
Enolate
If one reactant in the Michael reaction (or other reaction involving an alkoxide base) is
an ester, it is important to match your alkoxide base with the ester component.
If the enolate component in a Michael Reaction is an unstabilized ketone, it works better if you
use the corresponding enamine, which is called the Stork Modification after Gilbert Stork.
The Claisen Condensation: Reaction of Enolates with
Esters
When an enolate nucleophile reacts with an ester to give the product of nucleophilic
acyl substitution, it’s called the Claisen Condensation.
2
O
H3C
O
NaOCH3
OCH3
O
H3C
OCH3
CH3
122
+ CH3OH
The Driving Force for the Claisen Condensation is the
Formation of a Stable Anion
A full equivalent of base is employed, and the product is the dicarbonyl
anion and alcohol from which the alkoxide is derived.
Mixed (Cross) Claisen Condensation and the Dieckman
Condensation
O
H3C
O
CH3
O
H
H
H3C
O
O
NaOEt
O
H3C
O
Me
α-proton removed
NO α-protons!
to form enolate
H
H
O
O
O
H3C
CH3
O
H
O
NaOEt
H
α-protons of ketone are more acidic...
the enolate of the ketone will be formed preferentially
The intramolecular Claisen condensation is called the Dieckman condensation
123
CH3
Update: The Claisen Condensation is Nothing More
than a Special Case of Nucleophilic Acyl Substitution…
"Nucleophile Acyl Substitution"
O
R
O
Nu
Nu =
X
R
Nu
ONa
Nu =
R
O
R
X
R
O
R
Sample Problem
124
NH
R
The Claisen condensation
O
R
R
OH
H2O
Michael + Aldol = Robinson Annulation
Decarboxylation of Dicarbonyl Compounds
β-carbonyl acids will lose CO2, or “decarboxylate,” when heated…
O
O
R
O
OH
H
OH
O
R
O
Recall...
R
C
R
O
CO2
O
O
R
O
O
HCl/H2O
OMe
R
OH
Hydrolysis of a β-keto ester results in decarboxylation…
O
R
CO2
O
O
HCl/H2O
OMe
R
125
O
O
OH
R
CH3
CH3
Malonic Ester Synthesis: Alkylation of a Dicarbonyl
followed by Decarboxylation
"
X
"
O
+
H3C
R
OH
O
X
+
H3C
OH
R
O
Base
H3C
O
R
Malonic Ester Synthesis: Can be Used Iteratively to
Incorporate Two Electrophiles
126
Acetoacetic Ester Synthesis is Analogous to the Malonic Ester
Synthesis
O
2
H3C
O
NaOCH3
OCH3
O
H3C
OCH3
CH3
Sample Problem
127
Sample Problem
Biosynthesis of Polyketide Natural Products by Claisen
Condensations and Decarboxylations
O
O
O
O
O
AT
H3C
SCoA
-O
S
Acyl Transferase
S
H3C
ACP
ACP
Ketoreductase
acyl carrier protein
O
NADPH
OH
S
Dehydrogenase
ACP
Enoylreductase
O
H3C
H3C
S
ACP
ER
O
NAD+
KR
DH
H3C
NADPH
S
ACP
128
NAD+
Carbonyl Chemistry on One Slide
O
R
O
O
OMe
R
[H-]
O
R
R
H
[H-]
[O]
R
R
N
R1
N
R
R
[O]
OH
OH
OH
R1
R
R
R
R
R
OM
O
O
Nu
Nu
R
X
R
O
R1
O
Claisen
R1
R
OM
OH
R
R
O
Nu
Nu
R
OH
R1
R
R
R
OM
Nu
R
O
O
Nu
R
R
R1
O
Aldol
R1
O
1
R
Michael
O
R
R
Sample Problem
129
R
R
R1
R1O OR1
R
R1
R
Sample Problem
The Diels-Alder Reaction
•
•
•
•
Conjugated dienes react with alkenes in a process known as the Diels-Alder Reaction.
The product is a substituted cyclohexene
The mechanism is concerted and pericyclic (concerted = bond formation and bond breakage
occurs simultaneously in the same step)
The hydroboration reaction is the other concerted, pericyclic reaction that we have seen
previously.
diene
2
3
1
CH2
CH2
4
dienophile
CH2
6
1
2
∆
CH2
5
6
3
5
4
3 π-bonds
4 σ-bonds
1 π-bond
6 σ-bonds
2 π-bonds were "traded" for 2 σ-bonds
∆= heat: in this case temperatures of 30-250 °C are generally necessary
130
Molecular Orbitals of the DA Transition State
The Diels-Alder reaction is a [4π + 2π] cycloaddition
reaction.
Diene=
4π component
Dienophile=
2π component
The Diels Alder Reaction is Accelerated by Lowering the
LUMO of the Dienophile
LUMO
Energy
LUMO is lowered by the
electron-withdrawing group
(CO2Me)
lower ∆G(HOMO/LUMO) =
lower ∆G‡(reaction)
HOMO
ethene
H
1,3butadiene
methyl
acrylate
O
H
OMe
H
Dienophiles with EWGs (electron withdrawing groups) are good dienophiles.
EWGs = cyano, ester, aldehyde, ketone, etc
131
The Diels-Alder Reaction is a Stereospecific Syn Addition:
Dienophile Stereochemistry
O
H
H
H
∆
OMe
Me
H
O
Me
Me
H
CO2Me
Me
cis Diels-Alder products
(enantiomers)
cis-dienophile
(Z-alkene)
H
H
CO2Me
H
∆
OMe
H
H
CO2Me
H
Me
CO2Me
H
Me
trans Diels-Alder
products
(enantiomers)
trans-dienophile
(E-alkene)
Diene Stereochemistry/Syn Addition Transition States: Top Face or
Bottom face Addition gives Enantiomers
H
H
MeO2C
Me
H
H
H
H
H
MeO2C
H
H
H
Me
H
CO2Me
H
H
Me
H
H
CO2Me
H
H
Me
H
H
H
MeO2C
Me
MeO2C
H
H
H
H
H
H
H
H
Me
H
H
132
CO2Me
Me
The Diels-Alder Reaction is a Syn Addition:
Diene Stereochemistry
Me
H
Me
CO2Me
Me
CO2Me
H
CO2Me
H
H
Me
CO2Me
CO2Me
CO2Me
Me
E-E-diene
Me
cis Diels-Alder products
(enantiomers)
Me
Me
H
CO2Me
Me
CO2Me
CO2Me
H
CO2Me
Me
H
CO2Me
CO2Me
Me
H
Me
trans Diels-Alder products
(enantiomers)
E-Z-diene
Note: Alkynes can serve as dienophiles, but a double bond is produced, so no new
stereocenters are formed from the dienophile
The Diels Alder Reaction is Accelerated by Raising the
HOMO of the Diene
Energy
LUMO
HOMO is RAISED by adding an
Electron Donating Group (OMe)
HOMO
lower ∆G(HOMO/LUMO) =
lower ∆G‡(reaction)
ethene
1,3butadiene
1-methoxy-1,3butadiene
H
H
MeO
H
H
133
H
Regiochemistry of the Diels-Alder Reaction is Predicted by the
Substituents of the Diene and Dienophile
1. Draw out resonance contributors
+
OMe
H
OMe
H
H
H
H
H
OMe
H
H
H
+ H
H
H
-
H
O
H
OMe
H
-
H
O
H
OMe
+
O
OMe
O
O
OMe
H
OMe
-
CO2Me
H
H
+
OMe
H
H
OMe
H + H
OMe
H
H -
H + H
H
2. Match up charges (opposites attract)
H
-
OMe
H
+
H
OMe
X +
- X -
OMe
CO2Me
O
Diene Conformation: “s-cis” Required for the Diels-Alder Reaction
H
H
H
H
H
H
H
free rotation
H
s-trans conformation
H
Diels Alder Reaction
H
H
H
s-cis conformation
NO Diels-Alder REACTION
diene is locked in s-trans conformation
(no free rotation around σ-bond)
FAST DIELS-ALDER REACTION
diene is locked in s-cis conformation
More s-cis conformer of diene results in a faster Diels-Alder reaction
134
The “Endo” Rule: Endo and Exo Transition States Produce the Endo
and Exo Diastereomers of Product
H
H
H
H
H
H
CO2Me
"exo" (substituent of dienophile is
oriented away from double bond)
CO2Me
Me
H
H
CO2Me
CO2Me
H
Me
H
O
H
H
H
H
H
H
OMe
H
H
H
MeO2C
H
MeO2C
Me
CO2Me
H
H
H
H
Me
H
H
H
Me
"endo" (substituent of dienophile
is oriented toward double bond)
endo transition state, and thus
the endo product, is favored
Sample Problem
For the following three reaction, draw the DA product that results, including
RELATIVE stereochemistry. Circle the reaction that proceeds fastest.
CO2Me
+
CO2Me
+
O
CO2Me
+
135
H
H
H
CO2Me
Introduction to Pericyclic Reactions
1) Polar/Ionic Reactions: Heterolytic bond cleavage, formation of anionic or cationic
intermediates
O
Aldol
reaction
O
H3C
H
O
CH3
OH
H3C
CH3
2) Radical Reactions: Homolytic bond cleavage, formation of radical intermediates
Cl
H
2Cl
Cl
H
H
HCl +
H
Cl
Cl
H
H
H
3) Pericyclic Reactions: Cyclic transition structures where all bond-breaking and bond
forming occurs at the same time (in concert), reorganization of electrons in the reactants,
without formation of an intermediate
O
H
O
O
H
O
H
O
H3C
H
BH2
H3C
O
BH2
Hydroboration
Diels- Alder reaction
transition
state
Pericyclic Reactions
A concerted, single barrier,
one-transition state reaction
energy
starting
material(s)
A
B
(intramolecular)
A + C
B
(intermolecular)
product
progress of reaction
• Cyclic transition structures (therefore, SN2 is not a pericyclic reaction)
• Can be thermal or photochemical reactions
- A photochemical reaction takes place when a reactant absorbs light
- A thermal reaction takes place without the absorption of light
- In a thermal reaction the reactant is in its ground state; in a photochemical reaction,
the reactant is in its excited state
• Orbitals MUST have the same symmetry (be in-phase) to overlap and form products
(“conservation of orbital symmetry” theory)
• Can have suprafacial or antarafacial orbital interactions (bond formation or
rearrangements); always suprafacial for 6 atoms or less in transition state
136
3 Types of Pericyclic Reactions
1) Cycloaddition Reaction
Usually intermolecular
diene
dienophile
(Note: look for conjugated alkenes)
2) Electrocyclic Reaction
Always intramolecular
(Note: look for conjugated alkenes)
3) Sigmatropic Rearrangement
Always intramolecular
Molecular Orbitals: Thermal vs. Photochemical
(LUMO)
(HOMO)
Thermal HOMO and
photochemical
HOMO are different!
(HOMO)
(ground state)
thermal
energy levels
(excited)
photochemical
• The normal electronic state of a molecule is known as its ground state
• The ground state electron can be promoted from its HOMO to its LUMO by
absorption of light (excited state)
• In a thermal reaction the reactant is in its ground state; in a photochemical
reaction, the reactant is in its excited state
137
Molecular Orbitals of 1,3-Butadiene: Thermal vs. Photochemical
(LUMO)
(LUMO) (HOMO)
(HOMO)
The ground state HOMO
and the excited HOMO
have opposite symmetry
Pericyclic Type 1: Cycloaddition Reactions
Two π-bonds are converted into two σ-bonds and a new ring is formed
(usually intermolecular)
thermal
reaction
∆
forms new cyclohexene ring
photochemical
reaction (doesn’t
work with thermal
conditions)
forms new cyclobutane ring
[# + #] refers to the number of π-electrons in the cycloaddition reaction:
[4 + 2] = [4π + 2π] = 4π electrons + 2π electrons
[2 + 2] = [2π + 2π] = 2π electrons + 2π electrons
138
Review of Diels Alder Reaction
(Bruice chapter 8: pages 313-321)
•Conjugated dienes react with alkenes (dienophiles) in a process known as the Diels
Alder Reaction. The product is a substituted cyclohexene
• The mechanism is concerted and pericyclic - proceeds through a cyclic transition state
• The reaction is stereospecific: stereochemistry of the starting materials is conserved in
the products
∆
CH3
+
dieneophile
CH3
CH3
diene
cyclohexene
product
transition state
O
H3CO
CH3
trans-dieneophile
O
∆
+
H3CO
H3C
diene
trans-cyclohexene
product
A dienophile with an electron-withdrawing group (carbonyl or CN), will make the
Diels-Alder reaction faster because the LUMO of the dienophile is lowered to have
better overlap with HOMO of the diene
Molecular Orbitals for [4 + 2] Cycloaddition (Diels-Alder)
Consider the HOMO and LUMO (frontier molecular orbitals) of both reactants:
(This orbital
interaction is
most common)
Suprafacial bond formation =
formation of both bonds on the
same face (side) of the π-system
You can consider EITHER combination of HOMO and LUMO interactions,
as long as the overlapping orbitals are in-phase
139
Antarafacial vs. Suprafacial
• Only suprafacial orbital interactions
will occur if the transition state has six
or fewer atoms in the ring
Migrating group = C, H or other atoms
[2 +2] Cycloaddition Reactions
H3C
CH3
H3C
CH3
hv
+
H3C
H3C
CH3
CH3
Two π-bonds are
converted to two
σ-bonds
Must be a photochemical reaction for in-phase orbital overlap
[2+2] cycloaddition/dimerization reactions of your DNA:
DNA
photolyase
• Ultraviolet light (hv) can cause skin cancer by promoting thymine dimerization, a
structural modification of DNA that can lead to mutations
• DNA photolyase is an enzyme that repairs damaged DNA by reversing the [2+2]
cycloaddition reaction to regenerate the original thymine residues
Note: you will not be responsible for stereo- and regiochemistry for [2+2] cycloadditions
140
MO Analysis of the [2 + 2] Cycloaddition Reaction
Orbitals are NOT in-phase = NO reaction
X
Orbitals ARE in-phase = cycloaddition
Pericyclic Type 2: Electrocyclic Reactions
• Electrocyclic reactions are reversible: ring closing or opening
• Look for conjugation in reagents and/or products
Ring closing:
An intramolecular reaction in which
a new σ bond is formed between the
ends of a conjugated π system to give
a cyclic ring product
Ring opening:
An intramolecular reaction in which a
σ bond of a ring breaks and a
conjugated π system is formed in an
acyclic product
141
Electrocyclic Reactions: Conrotatory vs Disrotatory Ring Closure
The symmetry of the HOMO of the compounds undergoing ring closure determines
the stereochemical outcome of the electrocyclization.
HOMO (ground state)
H
H
H3C
Me Me
CH3
H
∆
HOMO (excited state)
H
H
H3C
Me Me
H
CH3H
The ground state HOMO is symmetric, so disrotatory closure occurs
The excited state HOMO is asymmetric, so conrotatory closure occurs
Woodward-Hoffman Rules Relate the Number of π-Bonds
to the Mode of Cyclization
For systems with an EVEN # of π-bonds, the ground state undergoes conrotatory closure
Me
Me
∆
Fortunately, a set of selection rules prevents the need to draw out the HOMO for
each specific case: These are known as the Woodward-Hoffman Rules
142
Pericyclic Type 3: [m,n] Sigmatropic Rearrangements
a [1,5] sigmatropic rearrangement
(a 1,5-hydrogen shift)
4
4
5
3
3
5
2
1
2
1
2
1
2
3
1
(note: do not worry
about the 1,3 shifts in
the book or previous
lecture note copy, the
book has an error and
the 1,3-shifts are less
useful so we will not
discuss them.)
3
2
1
3
1
3
2
Suprafacial rearrangement =
migrating group remains on the
same face (side) of the π-system
• Sigmatropic rearrangements have cyclic
transition states
• Rearrangement must be suprafacial if the
transition state has six or fewer atoms in the ring
[3,3] sigmatropic reactions
Count Carbons:
1) Count Carbons (1,2,3-3,2,1) in
the starting material to see where
alkenes will shift, and what the
product will look like
2) Count carbons in the product
(1,2,3,4,5) to help identify IF a [3,3]
sigmatropic rearrangment can be
used to synthesize the product
What is the driving force for these rearrangements?
More substituted double bonds in Cope rearrangement (usually)
Stronger bonds formed (C=O vs. C=C) in Claisen rearrangement
143
Ireland-Claisen Rearrangement
General Claisen reaction forms an alkene and an aldehyde:
Ireland-Claisen rearrangement forms an alkene and a carboxylic acid:
O
O
H3C
HO
O
O
acetylation of
alcohol with
acetic anhydride
forms ester
1. LDA
CH3
CH3
OH
2. H3O+
workup
O
O
Ireland-Claisen
Rearrangement
Mechanism:
O
O
O
CH3
LDA
O
CH2
O
Claisen
Rearrangement
H3O+
workup
O
OH
O
enolate
formation
(H starts at C-1 and
ends up on C-n)
[1, n] Hydrogen Shifts
4
1,5-hydrogen shift
4
3
3
2
2
1
1,7-hydrogen shift
5
5
3
One C–H σ bond
broken, one C–H
σ bond formed
1
2
3
1
4
4
5
5
2
1
6
6
7
144
7
Vitamin D Synthesis
Sunlight (hv) converts a cholesterol steroid to vitamin D using two
pericyclic reactions:
(Precursor to vitamin D)
Summary: How to Identify Different Pericyclic
Reactions
Are two molecules coming together to form a new ring?
That’s a cycloaddition
Is a ring opening or closing in an intramolecular fashion?
That’s an electrocyclic process
Is something shifting from one point in the molecule to another? Sometimes
products do not look like starting materials?
That’s a sigmatropic rearrangement (Hardest to identify - count carbons)
- Be able to identify different pericylic reaction types, (especially specific [1,5] and
[3,3] sigmatropic rearrangments)
- Know thermal vs. photochemical HOMO
- Be able to provide products from given starting materials
- you do NOT need to know how to draw the cyclic transition state, but you should
be familiar with the mechanistic details of the process
Vocabulary: Concerted, Pericyclic, Electrocyclic, Suprafacial , Antarafacial
Conservation of orbital symmetry
145
Sample Problems
1. a) What kind of pericyclic reaction is this?
b) What is the driving force of this reaction?
120°C
2. a) What is the product of this reaction?
b) Describe, as specifically as possible, what type pf pericyclic reaction this is.
170°C
O
H3C
CH3
3. Provide products and indicate what type of pericyclic reaction occurs.
O
170°C
CH2
CH2
+
hv
O
O
Introduction to Carbohydrates: Polyfunctional Compounds
general molecular formula = Cn(H2O)n
Carbohydrates (saccharides) are named based on their molecular formula,
which make them appear to be “hydrates of carbon”
Monosaccharide: the simplest carbohydrate unit or “single sugar”, classified by its
number of carbons (trioses, tetroses, pentoses and hexoses)
α- and β-notations: relative stereochemical designation at the anomeric center
D- or L-notations: the stereochemical designations for the configuration of a
sugar, based on glyceraldehyde (instead of R- and S-designations)
Reducing Sugars: have an aldehyde or hemiacetal group that can be oxidized
146
Classification by Carbonyl Type and # of Carbons
Aldoses: polyhydroxy aldehydes (an aldehyde sugar)
Ketoses: polyhydroxy ketones (a ketone sugar)
# of Carbons: triose, tetrose, pentose, and hexose
1
CHO
2
CHOH
3
CH2OH
an aldotriose
O
Nomenclature and Structure: Glyceraldehyde
H
OH
OH
Glyceraldehyde (an aldotriose) has a single stereogenic center and can exist in two
enantiomeric forms:
H
CHO
O
H
H
HO
H
H
HO
CH2OH
CH2OH
(R)-glyceraldehyde
CHO
HO
(S)-glyceraldehyde
D-notation:
hydroxy is on
the right side
D-glyceraldehyde
(Fischer projection)
O
CH2OH
OH
Fischer projections:
Horizontal lines are taken as
coming forward and vertical
lines are going backward
H
CH2OH
L-notation:
hydroxy is on
the left side
L-glyceraldehyde
(Fischer projection)
Note: “CHO” is a common shorthand for drawing an aldehyde group
147
Nomenclature and Structure: Enantiomers and Epimers
• Same D- and L-notation for aldoses with more carbons - look at lowest OH group
• Aldehyde group is always written at the top of the fischer projection and the
primary alcohol is at the bottom.
enantiomers
“L-series”
of sugars
“D-series”
of sugars
Epimers = diastereomers that differ in configuration at only one stereogenic
carbon center; epimers are NOT enantiomers (C-1 epimers are called anomers)
Hydroxy-Aldehydes Form Cyclic Hemiacetals
Intramolecular 5- and 6-membered ring (cyclic) hemiacetals are easily
formed, and are usually more stable than the open (acyclic) form.
a “pyran” ring
H 2O
H
O
H
a new stereocenter
is formed in the
hemiacetal product
OH
O
HO
hydroxy-aldehyde
(open form)
cyclic hemiacetal
(favored)
Cyclic product is the
predominant form
H 2O
H
O
H
H 2O
H
O
O
H
O
OH
OH
derived from
the alcohol
Equilibrium exists in aqueous solution, or with catalytic acid
148
derived from
the aldehyde
The Hemiacetal (cyclic) Form Predominates with Aldoses
β-glucose is favored because the anomeric
OH is in an equatorial position
H
HO
OH
OH
H
H
OH
H
OH
O
HO
HO
CHO
O
OH
CH2OH
(0.02%)
H
D-glucose
open/acyclic form
OH
β-D-glucopyranose
OH
H
β−D-glucose
(64%)
OH
HO
HO
beta = hydroxy up, equatorial
(more favored)
OH
anomeric center at C1
OH
HO
HO
O
H
OH
α-D-glucopyranose
OH
alpha = hydroxy down, axial
α-D-glucose
(more steric strain)
(36%)
The new chiral stereocenter of a hemiacetal is called the anomeric center (carbon-1).
Therefore, the two resulting α- and β-stereoisomers are called anomers
(anomers are specifically a C-1 epimers at an hemiacetal or acetal center)
Equilibrium (open-close-open) between the two anomers is called mutarotation - this
is NOT simply a chair flip - acetals can NOT mutarotate, only hemiacetals
Ketoses also exist predominantly in cyclic forms
Same Structures, Different Representations:
How to Draw Cyclic Monosaccharides
• hemiacetal (cyclic)
form is predominant
• β-anomer is more
favored
(α-D-glucopyranose)
(β-D-glucopyranose)
• Six-membered rings are called pyranoses
• Five-membered rings are called furanoses
149
Anomeric Effect
HO
HO
HO
O
HO
HO
HO
mutarotation
H
OH
OH
O
OH
OH
H
α-anomer,
OH axial (34%)
β-anomer,
OH equatorial (64%)
If the α-anomer (axial) is
disfavored because of
steric interactions, why do
we even see any of it??
We see even more α-anomer (axial) for more electron withdrawing groups:
HO
HO
HO
O
OH
X
HO
HO
HO
H
O
34
67
86
94
OH
OMe
OAc
Cl
X
OH
H
X
%axial
increasing
e- withdrawing
ability
Reason? Although an equitorial position is favored for steric reasons,
the axial position is favored for molecular orbital reasons
Anomeric Effect: Molecular Orbitals
axial lone pair
equatorial lone pair
O
O
X
H
good overlap between
axial lone pair of O and
axial σ* C-X bond
Look at σ* C–X bond in
Newman projections:
must have antiperiplanar
orientation between lone
pair and σ* C–X bond
X
σ* C-X
H
H
no overlap
with equatorial
C-X bond!
X
X
σ* C–X
σ* C–X
H
No overlap!!
good overlap!!
• The lone pair of oxygen stabilizes the axial anomer through donation of electron
density into the empty anti bonding orbital (σ* C-X)
• This effect is strongest for the most electronegative substituents (X) because
there is better overlap between the orbitals
(This alignment is similar to the conformation that is necessary for E2 elimination)
150
Reactions of Carbohydrates: Reductions
Reduction of a cyclic hemiacetal proceeds through a small amount of open (acyclic)
form. As long as an equilibrium exists between the cyclic and acylic form, the reduction
will take place and more open form will be regenerated until the reaction is complete
Reduction of an aldose:
OH
O
HO
HO
CHO
OH
H
OH
HO
HO
OH
OH
OH
H
β−D-glucose
HO
O
(0.02%)
H
H
CH2OH
OH
H
H
1. NaBH4
OH
2. H2O
H
OH
HO
H
H
OH
OH
H
CH2OH
OH
CH2OH
D-glucitol
Reduction of a ketose:
CH2OH
CH2OH
HO
CH2OH
O
HO
H
HO
HO
H
OH
H
α-D-fructofuranose
H
O
NaBH4
H
HO
OH
H
OH
H
HO
H
HO
+
OH
H
OH
H
H
H
OH
OH
CH2OH
CH2OH
D-glucitol
D-mannitol
CH2OH
open chain
ketone
CH2OH
OH
A mixture of sugar alcohols (alditols)
More Reductions of Carbohydrates
(H2/Ni can
also be used)
CHO
H
HO
OH
H
H
OH
H
OH
CH3
H
H2NNH2
HO
NaOH, heat
H
H
CH2OH
OH
H
OH
OH
CH2OH
Wolff-Kishner reduction (via hydrazone)
151
Reduces the aldehyde
group all the way to a
methyl group
Oxidations of Carbohydrates
Oxidation to gluconic acids:
Only the aldehyde
will be oxidized
This method will NOT
oxidize an alcohol or a
ketose, therefore, it is a
useful test to distinguish
between aldoses and
ketoses
Oxidation to glucaric acids:
BOTH the aldehyde AND
the primary alcohol will be
oxidized with HNO3
Oxidations of Carbohydrates: Reducing Sugars
A sugar with an aldehyde, a hemiacetal, a ketone or a hemiketal group is
considered a reducing sugar and can be oxidized. Hemiacetals and
hemiketals MUST be in equilibrium with the open form for oxidation.
Benedict’s and Tollen’s reagent will oxidize BOTH aldoses and ketoses, therefore,
they are good tests for reducing sugars
Oxidation to gluconic acids:
Benedict’s reagents = Na2CO3,
CuSO4, and sodium citrate
Cu(OH)2
+
(Benedict’s
solution)
1. Ag2O, NH3
2. H3O+ workup
+
(Tollen’s test)
Cu2O
A red solid
Ago
Silver mirror
Look for color change or
mirror to show proof of an
aldehyde (a positive test).
these will NOT oxidize the
primary alcohol
Tollen’s reagent = Ag(NH3)2+ -OH
152
Base-Catalyzed Enediol Rearrangments
How is a ketose a reducing sugar? How can a ketone be oxidized?
In a basic solution, ketoses are converted into aldoses (an enediol
rearrangement).
This rearrangement explains why a ketose (and a hemiketal) can be a reducing
sugar - the ketose can still provide an aldehyde for oxidation.
The conditions for the Benedict’s and Tollen’s test are basic enough to
promote the endiol rearrangement.
Enediol Rearrangement Mechanism
A “must know” mechanism
153
Base-Catalyzed Epimerization of an Aldose
This is known as the “Lobry de Bruijin-Alberda van Ekenstein reaction”
resonance stabilized enolate
Deprotonation of the alpha-proton will give an achiral enolate. Now,
reprotonation can occur from either face to give a mixture of epimers.
(must know mechanism)
Reactions of Carbohydrates: Alcohols
The OH groups of monosaccharides show the chemistry of typical alcohols.
For example, the OH groups can be acylated or alkylated:
Ester formation: acylation with acetic anhydride
Ether formation: alkylation with an alkyl iodide and Ag2O
Ag2O is a milder method to alkylate instead of using a base (NaH) to deprotonate the OH.
Ag2O associates with the iodide to make the CH3I a better electrophile.
154
Kiliani-Fischer Synthesis
The carbon chain of an aldose can be increased
by one carbon in a Kiliani–Fischer synthesis
Addition of the cyano group is not selective
and it will make two epimers. This means that
a mixture of aldose products will result in this
synthesis
O- and N-Glycoside Formation
A glycoside is an acetal or ketal, which do not equilibrate with the open form.
Therefore, glycosides cannot be oxidized and they are NON-reducing sugars
Acetal (O-glycoside) formation with glucose and ethanol:
Reducing sugar
NON-Reducing sugars
N-glycoside formation with ribofuranose and phenylamine:
A mixture of α- and βglycosides will form
155
Mechanism of Glycoside Formation
Step 1: Protonation of
anomeric OH at C-1
Step 2: Elimination of water
to form oxygen-stabilized
cation (electrophile)
Step 3: Nucleophile (O, N
or other) can attack from
either the top or the
bottom face.
Step 4: Deprotonation
A mixture of anomers will
be produced - acetals DO
NOT mutarotate
A “must know” mechanism
O-Glycoside Formation to make a Disaccharide
A disaccharide is composed of two monosaccharide subunits hooked together by an
acetal linkage
OH
OH
4
4
6
H+
O
5
HO
HO
1
3
2
OH
α-D-glucose HO
α-1,4'-glycosidic linkage
6
O
5
HO
HO
1
2
3
H
OH
H
4'
OH
6'
O
5'
O
HO
2'
3'
(two equivalents)
1'
H
OH
HO
β-1,4’-glycosidic linkage
HO
OH
OH
O
HO
4
+ HO
HO
OH
OH
H
β-D-galactose
(a C-4 epimer of glucose)
6
H+
O
5
1
3
2
OH
OH
H
β-D-glucose
HO
OH
HO
OH
6
4
O
5
3
2
4
1
6
O
OH
H
lactose
O
5
1
HO
3
2
OH
OH
H
1,2’- and 1,6’-glycosidic linkages are also possible
If a disaccharide glycoside still has an end with a hemiacetal group, then it is
still a reducing sugar - both maltose and lactose are reducing sugars.
156
Polysaccharides: Biopolymers
Amylose (a linear structure)
is a component of starch
Amylopectin is another polysaccharide component of starch that has a branched structure
Carbohydrate Summary
• Sugars (carbohydrates) are polyfunctional compounds that have similar reactivity to
their alcohol, aldehyde and ketone components (such as reductions, oxidations,
acetal reactions, ether and ester formation)
• Reducing Sugars (aldoses and ketoses) are hemiacetals (since they exist
predominantly in their cyclic form) and can mutarotate
• Non-reducing sugars (glycosides) are acetals (which do not equilibrate with an open
chain form) and they do not mutarotate
• Tollens’s and Bendedict’s test will distinguish between a reducing and non-reducing
sugar. The Br2/H2O oxidation will distinguish between aldoses and ketoses.
1)
2)
3)
4)
5)
Be able to draw the cyclic hemiacetal form from a Fischer projection
Be able to identify and draw anomers and epimers; identify D- and L-sugars
Be able to fill-in products or reagents for a given reaction
Mechanisms you need to know: hemiacetal formation, mutarotation, enediol
rearrangement, epimerization and glycoside formation
Know molecular orbital interactions for anomeric effect
Vocabulary: Epimers, Anomers, Anomeric center, Ketose, Aldose, Mutarotation, D- and
L-notations, alpha- and beta-notations, glycosides, glycosidic linkage
157
Summary of Reducing and Non-reducing Sugars
(Reducing gives positive test with Tollen’s or Benedict’s reagent)
Reducing sugars:
Look for an aldehyde, hemi-acetal, ketone or hemiketal. Hemiketal MUST be able to access
the open form for enediol rearrangement of the ketone to an aldehyde
HO
O
HO
H
HO
OH
OH
CH2OH
OH
HO
HO
O
HO
HO
OH
O
H OH
OH
O
O
OH
HO
β−D-glucose
OH
maltose
H
If a disaccharide glycoside still has a
hemiacetal, then it is still a reducing sugar
OH
H
α-D-fructofuranose
Non-Reducing sugars:
Alcohols and acetals are non-reducing and ketals cannot access the open form for enediol
rearrangement of the ketone to an aldehyde.
CH2OH
OH
HO
HO
HO
O
HO
O
OCH3
OH
H
HO
H
methyl β−D-glucopyranoside
CH2OH
H
HO
OCH2CH3
H
H
ethyl α-D-fructofuranoside
All monosaccharide glycosides are NON-reducing sugars
Sucrose
R
OH
H2N
R
O
α-amino acid
(prominant in nature)
H2N
O
H
N
R
R
N
H
OH
O
oligo/poly-peptide:
amino acids linked
by amide bonds
O
R β α
OH
β-amino acid
(uncommon in nature,
studied by synthetic chemists)
protein
(large peptide)
158
OH
D-glucitol
α-amino acid residue
(amino acid -H2O)
O
H
OH
CH2OH
Amino Acids, Peptides, and Proteins
H2N α
OH
Amino Acids are Chiral and Charged
O
H2N
Most amino acids have one stereocenter
OH
All “natural” amino acids are L-configuration,
most have the absolute configuration S
R
Amino acids have at least one basic and one acidic functional group, so as a
result they are always charged...positively at low pH (in acid) and negatively at
high pH (in base). At neutral pH, amino acids are net neutral, with one negative
and one positive charge (zwitterionic)
net charge = +1
net charge = +0
net charge = -1
Amino Acids 1: Hydrocarbon, Alcohol, Thiol, Acid and
Amide side chains
O
H2N
O
H2N
OH
O
H2N
OH
CH3
glycine
(Gly/G)
H3C
alanine
(Ala/A)
OH
H2N
CH3
CH3
OH
O
HS
H3C
cysteine
(Cys/C)
OH
S
methionine
(Met/M)
O
OH
HO
H2N
CH3
isoleucine
(Ile/I)
H2N
OH
O
H2N
OH
H3C
O
threonine
(Thr/T)
O
OH
CH3 leucine
(Leu/L)
H2N
OH
HO
serine
(Ser/S)
O
H2N
H3C
O
HO
H2N
OH
valine
(Val/V)
O
H2N
O
H2N
O
OH
H2N
OH
H2N
O
aspartic acid
(Asp/D)
HO
O
O
glutamic acid
(Glu/E)
159
asparagine
(Asn/N))
H2N
O
Glutamine
(Gln/Q)
Amino Acids 2: Basic, Aromatic, and Heterocyclic
Sidechains
O
H2N
O
H2N
OH
O
H2N
OH
O
H2N
OH
HN
H2N
H2N
lysine
(Lys/K)
HO
NH
phenylalanine
(Phe/F)
arginine
(Arg/R)
tyrosine
(Tyr/Y)
O
H
O
N
H2N
O
H2N
OH
OH
OH
N
HN
NH
proline
(Pro/P)
histidine
(His/H)
tryptophan
(trp/W)
The Acid-Base Properties of Amino Acids are
Important in Many Circumstances
160
OH
The Isoelectric Point (pI)
• When the AA has no ionizable
sidechain (no acidic or basic groups)
the pI is calculated by averaging the
pKas of the amino and acid groups.
• In the case of alanine, when the pH
is below 2.34, both groups are
mostly protonated. When the pH is
above 9.69, both groups are largely
deprotonated.
The Isoelectric Point (pI)
161
Isoelectric Point (pI) for Amino Acids with
Ionizable Sidechains
For groups with ionizable sidechains, the pI is the point at which the two groups
with similar pKas share one charge, which is then balanced with the charge of
the third group.
For lysine (basic side chain), at pH 9.87, the two amino groups are 50%
protonated [2 x (+0.5)], and the carboxylic acid is 100% deprotonated (-1), so the
net charge is 0.
For glutamic acid (acidic side chain), at pH 3.22, the two carboxylic acid groups
are 50% deprotonated [2 x (-0.5)], and the amino group is 100% protonated (+1),
so the net charge is 0.
Electrophoresis Separates Amino Acids and
Peptides Based on their pI Values
Amino acids will migrate toward electrodes when applied to a stationary surface that
is held at constant pH with a buffer. Amino acids with pIs higher than the buffer pH
migrate toward the cathode (- electrode), and amino acids with pIs lower than the
buffer pH move toward the anode (+ electrode).
The bigger the difference between pI and pH, the faster the amino acids will migrate.
A few drops of a mixture of
amino acids was placed here
Arginine, alanine, and aspartic acid separated by electrophoresis at pH 5.
162
Amino Acids are visualized in Electrophoresis with Ninhydrin
Ninhydrin is also a useful stain for distinguishing primary (purple), secondary
(yellow), and tertiary (no color) amines in TLC experiments
Analysis of Amino Acids and Peptides
By Ion Exchange Chromatography
•Charged resins are used to separate amino acids based on charge, in analogy to
silica gel chromatography separating organic molecules based on polarity
•Cation exhange resin (eg Dowex 50, shown below) has a negatively charged
group bound to the resin and an “exchangeable” cation (Na+).
•Anionic amino acids and peptides (low pI) flow through quickly, while cationic
amino acids and peptides (high pI) flow through slowly.
163
Automated Ion Exchange Chromatography: Determination of Relative
Amounts of Individual Amino Acids
Analysis of Amino Acid Content is Useful for Structure Determination
(based on the known structures of these amino acids)
A typical chromatogram obtained from the separation of amino acids using an
automated amino acid analyzer.
Determination of the Structure of Peptide Natural Products
N
OH
HO
O
H2N
OH
alanine
(Ala/A)
O
(6N HCl/reflux)
H2N
HO
O
H2N
OH
HN
2 tyrosine
(Tyr/Y)
H2N
O
H2N
OR
O
aspartic acid
(Asp/D)
(a cyclic peptide
natural product)
OH
arginine
NH (Arg/R)
O
H2N
OH
HO
Cyclonellin D
OH
CH3
2 proline
(Pro/P)
CH3
threonine
(Thr/T)
Hydrolysis
O
H
O
H2N
OH
H2N
O
asparagine
(Asn/N))
Readout from Amino Acid Analyzer
164
Synthesis of Amines: Reduction of Amides
H H
O
R
R
N
H
primary amines
(R1=R2=H)
H
OH
SOCl2
H
O
1
R
R
N
R
Cl
H H
O
R2 LiAlH4
R2
N
Et3N
R
N
R2 secondary amines
(R1=H)
H
R1
H H
R
N
R2 tertiary amines
R1
Ammonia Cannot be used to Synthesize Amines
from Alkyl Halides
H3C
NH3
Br
H3C
H3C
NH2
Br
H3C
(n-BuBr)
H3C
Br+
H3C
N
H3C
CH3
H3C
CH3
N
CH3
H3C
O
O
phthalimide; an "NH3" synthon,
or synthetic equivalent
R
NH2
amide
O
NH
O
1) KOH
2) n-BuBr
1) H+/H2O
2) NaOH
165
H3C
NH2
R
N
H
imide
O
R
NH
The Gabriel Amine Synthesis of Primary Amines
Synthesis of Primary Amines with Azide and Cyanide
Azide is also an NH3 Synthon (replaces Br with NH2)…
H3C
Br
NaN3
H3C
N3
H2; Pd/C
H3C
NH2
Cyanide is a CH2NH2 Synthon (replaces Br with CH2NH2)…
H3C
Br
NaCN
H3C
166
H2; Pd/C
N
H3C
NH2
Reductive Amination: Conversion of Aldehydes and Ketones
to Amines
H
O
H
+
H2N
NaHB(OAc)3
CH3
N
CH3
"H-"
N
CH3
H
imine intermediate
O
+
H3C
N
CH3
NaHB(OAc)3
CH3
N
H
CH3
H3C
N
CH3
"H-"
enamine intermediate
Synthesis of Amino Acids: From Acids and α-Keto Acids
O
O
OH
1) Br2/PBr3
O
OH
2) H2O
NH3
ONH3+
Br
(+/-)-phenylalanine
O
O
OH
NH3
O
OH
O
H2; Pd/C
ONH3+
NH
(+/-)-phenylalanine
Note: these syntheses will give racemic products
167
Synthesis of Amino Acids 2: From Aldehydes…The
Strecker Reaction
O
H
O-
2) H+/H2O
O
Imine
formation
1) NH3/HCN
NH3+
(+/-)-phenylalanine
NH3
H+/H2O
H
H
H
N
N
H-CN
H
H
N+
H
N
H
-CN
H
Nucleophilic
addition
Synthesis of Amino Acids 3: Amino Malonic Ester
Alkylation
O
O
O
EtO
OEt
N
O
O
1) KOEt/BnBr
O-
2)H+/H2O
NH3+
CO2
(+/-)-phenylalanine
O
O
HO
KOEt
Br
O
OK
EtO
O
OEt
N
O
OEt
Ph
H2N
O
EtO
O
EtOH
O
N
OEt
Ph
O
O
H+/H2O
H2N
phthalic acid
168
EtO
O
OEt
Ph
Preparation of Enantiomerically Pure Amino Acids:
Kinetic Resolution
•All of the amino acid syntheses discussed are racemic, but generally, enantiomerically
pure amino acids are desired.
•While they can sometimes be resolved (Bruice 5.14), or prepared using a chiral auxilliary
, a kinetic resolution can also be effective.
•A kinetic resolution is a reaction in which a catalyst (synthetic or enzyme) reacts more
quickly with one enantiomer than the other. Unlike a normal resolution, where a full
equivalent of a chiral reagent is required, a kinetic resolution uses only a small amount of
catalyst or enzyme.
Peptide Bond Synthesis: Regiochemical Problem
Normally, making amides from acids and amines is easy…
O
1
R
O
SOCl2
OH
1
R
H2N
Cl
R2
Et3N
O
1
R
N
R2
H
Since amino acids are bifunctional, there is a problem with regiochemistry…
which amine group reacts with which acid?
169
Peptide Synthesis Step 1: N-Protection
One standard protecting group for peptide synthesis is the t-butoxy carbonyl
group, also called a t-Boc or Boc group:
Peptide Synthesis Step 2: C-Activation
The imidate intermediate is an
activated ester “equivalent,” similar
to an acid chloride, but produced
under milder, more selective
conditions
170
Peptide Synthesis Step 3: Amide bond Formation
Peptide Coupling Agents Accomplish a Net Dehydration
O
BocNH
H
OH
CH3
N
C
H
O
N
O
OH
CH3
BocNH
CH3
CH3
OH
N
H
+ H2O
O
O
N
+ H2O
N
N
H
H
DCC is one of many different coupling reagents, but in all cases they work
by basically the same mechanism…activation of the carboxylic acid so that
a nucleophilic acyl substitution can take place. In all cases, the peptide
coupling agents produce a product that results from the addition of one
equivalent of water.
171
Peptide Coupling Reactions Can Be Repeated to Make
Long Chains
Peptide Couplings Are NOT Well-Suited to Large Peptide
Synthesis
Problems with peptide synthesis:
• Low yields from incomplete reactions
•Difficult separation of product, unreacted starting materials, and DCU
•Difficulty of purification. Chromatography is difficult with very polar molecules and
crystallization is limited to large scale
Bruce Merrifield received the Nobel Prize for solving all of these problems with a new
synthetic technique known as “solid phase synthesis” which is still widely used today.
The first step involves attaching the first amino acid to the resin:
172
Solid Phase Peptide Synthesis
In the next step, the N-protecting group is removed, and the amine is coupled to a
protected amino acid.
reaction
Solution
resin beads
(90 µM)
fritted glass
filter
stopcock
The incoming acid and DCC can
be used in gross excess to ensure
complete reaction;
The excess acid, and DCU are
washed away!
Primary Structure of Peptides: Sequence
Determination by Edman Degradation
Edman degradation removes one amino
acid residue from the N-terminus
O
R
R
H
H
N
N
R'
H
O
H+
S
+
H
N
N
H
S
PTH-amino acid
thiazolinone
derivative
+
H
O
N
H3N+
O
R''
peptide without the original
N-terminal amino acid
The Edman degradation can be performed a maximum of 50 times, so it is suitable
only for short peptides
173
Longer Peptides are Sequenced by Partial Hydrolysis
Dilute acid is used to perform a random partial hydrolysis, but various reagents and
enzymes can used to perform site-specific cleavage reactions:
Partial Hydrolyses Lead to Fragments Which are Lined
up to Determine the Complete Structure
Ala-Lys-Phe-Gly-Asp-Trp-Ser-Arg-Met-Val-Arg-Tyr-Leu-His
Trypsin:
Ala-Lys
Phe-Gly-Asp-Trp-Ser-Arg
Chymotrypsin:
Ala-Lys-Phe
Elastase:
Ala
Cyanogen
Bromide:
Ala-Lys-Phe-Gly-Asp-Trp-Ser-Arg-Met
Gly-Asp-Trp
Lys-Phe-Gly
Met-Val-Arg
Tyr-Leu-His
Ser-Arg-Met-Val-Arg-Tyr
Leu-His
Asp-Trp-Ser-Arg-Met-Val-Arg-Tyr-Leu-His
Val-Arg-Tyr-Leu-His
Modern peptide sequencing is largely done by Mass Spectroscopy. New
advances in MS have fueled many discoveries in Proteomics, or the study of
the protein products of gene expression.
174
Secondary Structure of Proteins: Amide Bond Rotation
The peptide chains that make up proteins might look like they are very
flexible, but in reality many factors contribute to their rigid structure
Cysteine Residues Form Disulfide Bonds
(Br2)
175
Disulfide Bonds Contribute to Secondary Structure
Hydrogen Bonding is an Important Secondary Structural
Element: The α-Helix
176
Hydrogen Bonding is an Important Secondary Structural
Element : The β-Pleated Sheet
Coil/Loop Conformation: >50% of Most Globular
Peptide Secondary Structure
177
Tertiary Structure: The 3-D Structure That results From
the Composition of various Secondary Structural
Elements
Summary of Protein Structure
178
Enzyme Active Site
Nucleic Acids Make up DNA and RNA
A nucleic acid is a polymer
of ribofuranoside rings,
phosphate ester groups
and nucleobases
NH2
7
N 5
6
8
a ribosephosphate
backbone
9
5'
HO
N
O
4'
3'
OH
4 N
3
1'
2'
A nucleoside
179
N1
2
Ribofuranoside Rings from Ribose
CHO
H
OH
H
OH
H
OH
HO
H
HO
CH2OH
CHO
H
H
OH
H
OH
CH2OH
D-2-deoxyribose
H
OH
nitrogen base
R
R
N
H
HO
O
H
HO
OH
H
β-D-2-deoxyribofuranose
R
HO
glycosidic
bond formation
O
R
a ribonucleotide
nitrogen base
R
R
N
H
glycosidic
bond formation
R
HO
O
H
HO
N
R
H
a deoxyribonucleotide
Phosphoester and Nucleobase Structures
180
N
H
OH
H
HO
β-D-ribofuranose
D-ribose
H
O
OH
Aromatic Amines and Basicity: 6-membered Rings
piperidine
+ H+
pyridine
N+
H H
+ H+
+
N
H
pKa
sp
morpholine
O
O
+ H+
5.2
+
N
pKa=9.3
N
H
H
N
3
H
N
sp2
pKa=11.2
N
H
The lone pair of pyridine is NOT part of the π-system of
the aromatic ring. The nitrogen is sp2 hybridized, and therefore less
basic.
Basicity = piperidine > morpholine > pyridine
nb: do not confuse acidity, basicity, low pKa, and stability of the conjugate
base…they are all ways of asking the same question!
Whenever ranking acidity or basicity, consider 4 effects: aromaticity,
resonance, hybridization, and induction (EWGs).
Nitrogen Atoms are Electron-Withdrawing in an
Aromatic Ring
pyridine
pyrimidine
N
+
N+
N
H
pKa
5.2
2
sp
H
sp2
1.0
Pyrimidine is significantly less basic
than pyridine due to the electron
withdrawing effect of the added nitrogen
181
Aromatic Amines and Basicity: 5-Membered Rings
Recall: EAS of 5-membered heterocycles (E+ = H+)
H
N+
H H
N H
H+
H+
+
N
2
sp
H
sp2
H
The lone pair of pyrrole IS part of the π-system of
the aromatic ring. Protonation destroys aromaticity,
and is therefore highly disfavored.
N H
pyrrolidine
pyrrole
N
N
sp2
17
pKa
H+
+
N
11.3
pKa -3.8
H
N+
H H
sp3
H
DEprotonation of pyrrole to
form an anion is facile. The
resultant anion is very stable.
36
Imidazole is Aromatic, and Behaves like a
hybrid of Pyridine AND Pyrrole
pyridinium
protonated
pyrrole
protonated
imidazole
+
N
H
pKa = 5.16
N+
H H
N+
H
H
H
pKa = 6.8
imidazole
N
N
N
H
H
pKa = 17
Aromaticity
Retained
N
pKa = -3.8
pyrrole
H
N+
N
Imidazole can serve as a weak
acid or as a moderate
base/nucleophile. It is
responsible for many important
biological functions.
pKa = 14.4
O
H2N
OH
N
NH
182
histidine
(His/H)
The Nucleosides of RNA and DNA
Nucleoside = ribofuranoside + nucleobase
NucleoTIDE = Nucleobase + Ribofuranoside +
Phosphate
183
Monomeric Nucleotides are Important Biological
Molecules
The Phosphate Linkage is Reactive…Kind of Like
an Anhydride
O
OH
OH
O
O
O
HO
HO
O
-O P O
-O
HO P O-O
O
HO
HO
OH
OH
∆G = + 3.3
kcal/mol
+ H2O
OH
Not a favorable reaction
O
P
P
P
-O
O
O
O
-O
-O
-O
O
A
O
+ H2O
O
P
P
O
O
O
-O
-O
A
O
∆G = - 7.3
kcal/mol
+ HO P O-O
OH
OH
ATP
O
ADP
Reaction becomes
favorable:
HO
HO
O
OH
-O P O
-O
O
+ ATP
OH
HO
HO
OH
OH
184
+ ADP
O
OH
∆G = - 4.0
kcal/mol
ATP is Thermodynamically Unstable (reactive), but
KINETICALLY Quite Stable (UNreactive)
Enzymes are necessary for the phosphoryl transfer reactions of ATP
Without ATP, No Phosphorylation Would Take Place
This phenomenon is reminiscent of alcohol activation by conversion to a
tosylate…you must have a good leaving group!
Nu:
R
OH
x
R
R
Nu
Unstable leaving group
TsCl/
Pyr
Nu:
OH-
TsO-
OTs
R
Nu
Stable leaving group
185
ATP’s Function in Biosynthesis
The following reaction is impossible in the absence of ATP…
Activation with ATP allows thioester formation to take place
Kinases are an Important Class of Enzymes that
Mediate Phosphoryl Transfer Reactions
Kinase +
ATP
P
Active Protein
Inactive Protein
Amino acids with alcohol groups in their side-chains are subject to
phosphorylation by kinases…
O
H2N
HO
serine
(Ser/S)
O
OH
H2N
HO
O
H2N
OH
CH3
threonine
(Thr/T)
HO
tyrosine
(Tyr/Y)
186
OH
Inhibition of Kinases Could lead to Cures for Many
Diseases, including some kinds of Cancer
Molecules that “look”
like ATP can occupy the
binding site and prevent
phosphorylation
The problem is
specificity: all
kinases (and many
other enzymes) have
ATP binding sites, so
most kinase inhibitors
are non-specific
GleevecTM (Novartis) is the one of the first SPECIFIC
Kinase Inhibitors Approved for the Treatment of Cancer
187
Several Other Nucleotides Play Important Biological Roles
Oligomerization of Nucleotide Triphosphates gives DNA
188
Hydrogen Bonding of Nucleobases
N
H
O
N
keto
H-Bond
donor, d
enol
H
N
X
H
N
H
N
B
H-Bond
acceptor, a
OH
NH
N
H
H
a
N
a
R
H
N
a
N
R
NH2
amine
imine
d
O
N
a
N
Hd
O
a
N
N
a
OH
d
R
cytosine
Keto-enol and imine-amine tautomerization of the nucleobase
determines the type of hydrogen-bonding that can occur
Base Pairing in DNA Occurs through Hydrogen Bonding
189
Complimentary Strands are Anti-parallel; π-Stacking
Interactions are Important
The attractive force between two proximal
aromatic rings is referred to as "π-stacking"
The Double Helical Structure of DNA
190
DNA Structural Unit: Chromatin
Histones combine with DNA to form nucleosomes,
the fundamental structural units of chromatin. All of
this points to the conclusion that chromatin in intact
nuclei is highly dynamic, with different folding
conformations that reflect its activity.
(Figure from Biology: Concepts and Connections by
Campell, Mitchell, and Reece. Text from Introduction
to Cell and Molecular Biology by Stephen L. Wolfe)
How Can Drugs React with DNA if it is Packed in a Nucleosome?
1) Outer surface of DNA is still
accessible to small molecules
2) Nucleosomes are in dynamic
equilibrium with uncoiled DNA so that the
drug can bind after uncoiling, which also
interferes with the binding of the DNA to
the histone
3 Types of DNA binders:
1) Reversible DNA binders
External electrostatic binding
Groove binding
Intercalation
2) Irreversible covalent reactions with DNA binders by Alkylation
3) Irreversible DNA strand breakers by radical reactions
191
Reversible DNA Binders
1)
2)
3)
External electrostatic binding:
cationic complexes, usually metals,
bind the negatively charged
phosphate backbone and lead to
disruption of the DNA structure
Groove binding: interactions with
minor groove by electrostatic and
hydrogen bonding
Intercalation: flat, aromatic
molecules insert in between base
pairs, stabilized by π-stacking and
charge transfer interactions
(anti-tumor agent)
Intercalation: Ethidium Bromide
Ethidium bromide is a
common fluorescent stain
used with double-stranded
DNA. Ethidium intercalates
between DNA bases. In the
intercalated state, ethidium
exposed to short-wave UV
light (302nm) will fluoresce
bright orange (595nm). The
intercalation of ethidium into
double-stranded DNA
causes the helix to extend
and unwind.
192
DNA as a Nucleophile: Alkylation
most nucleophilic
site in DNA
double helix
O
NH
N
O
N
NH2
N
O
H
O
N
H3C
H
O
H3C
N
H
I
O
N
NH2
O
H
alkylation
O P O-
NH
O
H
H
O P O-
O
O
Most reactive nucleophilic sites of DNA:
N-7 of guanine > N-3 of adenine > N-7 of adenine > N-3 of guanine > N-1 of adenine
> N-1 of cytosine. The amine on C-2, the O-6 of guanine and phosphate groups can
also be alkylated.
This reactivity is strongly controlled by a combination of steric, electronic and
hydrogen-bonding effects. For example, nucleophilicity is diminished by hydrogenbonding and nucleophilic sites on the interior of the DNA double helix are sterically
less accessible.
Recall: DNA Damage with other electrophiles
epoxide
hydrolase
p450
HO
O
benzo[a]pyrene
OH
O
N
O
NH
N
O
O P O
p450
NH2
N
HO
O
O-
OH
diol epoxide
O
O
N
N
O
O P O
NH HO
N
Cytochrome
p450
N
H
O
O
OO
covalently modified,
damaged DNA
193
benzene
benzene
oxide
Recall: Nitrogen Mustards as bis-Alkylating Agents that
Crosslink DNA
CH3
S
Cl
Nu:
Cl
N
Cl
Nu
intermolecular
N
Nu
Nu:
intramolecular
substitution (SN1)
N
CH3
SN2
CH3
intermolecular
H3C
Cl
Cl
mechlorethamine
treatment of advanced
Hodgkin's disease
Nu:
SN2
CH3
N
N
Cl
sulfur mustard
toxic nerve gas used
in World War II
Two methods:
Cl
Cl
CH3
H3C
Cl
N
Cl
Nu
N
Cl
aziridinium ion is
a great electrophile!!
Nu
Nu:
CH3
Nu
N
Nu
bis-alkylation product
Recall: Bis-Alkylation of DNA = Crosslinking
DNA
H3C
N
Cl
Nu:
intramolecular
substitution (SN1)
Cl
CH3
H3C
N
Cl
Cl
N
aziridinium ion
intramolecular
substitution (SN1)
DNA
O
Nu:
-O P O
O
H
H
H
O
CH3
HN
N
H2N
N
O
O P O
N
O
OH
O
H
H
N
N
O
O
N
H
CH3
N
-O
O P O
O
N
N
NH2
Crosslinked G-G residues of DNA double-helix
O P OO
194
DNA
DNA
DNA is Transcribed into RNA, Which is Used in the
Template Synthesis of Peptides
The DNA code: Each amino Acid is Described
by a 3-letter Codon
195
DNA is Very Stable (encyclopedia),
RNA is hydrolyzed very readily (post-itTM note)
Cell Biology Experiments Involve the Ability to Control
Gene Expression
DNA
Genetics (mutation/deletion)
RNA
Block Transcription (eg triple
helix)
Block Translation
(Antisense/siRNA)
Peptide
Chemical Genetics (small
molecules/drugs)
Function/Phenotype
196
Antisense Oligomers and siRNA bind to RNA and
Prevent Translation into a Protein
DNA-like strand still
around
5'-T-A-C-G-T-A-T-T-5'
A
3'-A-U-G-C-A-U-A-A-5'
Antisense
Oligonucleotide
(DNA-like)
U
C
G
T
A
A
A
RNAse (recognizes RNADNA duplex, destroys the
RNA strand)
5'-T-A-C-G-T-A-T-T-5'
3'-A-U-G-C-A-T-A-A-5'
X
Ribosome,
tRNA, etc
Ribosome
Protein
Chemically Modified RNA Analogs Are not Hydrolyzed, and are
More Effective at Blocking RNA Translation
O
O
O
O
base
O
P OO
O
O
O
base
O
base
O
P SO
O
S
O
base
O
base
O
H
C H
O
base
N
O
base
O P
O
Me N
Me
O
base
N
O
Phosphodiesterlinked DNA
O
O
Phosphothioatelinked DNA
197
Thioformacetallinked DNA
Morpholinolinked “DNA”
RNA Was Probably the First Biological Molecule:
Ribozymes
The Ribozymes are oligomers of RNA that can catalyze reactions…Tom
Czech received the Nobel Prize for this discovery.
X-ray crystal structure of the
Tetrahymena ribozyme. The secondary
structure is highly pre-organized for
substrate binding, much like an enzyme.
(T. Czech, et al, Sciene 282, p282, 1998)
RNA Was Probably the First Biological Molecule:
The Ribosome
The Ribosome is made up of mostly RNA, unlike most enzymes which are
made of amino acids. Gray=RNA, Yellow=Peptide
198
How, then, Did Life Begin, Chemically Speaking?
The Miller experiment demonstrated that simple organic molecules would
spontaneously form amino acids with the help of lightning
O
H
O
O
H2N
OH
OH
OH
O
O
H2N
HO
O
O
H3C
OH
CH3
OH
H3C
OH
O
H2N
OH
O
OH
H3 C
O
OH
H2N
O
HO
O
N
H
H
OH
H3C
O
HO
CH3
OH
O
OH
H3C
N
H
OH
O
N
O
NH2
H2N
NH2
CH3
O
H2N
O
OH
H2N
OH
CO2H
CO2H
The Oro Experiment Demonstrated That Prebiotic
Nucleobase Synthesis was Possible
In the presence of UV light, HCN forms adenine…
NH2
HCN
N
N
H
N
N
Adenine;
C5H5N5 or 5 x
HCN!
The conditions can be varied to produce other heterocycles, including other
nucleobases
199
The Formose Reaction Provides Ribose
O
O
H
HO
H
Base
H
OHO
H
Glycoaldehyde
Formaldehyde
(the simplest
carbohydrate)
O
H
H
OOH
O
HO
HO
H
HO
O
H
HO
H
OH
Ribozymes
HO
Ribose
Threose
NH2
NH2
N
N
HO
O
OH
N
H
OH
N
N
N
N
HO
N
O
OH
OH
OH
The yield of ribose is quite low, and many other sugars are formed at the
same time. The addition of borate to the reactions greatly increases the
selectivity for ribose formation (Science, 303, 196, 2004).
How Did we End Up as Single Enantiomers?
This is a raging origin of life debate, but the answer is probably not through the
influence of magnetic fields…
O
OH
H
RMgBr
R
New Stereocenter Created...
racemic (no magnetic field)
up to 90% ee (magnetic field)
Angew. Chem. Int. Ed. Engl. 1994, 33, 454; (original paper); Angew. Chem. Int. Ed. Engl. 1994, 33, 1458;
(results not reproducible); Angew. Chem. Int. Ed. Engl. 1994, 33, 1459; (results not reproducible) ;
Angew. Chem. Int. Ed. Engl. 1994, 33, 1376; (paper withdrawn); Angew. Chem. Int. Ed. Engl. 1994, 33,
1457; (paper withdrawn); Angew. Chem. Int. Ed. Engl. 2004, 43, 2194. (Ph.D. revoked)
“On February 7, 1996, as recommended by the commission, the
committee of the Faculty of Mathematics and Natural Sciences decided
to strip Guido Zadel of his doctorate because of grave deception during
(and after) his doctoral studies.”
“The doctoral certificate must be withdrawn and Guido Zadel is no
longer allowed to use the title of Doctor.”
200
Biosynthesis, Natural Products and Drug Action
Basic organic chemistry concepts can be applied to biological systems in two
main topics of discussion:
1)
Biosynthesis of natural products: How does nature synthesize all
of her molecules? Recall ATP…but there are also other methods.
Paul M. Dewick (2002) Medicinal Natural Products: A Biosynthetic Approach
John Wiley and Sons, New York, NY.
2)
Drug design and drug action: How do small molecules have such a
huge impact on biological systems? Recall aspirin…
Richard B. Silverman (2004) The Organic Chemistry of Drug Design and
Drug Action Elsevier Academic Press, Burlington, MA.
Sample Problem: Aflatoxin
Aflatoxin is formed in peanuts as a result of a mold that can grow inside the
shell. One mode of toxicity involves covalent modification of DNA by a
metabolite of aflatoxin, namely aflatoxin epoxide.
O
O
O
O
O
O
cytochrome
p450
H
O
O
O
O
O
O
H
H
aflatoxin
aflatoxin
a) Enzyme-mediated oxidation generally take place on
electron-rich alkenes. In the case of aflatoxin, why is only
one of the 3 possible alkenes epoxidized?
O
H
O
O
H
O
H
201
Sample Problem, continued
b) Aflatoxin epoxide damages DNA by covalent modification of N7 of adenine.
Draw the product.
NH2
N
N
HO
N
N
O
O
O P OO-
c) N7 of adenine is not the most nucleophilic nitrogen. What binding property
might aflatoxin have that would cause it to interact more selectively with DNA?
Chlorismate Mutase: Biosynthesis of Phenylalanine
and Tyrosine Using a Claisen Rearrangement
decarboxylative
aromatization and
transamination
CO2H
NH2
L-Phe
O
OH
chorismate
chlorismate
mutase
mutase
OH
O
OH
HO
HO
O
Claisen
rearrangement
chlorismic acid
O
O
O OH
OH
O
O
CO2H
NH2
OH
OH acid
prephenic
Count carbons to see which alkenes are
involved in the [3,3]-sigmatropic rearrangement
L-Tyr
decarboxylative
aromatization,
oxidation, then
transamination
OH
[3,3] sigmatropic rearrangements such as the Claisen rearrangment normally occur
under thermal reaction conditions. The rate of the Claisen rearrangment is increased
106-fold in the presence of the enzyme due to stabilization of the transition state.
202
Synthesis of Vitamin C from D-glucose
OH
HO2C
O
HO
HO
OH
HO
HO
NAD+
O
OH
OH
OH
NADH
D-glucuronic acid
D-glucose
HO2C
NAD+ = oxidant; NADH = reductant
OH
OH
HO
OH
HO
O
EKT
O
HO
HO
OH
OH
OH
L-gluconic acid
HO
HO O
O
[O]
HO O OH
O
CH2OH
OH
HO
O
2-oxogluconolactone
Ascorbic Acid
(Vitamin C)
H
L-gluconolactone
HO
Plants and most animals can convert glucose into ascorbic acid
(vitamin C) by the pathway shown. Humans and primates are deficient
in the enzyme oxidizing gluconolactone to the ketolactone, and are thus
dependent on a dietary source of Vitamin C.
H
H
OH
H
OH
OH
CO2H
Sample Problem: Ascorbic Acid’s Acidity
OH
HO
O
HO
O
Ascorbic Acid (Vitamin C) is not a carboxylic acid, yet
it is acidic enough to get “acid” in it’s name. Which
proton is most acidic and why?
OH
Ascorbic Acid
(Vitamin C)
203