* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Effects of activity-dependent strategies on regeneration and
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
End-plate potential wikipedia , lookup
Neurobiological effects of physical exercise wikipedia , lookup
Electromyography wikipedia , lookup
Synaptogenesis wikipedia , lookup
Proprioception wikipedia , lookup
Evoked potential wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Neural engineering wikipedia , lookup
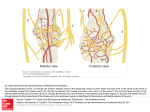
Annals of Anatomy 193 (2011) 347–353 Contents lists available at ScienceDirect Annals of Anatomy journal homepage: www.elsevier.de/aanat Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries Esther Udina a,b , Stefano Cobianchi a,b , Ilary Allodi a,b , Xavier Navarro a,b,∗ a Group of Neuroplasticity and Regeneration, Institute of Neurosciences and Department of Cell Biology, Physiology and Immunology, Universitat Autònoma de Barcelona, Bellaterra, Spain b Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), Spain a r t i c l e i n f o Article history: Received 9 December 2010 Received in revised form 14 February 2011 Accepted 24 February 2011 Keywords: Exercise Nerve regeneration Neuropathic pain Reinnervation Spinal reflexes Treadmill s u m m a r y Peripheral nerve injuries result in loss of motor, sensory and autonomic functions of the denervated limb, but are also accompanied by positive symptoms, such as hyperreflexia, hyperalgesia and pain. Strategies to improve functional recovery after neural injuries have to address the enhancement of axonal regeneration and target reinnervation and also the modulation of the abnormal plasticity of neuronal circuits. By enhancing sensory inputs and/or motor outputs, activity-dependent therapies, like electrostimulation or exercise, have been shown to positively influence neuromuscular functional recovery and to modulate the plastic central changes after experimental nerve injuries. However, it is important to take into account that the type of treatment, the intensity and duration of the protocol, and the period during which it is applied after the injury are factors that determine beneficial or detrimental effects on functional recovery. The adequate maintenance of activity of neural circuits and denervated muscles results in increased trophic factor release to act on regenerating axons and on central plastic changes. Among the different neurotrophins, BDNF seems a key player in the beneficial effects of activity-dependent therapies after nerve injuries. © 2011 Elsevier GmbH. All rights reserved. 1. Introduction Peripheral nerve injuries result in loss of motor, sensory and autonomic functions in the denervated territory. Moreover, as a consequence of the nerve injury positive symptoms also appear, such as hyperreflexia, due to enhanced spinal motor responses, and hyperalgesia and pain, influenced by plastic changes of afferent projections in the spinal cord, in addition to the development of distorted central somatotopic maps of the reinnervated regions (Lundborg, 2003; Navarro et al., 2007). The functional loss can be recovered if injured axons grow into the distal stump and reestablish functional connections with appropriate peripheral target organs. The degree of reinnervation primarily depends on the severity of the injury, related to length of nerve disruption and misalignment of nerve stumps, and on the repair procedure applied (Valero-Cabré and Navarro, 2002). The ultimate goal of peripheral nerve repair is an effective functional recovery, which requires a sufficient amount of regenerated axons, but also appropriate tar- ∗ Corresponding author at: Facultat de Medicina, Universitat Autònoma de Barcelona, E-08193 Bellaterra, Spain. Tel.: +34 935811966; fax: +34 935812986. E-mail address: [email protected] (X. Navarro). 0940-9602/$ – see front matter © 2011 Elsevier GmbH. All rights reserved. doi:10.1016/j.aanat.2011.02.012 get reinnervation, and restitution of adequate central connectivity in spinal circuits. Various forms of exercise training are used in rehabilitation medicine to help maintaining muscle properties during denervation or paralysis and to promote functional recovery after neural injuries and in neurodegenerative diseases. During the regeneration-reinnervation period, enhanced sensory inputs and/or motor activity by means of electrostimulation or exercise have been shown to positively influence the neuromuscular functional outcome after experimental nerve injuries (Al-Majed et al., 2000; Asensio-Pinilla et al., 2009; Marqueste et al., 2004; Vivo et al., 2008). Furthermore, activation of sensory afferents via electrical stimulation can result in modulation of spinal reflex circuits (Vivo et al., 2008) and amelioration of neuropathic pain (Nam et al., 2001; Sun et al., 2004). Intensive programs of sensory reeducation after nerve injury can improve tactile discrimination and threshold perception, although this effect may wane after cessation of training (Shieh et al., 1998). Combined rehabilitation of motor and sensory functions by passive or active exercise programs may eventually lead to a better coordination of sensorymotor tasks and restoration of adequate circuitry at the spinal level. This review is focused on the effects of activity-dependent treatments, particularly exercise training, on peripheral nerve function 348 E. Udina et al. / Annals of Anatomy 193 (2011) 347–353 Table 1 Summary of activity-induced effects in animal models of peripheral nerve injuries. Activity Injury Duration Nerve regeneration Swimming SNC 3d ↑ Axonal growth Collateral sprouting 25 m/d, 7 wk Wheel running Treadmill running Intact 2 h/d, 3–6 wk 1–2 h/d, 4–6 wk 3, 7 or 28 d ↓ Reinnervation ↑Reinnervation ↓MAG, ↑PKA ↑BDNF, GAP-43, CREB SNC 3, 7 d SNF 17/34 wk ↑Regeneration ↑BDNF, NT3, GAP-43 ↑Degeneration in tibialis a. (2 wk), ↑Regeneration in soleus muscle (8–12 wk) L4T, L5T 8 h/d, 4 wk LGST 30 or 90 d ↑Contraction Intact 1 h/d, 4 wk 9 h, 1–7 wk PNTR CCI L4T 3 h/d, 10 wk 1 h/d, 1 wk 1 h/d, 8 wk 10 wk pre-injury ↑BDNF, NT4, TrkB ↑Sprouting ↑ Fiber type change ↑ CNAPs ↑ Regeneration L4T, L5T 8 h/d, 3–28 d SNC 1–2 h/d, 2–6 wk 1 h/d, 14 d SNTR 1 h/d, 4 wk 20 m/d, 2 wk 60 m/d, 2 wk 1 h/d, 4 wk Bicycle training Mechanical stimulation PNC 2 h/d, 4 d SNTR 1 h/d, 4 wk FcTR Daily, 8 wk MTR DCNT 2 wk Neuropathic pain Reference ↓ Allodynia 5–7 wk Gutmann and Jakoubek (1963) Hutchinson et al. (2004) Herbison et al. (1974) Ghiani et al. (2007); Gomez-Pinilla et al. (2002) Molteni et al. (2004); Ying et al. (2005) Irintchev and Wernig (1987) ↓Sprouting in tibialis a. muscle ↑ MU enlargement Tam et al. (2001) Seburn and Gardiner (1996) Skup et al. (2002) Wernig et al. (1991) ↓ Allodynia; ↑Allodynia ↑Sprouting in plantaris, ↓in solueus ↓ Sprouting and Schwann cells bridging ↑Type II fibers in plantaris muscle ↑ DRGs neurite growth, Schwann cells proliferation, ↑GAP-43 ↑ Regeneration and reinnervation ↑Axonal elongation and sprouting ↑Axon elongation but not sprouting ↑Regeneration and reinnervation ↑ Sprouts and reinnervation ↑Regenerated axons and reinnervation ↑ Reinnervation Marqueste et al. (2004) Cobianchi et al. (2010) Gardiner et al. (1984) Tam and Gordon (2003) Herbison et al. (1980a,b) Seo et al. (2009) ↑Sensory recovery Asensio-Pinilla et al. (2009) Sabatier et al. (2008) Sabatier et al. (2008) ↓H-reflex excitability Udina et al. (2011) ↓H-reflex excitability Pachter and Eberstein, (1989) Udina et al. (2011) Angelov et al. (2007) No effect ↑Sprouting nociceptive fibers ↑Sensitivity Sinis et al. (2008) Nixon et al. (1984) Injury: SNC: sciatic nerve crush; SNF: sciatic nerve freezing; L4T: spinal L4 root transection; L5T: spinal L5 root transection; LGST: lateral gastrocnemius-soleus nerve transection; PNTR: peroneal nerve transection and repair; CCI: chronic constriction injury of sciatic nerve; SNTR: sciatic nerve transection and repair; PNC: peroneal nerve crush; FcTR: facial nerve transection and repair; MTR: median nerve transection and repair; DCNT: dorsal cutaneous nerve transection. Duration: m: minutes; h: hours; d: days; wk: weeks. Others: ↑: enhancement or increase; ↓: reduction or decrease; MU: motor units; CNAPs: compound nerve action potentials; DRGs: dorsal root ganglia. and regeneration and on plastic changes in spinal circuits (see Table 1). 2. Effects of exercise training on neuromuscular function Peripheral nerve lesion or spinal cord injuries induce inactivation and subsequent paralysis of affected skeletal muscles. Denervation or disuse leads to a progressive atrophy of skeletal muscles, with marked loss of muscle mass, decreased crosssectional area of muscle fibers and changes in the mechanical and biochemical muscle characteristics (Boudriau et al., 1996; Gordon and Mao, 1994; Marqueste et al., 2006). These adaptations are most evident in muscles predominantly of slow-twitch, which show the highest rate of recruitment during normal activity. Amongst the fast-twitch muscles, the ones that are normally more active (e.g., extensors versus flexors in the hind limb) are also more affected by inactivity (Roy et al., 1999). In general, slow muscles become faster and fast muscles become slower following disruption of their nerve supply (Pette and Staron, 2001). Reinnervation of a previously denervated muscle partially restores its phenotype, indicating the importance of neural activity in the maintenance of muscle fiber types (Wang et al., 2002). However, activity is not the only factor that influences the quality of the muscle. Alterations in the muscle are more marked after peripheral nerve injuries than after spinal cord lesions (Roy et al., 2002). The disruption of the neuromuscular unit after nerve lesion leads to the loss of both activity-dependent and -independent neural influences on the muscle. E. Udina et al. / Annals of Anatomy 193 (2011) 347–353 Artificially maintaining muscle activity during denervation/paralysis may improve neuromuscular rehabilitation. Electrostimulation of denervated muscle, passive exercise and locomotion training have been shown to be effective strategies in retarding muscle atrophy and improving contractile response after reinnervation (Eberstein and Pachter, 1986; Hennig and Lomo, 1987; Marqueste et al., 2004; Pachter and Eberstein, 1989; Soucy et al., 1996). In contrast to inactivity, increased neuromuscular activity or overloading (increased load to a muscle induced by surgical ablation of synergistic muscles) elicits fiber type transition from fast to slow (Pette, 2002). Muscle activity induced by stimulation may result in an autocrine release of trophic factors, thus avoiding the disuse effect following nerve or central lesions (Goldspink and Yang, 2001; McKoy et al., 1999). Muscle fibers are also capable of adjusting their phenotypic properties in response to muscle or nerve electrical stimulation (Marqueste et al., 2006; Pette and Vrbova, 1992), static stretching (Sakakima and Yoshida, 2003) and also to exercise (Booth and Thomason, 1991). To this respect, it was suggested that induced activity mimicking physiological motion would produce better preservation/recovery of muscle functional properties than conventional electrical stimulation (Marqueste et al., 2004, 2006). However, both forced exercise and electrical stimulation of the injured nerve had positive effects in promoting muscle reinnervation after nerve injury in rats (Asensio-Pinilla et al., 2009). Rather than the amount of electrical activity received, the effects seem to be mainly influenced by shortening or lengthening of the muscles (Gordon and Pattullo, 1993; Gordon et al., 2004). 3. Effects of exercise on peripheral nerve regeneration An overview of the literature shows conflicting evidence on beneficial and deleterious effects of exercise on peripheral nerve regeneration and muscle reinnervation. Variations in the type of nerve injury, the exercise applied, time and intensity of training appear to be the main factors explaining the controversial results reported. The amount and intensity of training play a key role in the positive effects of exercise after nerve injury. Hines (1942) reported beneficial results of daily running in a wheel only during the period of denervation, as trained rats showed a slightly but consistent increase in muscle mass and isometric muscle strength. Gutmann and Jakoubek (1963) found an early (3 days) increase in the axonal growth rate after bilateral sciatic nerve crush in rats forced to swim compared with that of sedentary controls. Nevertheless, long term hyperactivity induced by tenotomy (tendon section) of synergist muscles (Herbison et al., 1973), intensive swimming (Gutmann and Jakoubek, 1963; Herbison et al., 1974), or treadmill running (Herbison et al., 1980a,b) for 2–6 weeks after crush lesion of the sciatic nerve in rodents tended to show deleterious effects on muscle function recovery. On the other hand, mice running voluntarily after their sciatic nerves were cut and re-sutured, initially demonstrated a delayed reinnervation, whereas at later stages postlesion nerve maturation was improved (Badke et al., 1989). Voluntary running produced a decreased weight and force in extensor digitorum longus muscles but an increased force and weight in soleus muscles (Irintchev et al., 1990, 1991). These changes are typical of endurance training and could be interpreted as beneficial. Consistent with these observations, van Meeteren et al. (1997a) reported beneficial effects of exercise training, in the form of voluntary static standing on both hindpaws, on the recovery of motor and sensory functions during the early phase of reinnervation after sciatic nerve crush. After 10 weeks of standing exercise (van Meeteren et al., 1997a) or treadmill training (Marqueste et al., 2004) fast nerve fibers increased in proportion and improved their conduction velocity. In mice, continuous moderate or intermittent high-intensity training 349 promote axonal elongation after autograft repair (Sabatier et al., 2008). Besides, low but not high intensity treadmill training potentiates Schwann cell proliferation in the regenerating sciatic nerve in rats (Seo et al., 2009). It can be hypothesized that, when initiated in the denervation phase, moderate exercise results in accelerated functional sensorimotor recovery, because exercise might be beneficial for axonal outgrowth, nerve maturation, and recovery of muscle contractile properties, whereas forced intense exercise tends to have a detrimental effect, especially on muscle function, and overwork may even damage partially denervated muscles (Herbison et al., 1973; Irintchev et al., 1991; van Meeteren et al., 1997a). It is important to note that, in most of the studies on laboratory rodents, these were submitted to exercise from 3 to 5 days after nerve lesion, to avoid possible damage to the sutured nerve, and also due to the lack of clear evidence that exercise is beneficial at very early stages of regeneration. Little is known about the possible differential effects of passive, voluntary or forced exercise paradigms, differences that might affect the interpretation of results. Swimming and treadmill running increase physical activity, but may not merely represent physical overload, and, stress-related changes influence the results of training experiments (van Meeteren et al., 1997b). Some studies have shown that passive exercise of the denervated muscle before their reinnervation preserves the structure of the end-plates and enhance reinnervation (Pachter and Eberstein, 1989). In contrast, continuous passive motion of the hindlimb after tibial nerve section did not influence nerve regeneration, when this treatment was performed only during the first 14 days after injury (Kim et al., 1998). Recently, it has been demonstrated that daily brief manual stimulation (5 min/day for 2 months) of whisker pad muscles following section and repair of the pure motor facial nerve resulted in improved restoration of facial movements (whisking) and a reduction in motor endplate poly-innervation (Angelov et al., 2007; Guntinas-Lichius et al., 2007), indicating that activation of the intact sensory afferents could enhance motor regeneration. However, the same procedure did not influence the recovery, the degree of axonal sprouting or the extent of polyinnervation of motor endplates when applied to the mixed median nerve injured in the forelimb (Sinis et al., 2008). In a recent study, both passive and active exercise produced similar slight improvement in regeneration of the rat sciatic nerve, as indicated by increased recovery of compound muscle action potentials (CMAPs) a higher number of regenerated axons in the distal nerve, and a reduction in the increased excitability of spinal reflexes after nerve injury (Udina et al., 2011). Interestingly, there were differences in the recovery of flexor (gastrocnemius) and extensor (tibialis anterior) muscles depending on the type of exercise. Treadmill walking prevented hyperreflexia in both muscles, whereas, with passive cycling, it was only observed in the gastrocnemius muscle (Fig. 1). Cycling mainly acts by alternating lengthening and shortening of the triceps surae (Houle et al., 1999), whereas in active running, both extension and flexion of the ankle are alternated by the animal. The differential effects of exercise training on different muscle responses have been also reported in spinalized animals (Roy et al., 1998, 2005). On the other hand, cycling exercise is able to prevent muscle atrophy after spinal cord lesion but not the transition of soleus myofibers from slow to fast (Houle et al., 1999), whereas treadmill training prevents both atrophy and conversion of myofiber type (Roy et al., 1999). Regarding the mechanisms of action, Molteni et al. (2004) found that dorsal root ganglia (DRG) neurons exhibited increased neurite outgrowth when cultured from animals that had undergone 3 or 7 days of exercise compared with sedentary animals; neurite length in culture directly correlated with the distance that animals 350 E. Udina et al. / Annals of Anatomy 193 (2011) 347–353 Fig. 1. Plots of the recovery of compound muscle action potential (M wave) amplitude (top), and of the H/M ratio (ratio between the amplitude of the H reflex wave and the amplitude of the direct M wave after electrical nerve stimulation; bottom) of the tibialis anterior muscle after sciatic nerve transection and suture repair in the rat. One group received treadmill training at 5 m/min and another group received cycling exercise at 45 rpm, matching the number of step cycles in both activities. Note the increased levels of reinnervation in the late phase and the reduction of hyperreflexia during early phase of reinnervation (modified from Udina et al., 2011). ran. DRGs from exercised animals had higher levels of BDNF, NT3, and GAP43 mRNAs than DRGs from sedentary animals. Inhibition of Trk neurotrophin receptor activity before exercise attenuated the exercise-dependent increase in neurite growth, indicating that activation of neurotrophin signaling pathways during exercise plays a critical role in promoting plasticity and growth of neurons. Moreover, exercise significantly decreased the levels of myelinassociated glycoprotein (MAG), a potent axonal growth inhibitor, suggesting that downregulation of MAG is part of the mechanism through which exercise reduces growth inhibition (Ghiani et al., 2007). This effect was suppressed by blocking the action of BDNF during exercise, suggesting a potential role of BDNF in mediating the effects of exercise on axonal growth (Chytrova et al., 2008). 4. Effects of exercise on motoneurons and reflex motor responses Spinal motoneurons are sensitive to increased chronic activity and respond with phenotypic changes, which influence the manner in which these cells behave during voluntary recruitment (Gardiner et al., 2006). Motoneurons can adapt several functional relevant properties without losing the phenotype that designate them as fast or slow motoneurons. Some adaptations seem designed to have functional consequences during endurance exercise (increased capacity for axonal transport and for neurotransmitter release). Intense or mild endurance training in rats cause biophysical changes, such as hyperpolarized resting membrane potential and voltage threshold, and increase amplitude of afterhyperpolarization (Beaumont and Gardiner, 2003). This hyperpolarization may reduce the level of excitability for prolonged inputs. It is thus possible that exercise adapts motoneurons to a lower state of excitability. Although reflex responses evoked during voluntary contractions are potentiated following strength training, the corresponding H reflexes (i.e., the reflex response evoked by electrical stimulation of sensory afferents) and the F responses (late response induced by antidromic electrical stimulation of motor axons) at rest are not, suggesting that changes in intrinsic motoneuronal excitability are not involved in these responses (Sale et al., 1983). Changes in spinal reflexes persist after nerve injury and reinnervation, and may impair motor unit activation and control of movement, accounting in part for the poor clinical outcome achieved after severe nerve injuries (Valero-Cabré and Navarro, 2001). Forcing the activity of the injured neurons, by either passive or active exercise, decreases the hyperreflexia observed after peripheral nerve lesions (Fig. 1) (Asensio-Pinilla et al., 2009; Udina et al., 2011). This attenuation of hyperreflexia can be due to regulatory actions from the increased expression of neurotrophic factors in the spinal cord (Vaynman and Gomez-Pinilla, 2005) or to the beneficial effects of exercise on the muscles. Altered neuronal excitability of injured motoneurons is recovered after reinnervation (Foehring et al., 1986), suggesting that functional contact with muscle is required for the full expression of the normal neuronal electrical properties. Exercise, by maintaining the trophism and normal properties of the muscle can facilitate the expression of still unknown muscular signals to the innervating axons and attenuate the post-injury hyperreflexia. Even during the denervation period, exercise of the limbs stimulates muscle afferents of proximal innervated muscles, which may influence the axotomized motoneurons by spinal synaptic connections normally silent (Koerber et al., 2006). The effects observed with exercise training on the spinal H reflex are not likely caused by actions on motoneuronal excitability during the reinnervation phase. Probably, alterations in segmental inhibition, mainly involving modulation of Ia presynaptic inhibition, may serve as a mechanism responsible for chronic training-induced adaptations in the H reflex (Zehr, 2006). The effects of activity-dependent treatments on adaptive plasticity of spinal motor reflexes have been studied mostly after spinal cord injury, which usually leads to hyperreflexia and spasticity. Using an acute and chronic pattern of treadmill locomotor training, the enlarged H/M ratio (ratio between the amplitude of the H reflex wave and the amplitude of the direct M wave after electrical nerve stimulation) could be significantly suppressed in patients with spinal cord injury (Trimble et al., 2001). Passive bicycle exercise can also restore the frequency-dependent depression of spinal H reflex in a time-dependent manner following complete spinal transection in rats (Liu et al., 2010; Reese et al., 2006). Cycle training can likely be considered functionally equivalent to walking training in this context. Recent research on the effects of activity on functional recovery after neural injuries is focused on the importance of afferent information from the periphery as a source of control of posture or locomotor tasks, in conjunction with the spinal circuitry to which central pattern generation is routinely attributed (Edgerton et al., 2008). Expression of brain-derived neurotrophic factor (BDNF), neurotrophin-4 (NT4), their receptor tyrosine kinase B (trkB), and growth-associated protein-43 (GAP-43) are all increased in lumbar spinal cord and DRG neurons by voluntary exercise and decreased in muscle paralysis (Gomez-Pinilla et al., 2002; Skup et al., 2002). Then, it can be hypothesized that maintenance of denervated muscle activity increases the release of trophic factor that will benefit E. Udina et al. / Annals of Anatomy 193 (2011) 347–353 axonal regeneration and induce plastic changes in central synaptic connections. 5. Effects of exercise on collateral sprouting After a peripheral nerve injury, neighboring intact nerve fibers are able to extend collateral branches into regions previously occupied by nerve fibers that have undergone degeneration. This phenomenon allows reinnervation of denervated targets as an adaptive mechanism to functionally rescue muscle and sensory targets. Within partially denervated muscles, collateral sprouts emerge from the nodes of uninjured myelinated motor fibers above the motor nerve terminal to reinnervate nearby denervated muscles (Griffin and Thompson, 2008; Son and Thompson, 1995). The effects of neuromuscular activity on axonal sprouting and motor units enlargement have been controversial due to the conflicting findings of previous studies, which used different activity regimes and different muscles. Prolonged treadmill exercise was shown to have a preferential effect on promoting enlargement of fast-fatigable and fast-intermediate motor units in the partially denervated gastrocnemius (Einsiedel and Luff, 1994) and plantaris muscles (Gardiner et al., 1984). Since there was no evidence of muscle hypertrophy, the increased muscle force was attributed to changes in the extent of axonal sprouting. In contrast, other studies have reported that activity (wheeling exercise or functional overload) had no effects on motor unit enlargement by axonal sprouting in the plantaris muscle (Gardiner and Faltus, 1986; Michel and Gardiner, 1989). A thorough evaluation of active and passive neuromuscular activity on muscles subjected to different extent of partial denervation (Tam et al., 2002; Tam and Gordon, 2003) showed that daily protocols of high activity (8 h daily running exercise or functional electrical stimulation of the nerve) constrained axonal sprouting and consequent motor unit enlargement in extensively denervated muscles. The same detrimental effect was not evident in moderately denervated muscles, where motorunit size increased normally following partial denervation. They further demonstrated that the inhibitory effect of increased neuromuscular activity takes place at the early onset of axonal sprouting in extensively denervated muscles (Tam and Gordon, 2003). Following denervation, terminal Schwann cells elaborate a network of long processes that grow from the vacant end plate, forming “bridges” to nearby innervated end plates. Activity seems to alter at least two responses triggered by denervation in muscles: the ability of the processes of terminal Schwann cells to form bridges with innervated synaptic sites (Love et al., 2003; Tam and Gordon, 2003), and the growth of axons along these processes (Love et al., 2003). Therefore, increased activity during the early phase of denervation after nerve injury can be detrimental for axonal sprouting and collateral reinnervation of denervated muscles. On the other hand, neuromuscular inactivity also reduces the degree of motor axon sprouting (Tam et al., 2002). Thus, present evidence is that too much or lack of neuromuscular activity is counterproductive for effective collateral reinnervation in partially denervated muscles. Regarding collateral sprouting of sensory axons, unmyelinated and thin myelinated fibers show a strong capacity to sprout and enlarge their innervation territory (Diamond and Foerster, 1992; Nixon et al., 1984), whereas large myelinated fibers fail to produce functionally effective collateral reinnervation of adjacent denervated skin (Jackson and Diamond, 1984). Diamond and coworkers reported that collateral reinnervation of dorsal cutaneous “islands” of the rat skin was accelerated by repeated mechanical stimulation of the skin within the innervated regions, as well as by electrical stimulation of the uninjured nerves (Doucette and Diamond, 1987; Nixon et al., 1984). Activity-induced neurotrophic factor release 351 promotes neuronal growth and plasticity, and may partially antagonize the restrictions for axonal sprouting by changing the intrinsic growth state of neurons. Indeed, administration of NGF and other neurotrophins in vivo potentiates collateral sprouting in the denervated skin (Diamond et al., 1992). In sciatic nerve injury models, collateral reinnervation of denervated skin territories by nociceptors may be related to the underlying hyperalgesia (Casals-Diaz et al., 2009). Thus, inhibition or modulation of collateral sprouting may be of interest for antinociceptive effects. 6. Effects of exercise on neuropathic pain Peripheral nerve injuries often result in development of neuropathic pain symptoms, including spontaneous pain sensation, hyperalgesia and allodynia (painful perception caused by a normally non-painful stimulus). Neuropathic pain symptoms are also related to axonal regeneration mechanisms and functional recovery. After injury, mechanical allodynia affects the use of the injured paw and compromises successful rehabilitation (Vogelaar et al., 2004). However, only a few studies have investigated the effects of activity treatments on neuropathic pain after nerve injuries. Cobianchi et al. (2010) found that, after chronic constriction of the sciatic nerve in mice, early short-lasting treadmill running was able to reduce mechanical allodynia and to normalize the walking pattern of the injured hindpaw. Interestingly, the reduction of allodynia was maintained for 2 months, even when treadmill running was interrupted at day 7. Reduction of allodynia after early shortlasting exercise correlated with reduced microglia and astroglia reactivity in the dorsal horn, but astrocytosis was not prevented by long-lasting treadmill running. Other studies also suggest that microglia activation can be related to the hyperalgesia observed after peripheral nerve injuries, and astrocytes may be involved in its maintenance (Cao and Zhang, 2008). These findings suggest that the effectiveness of activity-dependent treatments on neuropathic lesions are related to specific time windows after nerve injury when increased activity may stimulate or inhibit regeneration of injured axons. In line with this view, acute brief electrical stimulation for 1 or 4 h after lesion to the injured nerve enhanced axonal regeneration and muscle reinnervation, whereas chronic daily electrical stimulation was detrimental for nerve regeneration (Asensio-Pinilla et al., 2009). Activity treatments can enhance the plasticity of intact axonal projections to compensate the loss of sensibility provoked by the disruption of neighboring axons. Since synaptic plasticity and sprouting of sensory fibers enhance pain perception during the denervation and reinnervation phases, different activity protocols could differently act on neuropathic pain compared to effects on recovery of motor function. The effects of treadmill running on pain symptoms may be attributable to its modulation on central and/or peripheral neurotrophin release like in nerve regeneration. Neurotrophins, such as BDNF, are up-regulated in several models of inflammatory and neuropathic pain (Ha et al., 2001; Pezet et al., 2002), and physical exercise is an important modulator of neurotrophin release in regenerating neurons (Molteni et al., 2004; Ying et al., 2005). There is evidence that BDNF may serve both pro- and antinociceptive roles in different contexts (Pezet and McMahon, 2006). After exercise, peripheral and/or central expression and delivery of BDNF depend on the site and model of injury (Vanelderen et al., 2010). BDNF redistribution between DRG neurons has been related to neuropathic pain; after nerve injury, BDNF is upregulated in smalland medium-sized neurons in rats with signs of neuropathic pain, whereas its expression was increased in large sensory neurons in rats without pain (Tender et al., 2010). The antinociceptive effects of central BDNF are thought to arise from its ability to increase the synthesis and release of serotonin in descending pathways, 352 E. Udina et al. / Annals of Anatomy 193 (2011) 347–353 or by rescuing GABAergic neurons and stimulating GABA release, both providing inhibitory modulation at the dorsal horn (Pezet et al., 2002; Vanelderen et al., 2010). Interestingly, treadmill exercise increases serotonergic immunoreactivity in medullary raphe nuclei and spinal cord of rats submitted to sciatic nerve section. The increased serotonergic nerve activity is parallel to the improvement in nociceptive responses and functional recovery. An alternative mechanism of action to explain the hypoalgesia induced by exercise could be the activation of endogenous opioids, specifically -endorphins (Goldfarb and Jamurtas, 1997; Koltyn, 2000). Otherwise, resistance exercise induced an antinociceptive effect mediated by -endorphin immediately after training, although it lasted only a few minutes (Galdino et al., 2010). Acknowledgments This research was supported by grant CP-FP-INFSO 224012 (TIME project) from the EU, grant SAF2009-12495 from the Ministerio de Ciencia e Innovacion of Spain, grant PI080598 and CIBERNED and TERCEL funds from the Fondo de Investigación Sanitaria of Spain, and FEDER funds. References Al-Majed, A.A., Neumann, C.M., Brushart, T.M., Gordon, T., 2000. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 20, 2602–2608. Angelov, D.N., Ceynowa, M., Guntinas-Lichius, O., Streppel, M., Grosheva, M., Kiryakova, S.I., Skouras, E., Maegele, M., Irintchev, A., Neiss, W.F., Sinis, N., Alvanou, A., Dunlop, S.A., 2007. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol. Dis. 26, 229–242. Asensio-Pinilla, E., Udina, E., Jaramillo, J., Navarro, X., 2009. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 219, 258–265. Badke, A., Irintchev, A.P., Wernig, A., 1989. Maturation of transmission in reinnervated mouse soleus muscle. Muscle Nerve 12, 580–586. Beaumont, E., Gardiner, P.F., 2003. Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle Nerve 27, 228–236. Booth, F.W., Thomason, D.B., 1991. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol. Rev. 71, 541–585. Boudriau, S., Cote, C.H., Vincent, M., Houle, P., Tremblay, R.R., Rogers, P.A., 1996. Remodeling of the cytoskeletal lattice in denervated skeletal muscle. Muscle Nerve 19, 1383–1390. Cao, H., Zhang, Y.Q., 2008. Spinal glial activation contributes to pathological pain states. Neurosci. Biobehav. Rev. 32, 972–983. Casals-Diaz, L., Vivo, M., Navarro, X., 2009. Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp. Neurol. 217, 84–95. Chytrova, G., Ying, Z., Gomez-Pinilla, F., 2008. Exercise normalizes levels of MAG and Nogo-A growth inhibitors after brain trauma. Eur. J. Neurosci. 27, 1–11. Cobianchi, S., Marinelli, S., Florenzano, F., Pavone, F., Luvisetto, S., 2010. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience 168, 273–287. Diamond, J., Foerster, A., 1992. Recovery of sensory function in skin deprived of its innervation by lesion of the peripheral nerve. Exp. Neurol. 115, 100–103. Diamond, J., Holmes, M., Coughlin, M., 1992. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J. Neurosci. 12, 1454–1466. Doucette, R., Diamond, J., 1987. Normal and precocious sprouting of heat nociceptors in the skin of adult rats. J. Comp. Neurol. 261, 592–603. Eberstein, A., Pachter, B.R., 1986. The effect of electrical stimulation on reinnervation of rat muscle: contractile properties and endplate morphometry. Brain Res. 384, 304–310. Edgerton, V.R., Courtine, G., Gerasimenko, Y.P., Lavrov, I., Ichiyama, R.M., Fong, A.J., Cai, L.L., Otoshi, C.K., Tillakaratne, N.J., Burdick, J.W., Roy, R.R., 2008. Training locomotor networks. Brain Res. Rev. 57, 241–254. Einsiedel, L.J., Luff, A.R., 1994. Activity and motor unit size in partially denervated rat medial gastrocnemius. J. Appl. Physiol. 76, 2663–2671. Foehring, R.C., Sypert, G.W., Munson, J.B., 1986. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J. Neurophysiol. 55, 947–965. Galdino, G.S., Duarte, I.D.G., Perez, A.C., 2010. Participation of endogenous opioids in the antinociception induced by resistance exercise in rats. Braz. J. Med. Biol. Res. 43, 906–909. Gardiner, P., Dai, Y., Heckman, C.J., 2006. Effects of exercise training on alphamotoneurons. J. Appl. Physiol. 101, 1228–1236. Gardiner, P.F., Faltus, R.E., 1986. Contractile responses of rat plantaris muscles following partial denervation, and the influence of daily exercise. Pflugers Arch. 406, 51–56. Gardiner, P.F., Michel, R., Iadeluca, G., 1984. Previous exercise training influences functional sprouting of rat hindlimb motoneurons in response to partial denervation. Neurosci. Lett. 45, 123–127. Ghiani, C.A., Ying, Z., de Vellis, J., Gomez-Pinilla, F., 2007. Exercise decreases myelinassociated glycoprotein expression in the spinal cord and positively modulates neuronal growth. Glia 55, 966–975. Goldfarb, A.H., Jamurtas, A.Z., 1997. Beta-endorphin response to exercise. An update. Sports Med. 24, 8–16. Goldspink, G., Yang, S.Y., 2001. Effects of activity on growth factor expression. Int. J. Sport Nutr. Exerc. Metab. 11 (Suppl.), 21–27. Gomez-Pinilla, F., Ying, Z., Roy, R.R., Molteni, R., Edgerton, V.R., 2002. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 88, 2187–2195. Gordon, T., Mao, J., 1994. Muscle atrophy and procedures for training after spinal cord injury. Phys. Ther. 74, 50–60. Gordon, T., Pattullo, M.C., 1993. Plasticity of muscle fiber and motor unit types. Exerc. Sport Sci. Rev. 21, 331–362. Gordon, T., Thomas, C.K., Munson, J.B., Stein, R.B., 2004. The resilience of the size principle in the organization of motor unit properties in normal and reinnervated adult skeletal muscles. Can. J. Physiol. Pharmacol. 82, 645–661. Griffin, J.W., Thompson, W.J., 2008. Biology and pathology of nonmyelinating Schwann cells. Glia 56, 1518–1531. Guntinas-Lichius, O., Hundeshagen, G., Paling, T., Streppel, M., Grosheva, M., Irintchev, A., Skouras, E., Alvanou, A., Angelova, S.K., Kuerten, S., Sinis, N., Dunlop, S.A., Angelov, D.N., 2007. Manual stimulation of facial muscles improves functional recovery after hypoglossal-facial anastomosis and interpositional nerve grafting of the facial nerve in adult rats. Neurobiol. Dis. 28, 101–112. Gutmann, E., Jakoubek, B., 1963. Effect of increased motor activity on regeneration of the peripheral nerve in your rats. Physiol. Bohemoslov. 12, 463–468. Ha, S.O., Kim, J.K., Hong, H.S., Kim, D.S., Cho, H.J., 2001. Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience 107, 301–309. Hennig, R., Lomo, T., 1987. Gradation of force output in normal fast and slow muscles of the rat. Acta Physiol. Scand. 130, 133–142. Herbison, G.J., Jaweed, M.M., Ditunno, J.F., 1974. Effect of swimming on reinnervation of rat skeletal muscle. J. Neurol. Neurosurg. Psychiatry 37, 1247–1251. Herbison, G.J., Jaweed, M.M., Ditunno, J.F., 1980a. Effect of activity and inactivity on reinnervating rat skeletal muscle contractility. Exp. Neurol. 70, 498–506. Herbison, G.J., Jaweed, M.M., Ditunno, J.F., 1980b. Histochemical fiber type alterations secondary to exercise training of reinnervating adult rat muscle. Arch. Phys. Med. Rehab. 61, 255–257. Herbison, G.J., Jaweed, M.M., Ditunno, J.F., Scott, C.M., 1973. Effect of overwork during reinnervation of rat muscle. Exp. Neurol. 41, 1–14. Hines, H.M., 1942. Effects of immobilization and activity on neuromuscular regeneration. JAMA 120, 515–517. Houle, J.D., Morris, K., Skinner, R.D., Garcia-Rill, E., Peterson, C.A., 1999. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve 22, 846–856. Hutchinson, K.J., Gómez-Pinilla, F., Crowe, M.J., Ying, Z., Basso, D.M., 2004. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 127, 1403–1414. Irintchev, A., Carmody, J., Wernig, A., 1991. Effects on recovery of soleus and extensor digitorum longus muscles of prolonged wheel running during a period of repeated nerve damage. Neuroscience 44, 515–519. Irintchev, A., Draguhn, A., Wernig, A., 1990. Reinnervation and recovery of mouse soleus muscle after long-term denervation. Neuroscience 39, 231–243. Irintchev, A., Wernig, A., 1987. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 249, 509–521. Jackson, P.C., Diamond, J., 1984. Temporal and spatial constraints on the collateral sprouting of low-threshold mechanosensory nerves in the skin of rats. J. Comp. Neurol. 226, 336–345. Kim, H.K., Kerr, R.G., Turley, C.B., Evans, P.J., Jay, V., Salter, R.B., 1998. The effects of postoperative continuous passive motion on peripheral nerve repair and regeneration. An experimental investigation in rabbits. J. Hand Surg. Br. 23, 594–597. Koerber, H.R., Mirnics, K., Lawson, J.J., 2006. Synaptic plasticity in the adult spinal dorsal horn: the appearance of new functional connections following peripheral nerve regeneration. Exp. Neurol. 200, 468–479. Koltyn, K.F., 2000. Analgesia following exercise: a review. Sports Med. 29, 85–98. Liu, H., Skinner, R.D., Arfaj, A., Yates, C., Reese, N.B., Williams, K., Garcia-Rill, E., 2010. L-Dopa effect on frequency-dependent depression of the H-reflex in adult rats with complete spinal cord transection. Brain Res. Bull. 83, 262–265. Love, F.M., Son, Y.J., Thompson, W.J., 2003. Activity alters muscle reinnervation and terminal sprouting by reducing the number of Schwann cell pathways that grow to link synaptic sites. J. Neurobiol. 54, 566–576. Lundborg, G., Richard, P., 2003. Bunge memorial lecture. Nerve injury and repair—a challenge to the plastic brain. J. Peripher. Nerv. Syst. 8, 209–226. Marqueste, T., Decherchi, P., Desplanches, D., Favier, R., Grelot, L., Jammes, Y., 2006. Chronic electrostimulation after nerve repair by self-anastomosis: effects on the size, the mechanical, histochemical and biochemical muscle properties. Acta Neuropathol. 111, 589–600. Marqueste, T., Alliez, J.R., Alluin, O., Jammes, Y., Decherchi, P., 2004. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J. Appl. Physiol. 96, 1988–1995. E. Udina et al. / Annals of Anatomy 193 (2011) 347–353 McKoy, G., Ashley, W., Mander, J., Yang, S.Y., Williams, N., Russell, B., Goldspink, G., 1999. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J. Physiol. 516 (Pt 2), 583–592. Michel, R.N., Gardiner, P.F., 1989. Influence of overload on recovery of rat plantaris from partial denervation. J. Appl. Physiol. 66, 732–740. Molteni, R., Zheng, J.Q., Ying, Z., Gomez-Pinilla, F., Twiss, J.L., 2004. Voluntary exercise increases axonal regeneration from sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 101, 8473–8478. Nam, T.S., Choi, Y., Yeon, D.S., Leem, J.W., Paik, K.S., 2001. Differential antinociceptive effect of transcutaneous electrical stimulation on pain behavior sensitive or insensitive to phentolamine in neuropathic rats. Neurosci. Lett. 301, 17–20. Navarro, X., Vivó, M., Valero-Cabré, A., 2007. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 82, 163–201. Nixon, B.J., Doucette, R., Jackson, P.C., Diamond, J., 1984. Impulse activity evokes precocious sprouting of nociceptive nerves into denervated skin. Somatosens. Res. 2, 97–126. Pachter, B.R., Eberstein, A., 1989. Passive exercise and reinnervation of the rat denervated extensor digitorum longus muscle after nerve crush. Am. J. Phys. Med. Rehab. 68, 179–182. Pette, D., Staron, R.S., 2001. Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 115, 359–372. Pette, D., Vrbova, G., 1992. Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev. Physiol. Biochem. Pharmacol. 120, 115–202. Pette, D., 2002. The adaptive potential of skeletal muscle fibers. Can. J. Appl. Physiol. 27, 423–448. Pezet, S., Malcangio, M., McMahon, S.B., 2002. BDNF: a neuromodulator in nociceptive pathways? Brain Res. Rev. 40, 240–249. Pezet, S., McMahon, S.B., 2006. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 29, 507–538. Reese, N.B., Skinner, R.D., Mitchell, D., Yates, C., Barnes, C.N., Kiser, T.S., Garcia-Rill, E., 2006. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord 44, 28–34. Roy, R.R., Talmadge, R.J., Hodgson, J.A., Oishi, Y., Baldwin, K.M., Edgerton, V.R., 1999. Differential response of fast hindlimb extensor and flexor muscles to exercise in adult spinalized cats. Muscle Nerve 22, 230–241. Roy, R.R., Talmadge, R.J., Hodgson, J.A., Zhong, H., Baldwin, K.M., Edgerton, V.R., 1998. Training effects on soleus of cats spinal cord transected (T12-13) as adults. Muscle Nerve 21, 63–71. Roy, R.R., Zhong, H., Monti, R.J., Vallance, K.A., Edgerton, V.R., 2002. Mechanical properties of the electrically silent adult rat soleus muscle. Muscle Nerve 26, 404–412. Roy, R.R., Zhong, H., Siengthai, B., Edgerton, V.R., 2005. Activity-dependent influences are greater for fibers in rat medial gastrocnemius than tibialis anterior muscle. Muscle Nerve 32, 473–482. Sabatier, M.J., Redmon, N., Schwartz, G., English, A.W., 2008. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 211, 489–493. Sakakima, H., Yoshida, Y., 2003. Effects of short duration static stretching on the denervated and reinnervated soleus muscle morphology in the rat. Arch. Phys. Med. Rehab. 84, 1339–1342. Sale, D.G., MacDougall, J.D., Upton, A.R., McComas, A.J., 1983. Effect of strength training upon motoneuron excitability in man. Med. Sci. Sports Exerc. 15, 57–62. Seburn, K.L., Gardiner, P.F., 1996. Properties of sprouted rat motor units: effects of period of enlargement and activity level. Muscle Nerve 19, 1100–1109. Seo, T.B., Oh, M.J., You, B.G., Kwon, K.B., Chang, I.A., Yoon, J.H., Lee, C.Y., Namgung, U., 2009. ERK1/2-mediated Schwann cell proliferation in the regenerating sciatic nerve by treadmill training. J. Neurotrauma 26, 1733–1744. Shieh, S.J., Chiu, H.Y., Hsu, H.Y., 1998. Long-term effects of sensory reeducation following digital replantation and revascularization. Microsurgery 18, 334–336. Sinis, N., Guntinas-Lichius, O., Irintchev, A., Skouras, E., Kuerten, S., Pavlov, S.P., Schaller, H.E., Dunlop, S.A., Angelov, D.N., 2008. Manual stimulation of forearm muscles does not improve recovery of motor function after injury to a mixed peripheral nerve. Exp. Brain Res. 185, 469–483. 353 Skup, M., Dwornik, A., Macias, M., Sulejczak, D., Wiater, M., Czarkowska-Bauch, J., 2002. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp. Neurol. 176, 289–307. Son, Y.J., Thompson, W.J., 1995. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron 14, 133–141. Soucy, M., Seburn, K., Gardiner, P., 1996. Is increased voluntary motor activity beneficial or detrimental during the period of motor nerve regeneration/reinnervation? Can. J. Appl. Physiol. 21, 218–224. Sun, R.Q., Wang, H.C., Wan, Y., Jing, Z., Luo, F., Han, J.S., Wang, Y., 2004. Suppression of neuropathic pain by peripheral electrical stimulation in rats: mu-opioid receptor and NMDA receptor implicated. Exp. Neurol. 187, 23–29. Tam, S.L., Archibald, V., Jassar, B., Tyreman, N., Gordon, T., 2001. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J. Neurosci. 21, 654–667. Tam, S.L., Archibald, V., Tyreman, N., Gordon, T., 2002. Tetrodotoxin prevents motor unit enlargement after partial denervation in rat hindlimb muscles. J. Physiol. 543, 655–663. Tam, S.L., Gordon, T., 2003. Neuromuscular activity impairs axonal sprouting in partially denervated muscles by inhibiting bridge formation of perisynaptic Schwann cells. J. Neurobiol. 57, 221–234. Tender, G.C., Li, Y.Y., Cui, J.G., 2010. Brain-derived neurotrophic factor redistribution in the dorsal root ganglia correlates with neuropathic pain inhibition after resiniferatoxin treatment. Spine J. 10, 715–720. Trimble, M.H., Behrman, A.L., Flynn, S.M., Thigpen, M.T., Thompson, F.J., 2001. Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 24, 74–80. Udina, E., Puigdemasa, A., Navarro, X., 2011. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve 43, 500–509. Valero-Cabré, A., Navarro, X., 2001. H reflex restitution and facilitation after different types of peripheral nerve injury and repair. Brain Res. 919, 302–312. Valero-Cabré, A., Navarro, X., 2002. Changes in crossed spinal reflexes after peripheral nerve injury and repair. J. Neurophysiol. 87, 1763–1771. van Meeteren, N.L., Brakkee, J.H., Hamers, F.P., Helders, P.J., Gispen, W.H., 1997a. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch. Phys. Med. Rehab. 78, 70–77. van Meeteren, N.L., Brakkee, J.H., Helders, P.J., Wiegant, V.M., Gispen, W.H., 1997b. Functional recovery from sciatic nerve crush lesion in the rat correlates with individual differences in responses to chronic intermittent stress. J. Neurosci. Res. 48, 524–532. Vanelderen, P., Rouwette, T., Kozicz, T., Roubos, E., Van Zundert, J., Heylen, R., Vissers, K., 2010. The role of brain-derived neurotrophic factor in different animal models of neuropathic pain. Eur. J. Pain 14, 1–9. Vaynman, S., Gomez-Pinilla, F., 2005. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehab. Neural Repair 19, 283–295. Vivo, M., Puigdemasa, A., Casals, L., Asensio, E., Udina, E., Navarro, X., 2008. Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp. Neurol. 211, 180–193. Vogelaar, C.F., Vrinten, D.H., Hoekman, M.F.M., Brakkee, J.H., Burbach, J.P., Hamers, F.P.T., 2004. Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome. Brain Res. 1027, 67–72. Wang, L., Copray, S., Brouwer, N., Meek, M.F., Kernell, D., 2002. Regional distribution of slow-twitch muscle fibers after reinnervation in adult rat hindlimb muscles. Muscle Nerve 25, 805–815. Wernig, A., Salvini, T.F., Irintchev, A., 1991. Axonal sprouting and changes in fibre types after running-induced muscle damage. J. Neurocytol 20, 903–913. Ying, Z., Roy, R.R., Edgerton, V.R., Gomez-Pinilla, F., 2005. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 193, 411–419. Zehr, E.P., 2006. Training-induced adaptive plasticity in human somatosensory reflex pathways. J. Appl. Physiol. 101, 1783–1794.