* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download INTRODUCTION - international journal of advances in
Radical (chemistry) wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Magnesium transporter wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Paracrine signalling wikipedia , lookup
Lipid signaling wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Proteolysis wikipedia , lookup
Biochemical cascade wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
Electron transport chain wikipedia , lookup
Reactive oxygen species wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Western blot wikipedia , lookup
Signal transduction wikipedia , lookup
Free-radical theory of aging wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Anthrax toxin wikipedia , lookup
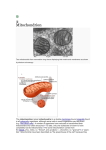
MITOCHONDRIA AS A DRUG SITE IN THE ISCHEMIC REPERFUSION INJURY IN HEART Krunal P. Pawar*1, Ashish V. Jaydeokar1, Dipak G. Gangurde1, Hitesh N. Thakar1, Unnati S. Patil2, Pranav Samuel3, Chitrakala U. Dhuri4 M.E.S’s H.K. College of pharmacy, Prateeksha Nagar, Oshiwara, Jogeshwari (west), Mumbai-400102 2 MGV’s S.P.H College of Pharmacy, Malegaon, Dist. Nashik. 3 Parshvanath Charitable Trust's V.M.H.P. Shah College of Pharmacy, Kasarvadavali, Ghodbunder Road, Thane. 4 S.V.B. College of Pharmacy, Kalyan Shil Road, Dombivli (E), Dist:Thane - 421 203. 1 *Address for Correspondence Krunal Pradeep Pawar H.K. College of Pharmacy, Oshiwara, Jogeshwari-west, Mumbai- 400 102, Maharashtra, India. Phone: +91-9869925806 Email: [email protected] ABSTRACT Mitochondrial medicine is a unique disciple in the nascent stage and is growing with the support of the technological advances and our knowledge of the role in the mitochondrial role in the ischemic reperfusion injury as well. Cardioprotection operates with activation of various signaling pathways. The MPTP is considered as the major mechanism of the cardiac myocyte death. The pharmacological strategy inhibiting mitochondrial outer membrane permeability and thus apoptosis, by manipulation of the Bcl-2 family proteins and Mitochondrial Permeability Transition Pore to protect the myocardium against ischemia-reperfusion is very recent. It has already provided interesting results, which support the idea that a clinical benefit might be obtained in the near future. However, with the current paucity of knowledge about the identity of the MPTP, preparing a candidate for its inhibition needs more research and thus the major area of research in future would be identifying the MPTP comprehensively. Keywords: Mitochondria, free radicals, ischemia,apoptosis. INTRODUCTION Cardioprotection in ischemia has been the subject for research since last decade, but till date the elucidated pathways still lack the understanding of the mechanism by which cells reduce cell death. Considerable research led down the results that mitochondria is a potential player in cell death. If mitochondrion is responsible for the apoptotic and necrotic cell death then opposing the mitochondrial death pathways will help in cardioprotection. The ischemic cell death is suggested due to the opening of a large pore in the inner mitochondrial membrane known as mitochondrial permeability transition pore. Inhibition of mitochondrial permeability transition pore and its components enhances cardioprotection which thus leads to a potential drug target [1]. MITOCHONDRIA AND CELL There are number of the mitochondria in the somatic cells. The mitochondria are called as the ‘power house' of the cells [2]. Organs with more physiological energy need they have more mitochondria such as muscles, brain, heart and liver. Detailed structure of mitochondria Mitochondria have dimensions of 0.5-1 µm in diameter and length of 7µm. The shape of the mitochondria depends on the location of the tissue in which it is located and the number of the mitochondria also depends on the energy requirement of the cell if the organs has the requirement of more aerobic respiration then the number of mitochondria will be more. The heart and kidneys which has more metabolic requirement has more number of mitochondria. Fig.1: structure of mitochondria: an overview In fig.1, the mitochondrion contains outer membrane and inner membrane which are made up of phospholipids bilayers and proteins. The two membranes have different biochemical and physiological properties. There is an outer membrane, intermembrane space and inner membrane, cristae space and matrix within the inner membrane space. Outer membrane The outer membrane is surrounds the entire organelle. The outer membrane is made up of protein and lipids in the ratio 1:1. It contains numerous integrated proteins known porins. The porins form channels that allow molecules 5000 Daltons or less in molecular weight to diffuse freely from one side of membrane to the other side. Large protein move across the membrane actively with the help of translocase enzyme [3]. Intermembrane space The intermembrane space is between the inner and outer membrane. The intermembrane space has ions and sugars same as that in cytosol but the protein constitution is different as that of cytosol. The protein in the intermembrane space is cytochrome c [3]. Inner membrane The inner membrane consists of 151 polypeptide and these peptides have high protein to phospholipids ratio. The inner membrane consists of cardiopilin. In addition to this there is membrane potential across the inner membrane formed by the action of the enzymes of the of the electron transport chain [3]. Cristae The inner mitochondria is compartmentalized in to structures called cristae which further increases the surface area for the mitochondria enhancing the ability to produce ATP. The inner membrane consists of F1 particles or oxysomes. Matrix As shown in fig.2, the matrix is enclosed in the inner membrane. It contains major part of proteins. The matrix is necessary for the production of ATP with the help of ATP synthase contained in the matrix. The matrix functions for the oxidation of pyruvate, fatty acids and Citric Acid Cycle. The mitochondria has its own genetic material and manufactures its own RNA and proteins Fig.2: structure of mitochondria: internal Energy production The main functions of mitochondria are to produce ATP and to regulate cellular metabolism. The core reaction involves Citric Acid Cycle or Krebs cycle. The production of ATP is done by oxidizing glucose, pyruvate and NADH which is done in cytosol. This process is called cellular respiration and aerobic respiration which requires oxygen. Pyruvate and citric acid cycle Pyruvate molecule which was produced by glycolysis is oxidized and combined with coenzyme A to form CO2, Acetyl-CoA and NADH. The Acetyl-CoA then enters the Citric Acid Cycle. Citric Acid Cycle oxidizes Acetyl-CoA to from CO2, three molecules of NADH and one FADH2. NADH and FADH2: The Electron Transport Chain Fig.3: The electron transport chain The redox energy from NADH and FADH2 is transferred to oxygen (O2) in several steps via the electron transport chain. This electron transport chain is shown in fig.3. These energy-rich molecules are produced within the matrix via the Citric Acid Cycle but are also produced in the cytoplasm by glycolysis. Reducing equivalents from the cytoplasm can be imported via the malate-aspartate shuttle system of antiporter proteins or feed into the electron transport chain using a glycerol phosphate shuttle. Protein complexes in the inner membrane (NADH dehydrogenase, cytochrome c reductase, and cytochrome c oxidase) perform the transfer and the incremental release of energy is used to pump protons (H+) into the intermembrane space. This process is efficient, but a small percentage of electrons may prematurely reduce oxygen, forming reactive oxygen species such as superoxide. As the proton concentration increases in the intermembrane space, a strong electrochemical gradient is established across the inner membrane. The protons can return to the matrix through the ATP synthase complex, and their potential energy is used to synthesize ATP from ADP and inorganic phosphate (Pi) [4]. Role of mitochondria in ischemic heart injury Mitochondria play a very important role in energy generation within the cell. Recent literature states that mitochondria have roles in cardioprotection and apoptosis. Fig.4: mitochondrial death pathway and apoptosis Fig.4 simplifies the mitochondrial death pathway and apoptosis. The pathway is triggered by various “death signals”, such as ROS, DNA damage, that promote binding of the proapoptotic protein Bax with the outer mitochondrial membrane, most likely at the contact sites between the two membranes, and its association with the PTP. This enables the release of cytochrome c (•) and the apoptosis-inducing factor (AIF) (■) from the intermembrane compartment to the cytosol. An increased intramitochondrial Ca2+ level and ROS production facilitate this process by promoting PTP opening. Once in the cytosol, cytochrome c and AIF, in cooperation with a cytosolic factor, Apaf-1 (not indicated), activate caspase-9 and subsequently other members of the caspase family, thus initiating self-digestion of the cell and nuclear DNA fragmentation, eventually leading to apoptotic cell death. Association of Bax with mitochondria is prevented by the antiapoptotic protein Bcl-2. ROS can be decomposed by Mn-containing (mitochondrial) and Cu, Zn-containing (cytosolic) superoxide dismutases (SOD), catalase, and glutathione peroxidase (GPx). Stimulation of ROS production is exemplified here by UV and ionizing radiation and by two anticancer drugs, Adriamycin and BMD188. Activation is indicated as [+] and inhibition as [-] [5] [6]. HYPOXIA AND ISCHEMIA Biology of hypoxia Hypoxia occurs from conditions like ischemia and cardiovascular complications leading to cell death. Hypoxia has shown to lead increase intracellular free calcium concentration, [(Ca2+)i], 5-lipooxygenase, lipid peroxidation, cyclooxygenase constitutive nitric oxide synthase (cNOS), leukotriene B4 (LTB4), prostaglandin E2 (PGE2), interleukins, tumor necrosis factor-α (TNF-α), caspases, complement activation, kruppel-like factor 6 (KLF6), inducible nitric oxide synthase (iNOS), heat shock protein 70 kDa (HSP-70), and hypoxia-inducible factor-1α [(HIF-1α). The sequence of their occurrence provides understanding of the mechanism in hypoxia induced injury. Calcium Effect Intracellular calcium has considered to the most prominent secondary messenger. The external signals are transmitted to the cell with the help of intracellular free Ca2+. The resting [Ca2+]i is regulated at three different Levels: 1) At cell membrane; 2) At the intracellular Ca2+ pools in the cytoplasm; and 3) By Ca2+ -binding proteins in the cytoplasm and the nucleus. First, there are Na+/Ca2+ exchanger, Ca2+/H+ antiporter, Ca2+-ATPase pumps, and Ca2+ channels present at the cell membrane. Normally, activation of the Na+/Ca2+ exchanger stimulates the import of three molecules of Na+ and the export of two molecules of Ca2+. However, this ions exchanger is concentration driven, so the Na+ and Ca2+ concentration Gradients between the cytoplasm and the extracellular space can reverse the direction of Na+/Ca2+ exchange. The Ca2+/H+ antiporter takes in two molecules of H+ and expels one molecule of Ca2+. This antiporter is pH-sensitive. Ca2+ ATPase pumps are energy driven and remove Ca2+ from the cell. Voltage-gated Ca2+ channels are activated by changes in membrane potential. There are L-type, N-type, and T-type messenger- operated Ca2+ channels are activated by inositol 1,4,5-trisphosphate (IP3) or cAMP-stimulated protein kinase (PKA); receptor-operated Ca2+ channels are activated by ligand-binding to receptors on the membrane. Secondly there are intracellular Ca2+ pools in the cytoplasm. The increase in [Ca2+]i induce Ca2+ mobilization from intracellular Ca2+ pools to further elevate [Ca2+]i which could be simplified as this Ca2+ pools inducible. However, Ca2+ sequestration into the intracellular Ca2+ pools to lower [Ca2+]i also takes place. Third, Ca2+-binding proteins are present in the cytoplasm and nucleus. The glutamic acid- and phenylalanine-rich proteins, like calmodulin and S100 proteins are considered to exert Ca2+-dependent actions in the nucleus or the cytoplasm. Hypoxia has been shown to perturb calcium homeostasis. It is believed that a sudden increase in [Ca2+]i is associated with cell death and the death is mediated by activation of Ca2+-dependent proteases [7]. Bcl-2 and p-53 Hypoxia alters expression of Bcl-2 and p-53. Expression of Bcl-2 is accompanied by tumor progression protein p-53. p-53 inhibits cell growth and promotes programmed cell death [7]. Caspases Caspases are the front line executioner of apoptosis. Caspases are divided into functional subgroups that are activated during the apoptosis and those which are implicate the processing of the proinflammatory immune response. Caspases are in the inactive forms which are known as procaspase which then turns into caspase after proteolytic cleavage of the large and small units of the active enzyme [7]. fig.5: molecular abnormalities in a hypoxic event Fig.5 describes the molecular abnormalities in a hypoxic event. Hypoxia increases KLF6 and NF-κB and decrease KLF-4 which results in an increased iNOS. This increases the NO generation which then reacts with O2 to form ONOO- which in turns swells up the mitochondria and release the cytochrome C and the caspase 9 to form apoptosome. This apoptosome in turn activates caspase-3 leading to the apoptosis. KLF4: Kruppel-like factor 4; KLF6: Kruppel-like factor 6; NF-κB: nuclear factor-kappa B; iNOS: inducible nitric oxide; cyto-c: cytochrome c; casp-9: caspase-9; ↑: increase; ↓: decrease [7]. Adenosine Triphosphate (ATP) ATP is considered as the energy cash of the cell. According to fig.6, the hypoxia increases inflammatory mediators like LTB4 and decreases the enzyme activity and the protein expression of pyruvate dehydrogenase (PDH) an enzyme that is responsible for the oxidative decarboxylation of pyruvate molecule and formation of the Acetyl-CoA which then moves in the Citric Acid Cycle. Thus the decrease enzyme activity of pyruvate dehydrogenase leads to less generation of Acetyl-CoA molecule and low levels of the ATP. Apoptosis requires energy if the levels of cellular ATP remains at levels high than 15% of baseline, then apoptosis ensues. If the cellular ATP levels fall below 15% baseline, then necrosis ensues [7]. Fig.6 effect of hypoxia on mitochondria Free Radicals Free radicals are molecules with unpaired electrons. These free radicals are highly reactive which reacts with the biomolecules and causing oxidative damage to these molecules. The hypoxia induced ATP depletion promotes the formation of free radicals. These free radicals then react with the cellular targets which then change the major configuration of the critical molecules. The free radicals which consist of reactive oxygen and nitrogen species cause mitochondrial disruption so that the cytochrome c, AIF or EndoG translocates to the cytoplasm. The cytochrome c then binds to Apaf-1 and caspase -9 to form apoptosome that activates other caspase to initiate apoptosis. Understanding the biology of hypoxia will certainly help to understand the mechanism of ischemic injury and targeting the site for cardioprotection [7]. MYOCARDIAL ISCHEMIA AND INJURY Introduction to ischemia and reperfusion Myocardial ischemia is a clinical condition that is observed when there is reduced coronary blood flow which then results in hypoxia and accumulation of the toxic cellular metabolites. The accumulation of the lactic acid leads to the decrease in the intracellular pH and increase in intracellular Na+ ([Na+]i) and Ca2+ ([Ca2+]i). The ionic and metabolic disturbance leads to reduction of myocardial function. If the disturbance is resolved quickly then the reestablishment and recovery occurs but if reperfusion is followed itself by irreversible damage. Depressed function and energy production during ischemia The onset of ischemia there is a decline in developed pressure and increase in the enddiastolic pressure that is linked with regional and global akinesia. If the coronary flow is not restored than there is increase in the pressure, full contracture develops and tissue necrosis occurs. Ischemia leads to decrease in the energy producing mechanism since the availability of oxygen is limited which in turns decreases the oxidative phosphorylation. Intracellular creatine phosphate is rapidly decreased and intracellular phosphate is increased. The glycolysis and lactic acid production are stimulated; the increase lactic acid production leads to decrease of the intracellular pH decreases further inhibition of glycolysis and reduction of ATP. The main reason for the ischemic injury is increase in the intracellular phosphate and decreased intracellular pH [8]. Consequences of reperfusion Consequences of the reperfusion injury include arrhythmias and loss of the intracellular proteins. Reperfusion following prolong ischemia leads to the death of the cardiac myocytes by necrosis or apoptosis. The mechanism of the necrosis and apoptosis in the cardiac myocytes will be discussed in the proceding sections. Disruption of ionic homeostasis during ischemic injury Ischemia leads to decrease in the intracellular pH and decrease in ATP levels as well. The increase in the acidic environment in the cell leads to activation of Na+/H+ exchanger as the cell tries to restore the intracellular pH. The decrease in ATP levels and increase intracellular pH leads inhibition of the Na2+/K+ ATPase so that the Na+ that enters the cell cannot be pumped out through Na+/H+ exchanger and there is increases in the levels of the [Na+]i. Prolong ischemia leads to the gradual increases in the intracellular Ca2+ due to the inhibition of the Na+/Ca2+ exchanger, which normally pumps the Ca2+ out is stopped due to increased [Na+]i. Elevated intracellular Ca2+ leads to the activation of the degradation enzymes such as phospholipases, proteases and nucleases which ultimately damages the tissue irreversibly. The irreversible tissue damage proceeds by blebbing of plasma membrane and also by loss of ionic disturbances, swelling, de-energizing mitochondria and release of intracellular enzymes which in turn exhibits loss of the membrane integrity [8]. Effect of free radical on cellular components a. Cell death Two types of the cell death are necrosis and apoptosis [9]. Necrosis is associated with inflammatory cell infiltration and subsequent collagen deposition and scar formation whereas the apoptosis is accompanied by ultrastructural changes and biochemical features, cytoplasmic and nuclear condensation. b. Apoptosis Apoptosis accounts for a great proportion of cell death associated with myocardial ischemia/reperfusion injury. Apoptosis contributes to the impairment of cardiac performance, and also plays an important role in the myocardial and vascular remodeling processes. Radical oxygen species induced cardiac apoptosis which is mediated through signaling systems, like intracellular Ca2 +, cytokines, lipid oxidation, and proto-oncogene activation. The perturbation of intracellular Ca2 + homeostasis by the cellular redox state can aggravate free radical reactions and can activate endonucleases via activation of caspases-key enzymes in the apoptotic pathway. Additionally, intrinsic degree of oxidant production regulates cellular susceptibility to apoptosis through both p53-dependent and p53-independent pathways. Effect of free radicals on lipids and lipid metabolism Free lipids and membrane bound lipids are targets for ROS. ROS attacks on lipids leads to lipid peroxidation thus leading to decreased membrane fluidity, increased membrane permeability, inducing immune response and destabilized membrane receptors. Effect of free radicals on long chain fatty free acids Elevated plasma levels of free fatty acids (FFA) are culprit in myocardial ischemia. Under normal physiological conditions of the heart, FFA is the first line source of energy generated via β-oxidation within the mitochondrial matrix. FFA requires 60-70% of the oxygen for energy production. However, β-oxidation cannot proceed under oxygen- deprived conditions, such as in ischemia, where FFA and their metabolites become toxic. When FFA metabolism is inhibited, toxic metabolic intermediates are accumulated and incorporated into cell membranes, sarcolemma and mitochondrial membrane. High levels of FFA and their metabolites impair Ca2 + homeostasis and ion gradients, which may lead to cardiac arrhythmias during ischemia reperfusion [10]. Membrane lipids Free radicals also react with membrane bound lipids leading to lipid peroxidation. These reactive species react with polyunsaturated lipid membrane generating products which lead to inhibition of protein synthesis and alter enzyme activities. Oxidation of polyunsaturated fatty acids can cause membrane disintegration, mitochondrial dysfunction and Ca2+ overload. Free radicals may act as signal transduction molecules. Cellular H2O2 was transiently increased upon activation of platelet derived growth factor [11] . Numerous signaling pathways are affected by the platelet-derived growth factorinduced increase in H2O2 concentration. H2O2 activates apoptotic and hypertrophic signaling pathways in cardiac myocytes [12] Ca2+ dysregulation and mitochondrial Ca2+ transport In normal cardiac function, mitochondrial Ca2+ transport pathways are thought to play a significant role in the coordination of ATP supply and demand [17]. The relationship of the [Ca2+]i and energy demands is illustrated in fig.6 [6] Increased energy demand and physical and metabolic activity of cardiac myocytes Increased mitochondrial Ca2+ Increased cytosolic Ca2+ Increased NADH Increased energy supply fig.6: The relationship of the [Ca2+]i and energy demand Mitochondrial Ca2+changes in ischemia The mitochondria have a capacity to take large quantities of Ca2+. However excessive accumulation of Ca2+ in mitochondria leads to damage of the mitochondria by competing for ATP production and inducing the Mitochondrial Permeability Transition Pore [6] [8]. MITOCHONDRIAL TRANSITION PERMEABILITY PORE Introduction to Mitochondrial Transition Permeability Pore Mitochondrial dysfunction is the underlying cause of ischemia-reperfusion injury. Dramatic events occur in the mitochondria which lead to a chain of events which leads apoptotic and necrotic cardiomyocyte death. The mPTP is a large conductance pore formed apparently through a conformational change of several constituent proteins of mitochondrial membrane. The pore opens most clearly under specific and usually pathological conditions, and spans the inner and outer mitochondrial membranes [18]. This section will elaborate on the on the role of the MPT pore in ischemia reperfusion injury, the mechanisms involved, and regarding the pore's molecular composition. Mitochondrial death pathways There is molecular machinery in the mitochondria which leads to the cardiac myocytes death. The mitochondrial death mechanism is the so-called “intrinsic” pathway that mediates apoptosis. Toxic stimuli such as oxidative stress induce translocation and integration of the pro-death members of the Bcl-2 family. These proteins, by a mechanism permeabilize the outer membrane to an extent that allows the release of proapoptotic proteins from the inter-membrane space, cytochrome c, Smac/DIABLO, htrA2/Omi protease, and endonuclease-G (endoG). Cytochrome c binds to the cytosolic protein apaf1 and the resultant “apoptosome” activates the caspase-9 and -3 protease system. Smac/DIABLO and htrA2/Omi activate caspases by either sequestering or degrading caspase-inhibitory proteins. EndoG translocates to the nucleus and mediates DNA fragmentation. The coordinated action of these proteins subsequently results in the death of the cardiomyocyte by apoptosis. Mitochondrial rupture can lead to the release of pro-apoptotic inter-membrane proteins described above, which would initiate the apoptosis. However, if the stress is severe or prolonged, ATP which is required for apoptosis to occur will be depleted and the cell will instead die by necrosis. Indeed, the relative contribution of MPT to apoptosis versus necrosis is still the subject of debate [19]. Mitochondria in ischemia-reperfusion injury The following description is focused on the main factors that contribute to the induction of MPT and cardiomyocyte death. a. Adenine nucleotides The ischemia can be explained as the lack of oxygen supply to the affected area of the myocardium. As the electrons generated by the electron transfer chain in the mitochondria cannot be transferred to molecular oxygen there is a cessation of oxidative phosphorylation and inhibition of mitochondrial ATP synthesis. Also the inhibition of electron transfer prevents the pumping of H+ across the inner membrane, which is required to generate the Δψm. In an effort to maintain the Δψm the mitochondrion runs the F1F0 ATP synthase in reverse thereby hydrolyzing the remaining ATP. [14] b. Mitochondrial Ca2+ After inhibition of the of oxidative phosphorylation in ischemic condition, the cardiac myocytes has to rely on anaerobic glycolysis which leads to generation of the lactate which leads to acidification of the cytosol, this leads ionic disturbances leading to slow increase of cytosolic Ca2+ that is then accelerated upon reperfusion. Application of a contractile stimulus to smooth muscle produces an elevation in the free cytoplasmic Ca2+ concentration ([Ca2+]i), which subsequently leads to activation of the contractile machinery. Depending upon the nature of the contractile stimulus, the increase in [Ca2+]i may reflect influx of Ca2+ from the extracellular medium, and/or release of Ca2+ from the sarcoplasmic reticulum (SR) (17). c. Mitochondrial ROS It is paradoxical that the restoration of the oxygen supply to the ischemic region by reperfusion is most likely the biggest cause of myocyte death [20]. The reperfusion is associated with outburst of mitochondrial derived free oxygen radicals. These radicals will damage the mitochondrial proteins which includes electron transfer chain and lipid peroxidation. The free oxygen radicals are the inducer for the MPTP. d. Apoptotic Bcl-2 proteins Ischemia reperfusion induces cardiac myocyte death. The activation of Bcl-2 proteins such as Bax, Bid, Puma and BNIP3, translocation of these proteins in the mitochondrial membranes leads to death of cardiomyocyte. The activation of pro-death Bcl-2 proteins such as Bax, Bid, Puma, and BNIP3, and the translocation and integration of these proteins into mitochondrial membranes has been causing ischemic damaged to myocytes. The MPT pore The MPT phenomenon is mediated by the MPT pore which is a non-specific channel and is thought to span the inner mitochondrial membrane. The pore itself is permeable to solutes up to 1.5 kDa. This pore helps in the equilibration of H+. The mitochondrial pore is sensitive to redox, Ca2+, voltage, adenine nucleotide, Pi, and pH sensitive [21][22][23]. The pore consist the voltage-dependent anion channel (VDAC) [24] in the outer membrane, the adenine nucleotide translocase (ANT) in the inner membrane, plus cyclophilin-D (CypD) [25] in the matrix. The increased Ca2+ concentration and the presence of the free radical species induce pore opening and the adenine nucleotides inhibit the pore opening. PHARMACOLOGICAL STRATEGIES TO PREVENT ISCHEMIC REPERFUSION INJURY The importance of limiting myocardial ischemia–reperfusion injury in clinical practice has been appreciated for a very long time. Unfortunately, till date no therapeutic approach has been demonstrated clinically effective in protecting heart muscle at risk of ischemic and reperfusion injury. Following strategies can be hypothesized in order to prevent the ischemic and reperfusion [20]. a. Proteins of the mitochondrial outer membrane Targeting mitochondrial permeability transition pore Triggering mitochondrial permeability transition pore induces the release of cell death effectors and the loss of mitochondrial functions which are necessary for cell survival [26]. Thus, drugs should be design in a fashion which will be able to block or to limit mitochondrial membrane permeabilization during ischemia-reperfusion and can be a possible cytoprotective. Another approach could be determining the genetic makeup of the mitochondrial permeability transition pore and targeting the drug [27]. The MAC/Bak channel Mitochondrial membrane permeabilization is under the control of the Bcl-2 family of proteins. Proapoptotic members (e.g. Bax, Bak or the BH3-only subfamily proteins Bid, Bad, Bnip3) facilitate membrane permeabilization and promote the release of cytochrome c and other intermembrane space components. Cytochrome c triggers formation of the apoptosome complex and activation of caspase-9. In addition to this, there is another pathway which can be activated by two other proteins, apoptosis inducing factor (AIF) and endonuclease G, which are also released after outer membrane permeabilization, can induce apoptosis in a caspase-independent manner. The anti-apoptotic members Bcl-2 and Bcl-xL are mainly localized to the mitochondrial outer membrane where they antagonize the pro-apoptotic effect of Bax and Bak. Designing the anti-apoptotic intervention targeting the Bcl protein may lead to successful lead. BH3-only Bcl-2 proteins The activation of Bak and Bax is regulated by Bcl-2 proteins that consist of BH-3 domains [28]. These proteins regulate the signals of the apoptosis of the mitochondria and hence can be the perfect intervention as the drug target which would prevent ischemicreperfusion injury. Another potential target from outer mitochondrial membrane permeabilization might involve VDAC which is the major permeability pathway for metabolites through the mitochondrial outer membrane. Different isoforms of VDAC have been identified as mentioned in the above section. The role of VDAC in cell death induced by mitochondria seems to be isoform specific. VDAC1 has been implicated in the mitochondrial release of proapoptotic protein whereas VDAC2 displays antiapoptotic properties [29] [30], however VDAC is the hypothetical component of the MPTP therefore intense research is to be done. b. Proteins of the mitochondrial inner membrane The inner membrane of the mitochondria consists of the proteins which regulate the electron transport chain (ETC) and oxidative phosphorylation. The inner membrane has high ψm, the lipid cardiopilin and is compartmentalized with the cristae. The ETC is the important source of the mitochondrial free radical generation that occurs due to leakage of the molecular oxygen. The free radicals include superoxide anion (O-2) and hydrogen peroxide which are continuously generated, as the byproducts of the normal aerobic metabolism. Normally the byproducts are detoxified by the help of manganese superoxide peroxidase (MnSOD) and mitochondrial GSH-dependent peroxidase which ensures that free radical levels are normal and do not reach the toxic levels. However, the rise in the free radicals production beyond the cellular defenses then the condition is termed as ‘oxidative stress’ which can cause cell damage and lead to cell death. The respiratory complex Ι, known as the NADH dehydrogenase generates significant levels of free radicals. Hence ETC can be a target in preventing the myocyte death. The complex ΙΙΙ (bc1) regulates the MPTP and also controls the mitochondrial calcium influx. The other targets could be the UCP (uncoupling protein) which regulates the protons gradient and also the optic atrophy protein (OPA1) has been recently identified as the regulator of the cytochrome c release and thus can be the potential target in the ischemic reperfusion injury. In the recent work it has been found that the OPA1 is regulated by the MPTP. Thus, the membrane dynamics of the inner mitochondria are involved in apoptotic regulation and can be the potential targets for therapeutic intervention [31] [32]. Other possible Interventions to prevent the ischemic reperfusion injury: Conjugation of the antioxidants with lipophilic compounds and delivering it in the cardiac myocytes to prevent the ischemic reperfusion injury and efficient methods to regulate drug delivery to the cardiac myocytes. Extensive research on the PKCε [33] which focuses and modulates the functionality of MPTP. Designing the agents in order to lower the pH in the cardiac myocyte which helps in inhibiting the opening the MPTP. Inhibiting the xanthine oxidase with the help of designing a candidate with specificity to the enzyme in the cardiac myocytes that leads to production of the free radicals which in turn opens the MPTP further initiating the apoptotic events in the myocyte. Use of proteomic approach to examine the MPTP structure and try to isolate the ‘event’ of the MPTP opening and isolating the proteins in the event. Designing the specific Ca2+ inhibitors in the myocytes which are responsible for the opening of the MPTP. REFERENCES 1. Szewczyk A, Wojtczak L, “Mitochondria as a Pharmacological Target” The American Society for Pharmacology and Experimental Therapeutics, 2002; 54(1): 101-27. 2. Armstrong J, “Mitochondrial Medicine: Pharmacological targeting of the mitochondria in disease”, British Journal of Pharmacology, 2007; 151(8): 1154-65. 3. Suleiman M, Halestrap A, Griffiths E, “Mitochondria: a target for the myocardial protection” Pharmacology & Therapeutics, 2001; 89(1): 29-46. 4. Baines C, “The mitochondrial permeability transition pore and ischemia reperfusion injury” Basic Res. Cardiol., 2009; 104(2): 181-8. 5. Zorov D, Juhaszova M, Yavin Y, Bradley Nuss H, Wang S, Steven J, “Regulation and pharmacology of the mitochondrial permeability transition pore” European Society of Cardiology, 2009; 83(2): 213-25. 6. Morin D, Assaly R, Paradis S, Berdeaux A, “Inhibition of mitochondrial membrane permeability as a putative pharmacological target for cardioprotection” Curr. Med. Chem., 2009; 16(33): 4382-98. 7. Wattanapitayakul S, Bauer J, “Oxidative pathways in cardiovascular disease Roles, mechanisms and therapeutic implications” Pharmacology & Therapeutics, 2001; 89(2): 187-206 8. Juliann G, Kiang K, Tsen T, “Biology of Hypoxia” Chinese Journal of Physiology, 2006; 49(5): 223-33. 9. Moncada S, “Mitochondrial as pharmacological targets” British Journal of Pharmacology, 2010; 160(2): 217-219. 10. Elizabeth M, Steenbergen C, “What makes the mitochondria a killer? Can we condition them to be less destructive?” Biochimica et Biophysica Acta, 2011; 1813(7): 1302-1308. 11. Di Lisa F, Canton M, Menabò R, Kaludercic N, Bernardi P, “Mitochondria and cardioprotection” Heart Fail Rev., 2007; 12(3-4): 249-260. 12. Gustafsson A, Gottlieb R, “Heart mitochondria: gates of life and death” Cardiovasc. Res., 2008; 77(2): 334-43. 13. Halestrap A, Clarke S, Javadov S, “Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection” Cardiovasc. Res., 2004; 61(3): 372-85. 14. Kroemer M, “The mitochondrial permeability transition pore complex as a pharmacological target” Curr Med Chem., 2003; 10(16): 1469-1472. 15. Wang P, Heitman J, “The cyclophilins” Genome Biol., 2005; 6(7): 226. 16. Blachly-Dyson E, Forte M, “VDAC channels” IUBMB Life., 2001; 52(3-5): 113118. 17. Shimizu S, Narita M, Tsujimoto Y, “Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC” Nature, 1999; 399(6735): 483-7 18. Cheng E, Sheiko T, Fisher J, Craigen W, Korsmeyer S, “VDAC2 inhibits BAK activation and mitochondrial apoptosis” Science, 2003; 301(5632): 513-7 19. Maitrayee S, Zu-Xi Y, Ferrans V, Irani K, Finkel T, “Requirement for Generation of H2O2 for Platelet-Derived Growth Factor Signal Transduction” Science., 1995; 270(5234): 296-9. 20. Hunter J, Chien K, “Signaling Pathways for Cardiac Hypertrophy and Failure” N.Engl. J. Med., 1999; 341(17): 1276-83. 21. Drummond R, Richard A, Tuft R, “Mitochondrial Ca2+ homeostasis during Ca2+ influx and Ca2+ release in gastric myocytes from Bufo marinus” J. Physiol., 2000; Feb; 522(3): 375–390. 22. Rouslin W, Ranganathan S, “Impaired function of mitochondrial electron transfer complex I in canine myocardial ischemia: Loss of flavin mononucleotide” Journal of Molecular and Cellular Cardiology, 1983; 15(8): 537-542. 23. Gohil V, Hayes P, Matsuyama S, Schagger H, Schlame M, Miriam L, Greenberg M, “Cardiolipin Biosynthesis and Mitochondrial Respiratory Chain Function are Interdependent” The journal of biochemistry, 2004; 279( 41): 42612–42618. 24. Veitch J, McColl S, “A critical examination of perceptual and cognitive effects attributed to full-spectrum fluorescent lighting” Ergonomics, 2001; 44(3): 255-279. 25. Duchen M, “Mitochondria in health and disease:perspectives on a new mitochondrial biology” Molecular Aspects of Medicine, 2004; 25: 365–451. 26. Mohar Z, “The mitochondrial permeability transition pore components” The Plymouth Student Scientist, 2010; 3(1), 245-254. 27. Darley-Usmar V, “The Powerhouse Takes Control of the Cell: the Role of Mitochondria in Signal Transduction” Free Radical Biology & Medicine, 2004; 37, (6): 753 – 754. 28. Frey T, Renken C, Perkins G, “Insight into mitochondrial structure and function by electron tomography” Biochemica et Biophysica Acta, 2002; 1555: 196– 203. 29. Chen Y, Ward E, Kong J, Israels S, Gibson S, “Mitochondrial electron-transportchain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species” Journal of Cell Science, 2007; 120: 4155-4166. 30. Golstein P, Kroemer G, “Cell death by necrosis: towards a molecular definition” Trends in Biochemical Sciences, 2006; 32 (1):1-7. 31. Scislowski V, Bauchart D, Gruffat D, Laplaud P, Durand D, "Effect of dietary n-6 and n-3 polyunsaturated fatty acids on peroxidizability of lipoproteins in steers" Lipids, 2005; 40 (12): 1245–56. 32. Dai H, Smith A, Wei Meng X, Schneider P, Yuan-Ping P, Kaufmann S, “Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization” The jrnl. of physiol., 2011; 194 (1): 39-48 33. Chalah A, Khosravi-Far R, “The mitochondrial death pathway” Adv Exp Med Biol., 2008; 615:25-45.