* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Metabolic Diversity
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Cyanobacteria wikipedia , lookup
Phosphorylation wikipedia , lookup
Magnetotactic bacteria wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Citric acid cycle wikipedia , lookup
Electron transport chain wikipedia , lookup
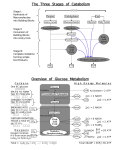
Life is based on redox • All energy generation in biological systems is due to redox (reduction-oxidation) reactions Aerobic Respiration: C6H12O6 + 6 H2O ==> 6 CO2 + 24 H+ +24 e(O2+ 4H+ + 4e- ==> 2H2O) x6 --------------------------------------C6H12O6 + 6 O2 ==> 6 CO2 + 6 H2O oxidation electron donor (aka energy source) reduction electron acceptor overall reaction (24 electrons) Types of bacterial metabolisms • While eukaryotes only reduce O2 and oxidize organic compounds, prokaryotes can use a variety of electron donors and acceptors, organic and inorganic. • Aerobic respiration: e- acceptor is O2 • Anaerobic respiration: e- acceptor is not O2 • Fermentation: e- donor and acceptor are organic molecules • Chemolithotrophy: e- donor and acceptor are inorganic molecules • Phototrophy: e- donor is light and e- acceptor is either organic or inorganic all microorganisms energy source? chemical light chemotroph phototroph carbon source? organic compound carbon source? CO2 chemoheterotroph chemoautotroph e- acceptor? Nitrifying and sulfuroxidizing bacteria O2 most bacteria fermentative organism photoheterotroph CO2 photoautotroph use H2O to reduce CO2? green non-sulfur and purple non-sulfur bacteria Other than O2 Organic compound organic compound Inorganic compound anaerobic respiration: nitrate, sulfate, Fe(III) oxygenic photosynthesis: cyanobacteria anoxygenic photosynthesis: green sulfur and purple sulfur bacteria Aerobic or anaerobic respiration Chemolithotrophy Important molecules Redox Electron Carrier: for example the NAD/NADH couple Energy storage compounds: ATP Coenzyme A NAD as a Redox Electron Carrier • freely diffusible carrier • nicotinamide-adenine dinucleotide NAD+ • transferring electrons from one place to another in the cell • carry 2 e- and 2 protons (H+) • NAD+/NADH -0.32 V - NADH is a good edonor •NAD+ + 2 e- +2 H+ ==> NADH + H+ 2 [H] Coenzyme A • Conserve energy released in energy-producing reactions • Energy stored on thioester bond • Can store enough energy to drive the synthesis of ATP Acetyl-S-CoA + H2O +ADP+Pi ==> acetate + HS-CoA + ATP Metabolism fermentation lactate glucose glycolysis Substrate-level phosphorylation ATP pyruvate Substrate-level phosphorylation butyrate ATP acetate NADH Acetyl-coA TCA cycle GTP Substrate-level phosphorylation NADH/ FADH2 Oxidative phosphorylation Proton motive force ATPase ATP e- acceptor: O2, NO3- or SO42- ATP CAC= citric acid cycle Glycolysis Citric acid cycle • NADH and FADH coming from glycolysis will bring electrons NADH ==> NAD+ + eFADH2 ==> FAD+ + e• These electrons are transported down the chain until they oxidize O2 • At each step, protons are translocated to outside the membrane • Thus, a proton gradient is established between inside and outside the cell Oxidative Phosphorylation • This proton gradient is termed the proton motive force (PMF) anaerobic respiration ATP generation with PMF • The proton motive force is used by ATP synthase to produce ATP. • Process called chemiosmosis Substrate level phosphorylation Other metabolisms Anaerobic food chain • • In contrast to aerobic organisms, no single anaerobe is able to take glucose to CO2 Need an anaerobic food chain that takes each compound part of the way. Various organisms participate in the degradation of a polymeric sugar such as cellulose cellulose concentration • CH4 acetate H2 fatty acids time Methanogenic environments 1 Fermentative bacteria Carbohydrates, nucleic acids Proteins, lipids 2 Syntrophic bacteria 3 Homoacetogenic bacteria 1 4 Methanogenesis Lactate Propionate Alcohols, … 2 H2, CO2 3 4 CH4, CO2 Acetate Types of metabolisms • Fermentative bacteria: Diversity of fermentation types- use sugars, amino acids, nucleic acids- produce any combination of acids, alcohol, CO2, H2, NH3 • Syntrophic: Organisms that produces H2 and needs other organisms in coculture to remove the H2 produced • Homoacetogenic: 4 H2+ H+ + 2 HCO3- ==> CH3COO- + 4 H2O acetate • Methanogenic (archea) 4 H2+ CO2 ==> CH4 + 2 H2O methane Sulfidogenic environments 1 Fermentative bacteria 2 Syntrophic bacteria Carbohydrates 3 Sulfate-reducing bacteria 1 2 H2, CO2 Lactate Propionate Alcohols, … 2 Acetate 3 SO42H2S and CO2 and/or acetate Important molecules pyruvate OH O OH C C C C C O O OH C C C acetate O OH C C lactate OH ethanol OH OH OH O C C C C O O C C C C O fumarate OH O C succinate OH C C C O OH malate OH OH C O formate