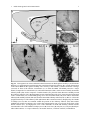
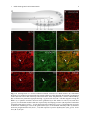
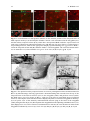
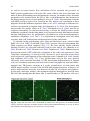
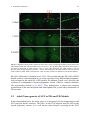
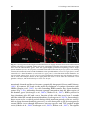
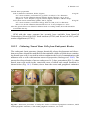
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1 - BrainMaster
Neuroplasticity wikipedia , lookup
Synaptogenesis wikipedia , lookup
Synaptic gating wikipedia , lookup
Haemodynamic response wikipedia , lookup
Multielectrode array wikipedia , lookup
Limbic system wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neural engineering wikipedia , lookup
Nervous system network models wikipedia , lookup
Metastability in the brain wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neurogenesis in the Adult Brain II Tatsunori Seki Kazunobu Sawamoto Jack M. Parent Arturo Alvarez‑Buylla ● ● Editors Neurogenesis in the Adult Brain II Clinical Implications Editors Tatsunori Seki, Ph.D. Professor Department of Histology and Neuroanatomy Tokyo Medical University Tokyo 160-8402, Japan [email protected] Jack M. Parent, M.D. Associate Professor Department of Neurology University of Michigan 109 Zina Pitcher Place 5021 BSRB Ann Arbor, MI 48109, USA [email protected] Kazunobu Sawamoto, Ph.D. Professor Department of Developmental and Regenerative Biology Institute of Molecular Medicine Nagoya City University Graduate School of Medical Sciences 1 Kawasumi, Mizuho-cho, Mizuho-ku Nagoya 467-8601, Japan [email protected] Arturo Alvarez-Buylla, Ph.D. Professor Department of Neurosurgery University of California, San Francisco San Francisco, CA 94143, USA [email protected] ISBN 978‑4‑431‑53944‑5 e-ISBN 978‑4‑431‑53945‑2 DOI 10.1007/978‑4‑431‑53945‑2 Springer Tokyo Dordrecht Heidelberg London New York Library of Congress Control Number: 2011928783 © Springer 2011 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Product liability: The publishers cannot guarantee the accuracy of any information about dosage and application contained in this book. In every individual case the user must check such information by consulting the relevant literature. Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Preface The phenomenon of adult neurogenesis, or persistent generation of neurons in the adult brain, is attracting more and more attention every day. Adult neurogenesis has become increasingly important to studies of brain development and diseases, learning and memory, and aging. A considerable number of papers on the mechanism of adult neurogenesis or linking this process to various physiological and pathological events are published monthly. Most recent popular textbooks in the neurosciences also provide ample coverage of basic principles of adult neurogenesis, a topic which just 20 or 30 years ago was considered tangential, controversial, or unimportant. The discovery that some populations of neurons continue to be produced post‑ natally has dramatically changed previous fundamental concepts of neuroscience. For example, it was widely believed that once development is complete and the embryonic and fetal scaffolding for neuronal generation, migration, and integration are dismantled, these processes cannot be reenacted; therefore, once neurons die they never regenerate. This dogma has been overturned, and studies on adult neuro‑ genesis have opened up the possibility of newborn neurons participating in brain tissue repair and processes of learning and memory. Investigations of adult neuro‑ genesis have also led to basic new principles on the identity of neural stem cells, the function of transit amplifying progenitors, and new forms of neuronal migration. Furthermore, some neuropsychiatric disorders are suspected to be associated with defects in adult neurogenesis. Despite the existence of such a broadly applicable and fundamentally important phenomenon, 20 years ago only a few groups in the world studied adult neurogen‑ esis. It was the pioneering [3H]-thymidine autoradiography studies of Joseph Altman that showed in the 1960s how newborn neurons continue to be formed post‑ natally in the rodent hippocampus and olfactory bulb. Adult neurogenesis, however, did not become widely accepted and remained a controversial field for more than a decade. In the 1980s a series of rigorous studies, inspired by the neurobiology of song learning in birds, led Fernando Nottebohm and his group to demonstrate the origin, migration, and recruitment of new neurons in song-control nuclei and the rest of the telencephalon of adult canaries. Unfortunately, the history of the field is either ignored or underappreciated by the many neuroscientists who are now interested in or working on adult neurogenesis. To redress that, this book contains historically crucial and memorable articles by Drs. Altman and Nottebohm describing from a v vi Preface very personal perspective the motivations and excitement that triggered these seminal discoveries. They also highlight some of the scientific and funding challenges posed by the strong early opposition to adult neurogenesis. Their two articles should not only be the primary source for neuroscientists interested in the initial discoveries in adult neurogenesis and how they came about, but should also be of value to those interested in science history, funding, and policy. Novel methods for labeling new neurons – new thymidine analogs such as BrdU, immunohistochemical markers for immature neurons, retrovirus and genetic tagging techniques – resulted in the 1990s in an explosion of studies on the mechanism and func‑ tion of adult neurogenesis. Stunningly beautiful preparations revealed the entire process of neuronal formation in the adult and revealed the nature and connectivity of individual newly formed neurons. Physiological studies have begun to decipher the unique contribution of new neurons to adult neural circuits. Despite the enormous progress made over the past decade, it is clear that we are still in the early days of understanding the functional meaning and molecular mechanisms of adult neurogenesis. In prepara‑ tion for this next stage of discovery, we thought it was fitting to compile a collection of thoughtful reviews from leading laboratories working in this area of research. Contributing researchers describe their current work in 27 chapters that are grouped into two volumes, which cover a wide array of topics concerning adult neurogenesis. The first volume, in addition to the two articles by Drs. Altman and Nottebohm on the history of adult neurogenesis, comprises a comprehensive pre‑ sentation of the basic biology of adult neurogenesis: basic aspects of neurogenesis, adult neurogenesis in non-mammalian vertebrates, in the mammalian hippocampus and olfactory bulb. In the second volume, clinical implications of adult neuro‑ genesis are considered, including neurogenesis in the adult monkey and human brain, Parkinson’s disease, epilepsy, stress, depression, schizophrenia, stroke, brain injury, and neurodegenerative and neuropsychiatric pathology. A small research group in Japan, the Adult Neurogenesis Kondankai (Conference), working on various aspects of adult neurogenesis initially conceived these volumes. Later, two new editors (A.A.-B. and J.M.P.) joined the project and the original idea was expanded and appropriately shaped. Our goal is not only to provide a compre‑ hensive knowledge base on adult neurogenesis, but also to share our excitement and motivation for an extraordinary field in the neurosciences. We believe that these ingredients will be fundamental to future research in this exciting field towards a better understanding of how adult neurogenesis is maintained and regulated, and how it contributes to plasticity and possibly one day to brain therapy. During the editing of these two volumes, a massive 9.0-magnitude earthquake struck northeastern Japan on March 11, 2011, and tens of thousands of people died in the resulting tsunami. We dedicate this book to their memory, and offer our deepest condolences to those who lost loved ones. Tatsunori Seki Kazunobu Sawamoto Jack M. Parent Arturo Alvarez-Buylla Contents 1 Neurogenesis in Monkey and Human Adult Brain............................... Andréanne Bédard, Patrick J. Bernier, and André Parent 1 2 Adult Neurogenesis in Parkinson’s Disease........................................... Hideki Mochizuki 23 3 Adult Neurogenesis in Epilepsy.............................................................. Sebastian Jessberger and Jack M. Parent 37 4 Stress Disorders........................................................................................ Muriel Koehl, Michel Le Moal, and Djoher Nora Abrous 53 5 Depression................................................................................................. Shin Nakagawa and Ronald S. Duman 99 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia and Related Mental Diseases.................................................................. 109 Noriko Osumi and Nannan Guo 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke: A Future Therapeutic Target to Promote Functional Restoration?.............................................................................. 133 Olle Lindvall and Zaal Kokaia 8 Perspectives of “PUFA-GPR40 Signaling” Crucial for Adult Hippocampal Neurogenesis.................................................... 149 Tetsumori Yamashima 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex....................................................................................... 173 Noriyuki Kishi, U. Shivraj Sohur, Jason G. Emsley, and Jeffrey D. Macklis vii viii Contents 10 Culturing Adult Neural Stem Cells: Application to the Study of Neurodegenerative and Neuropsychiatric Pathology....................... 189 Seiji Hitoshi, Tod Kippin, and Derek van der Kooy Index.................................................................................................................. 209 Contents of Volume I Part I History 1 The Discovery of Adult Mammalian Neurogenesis.............................. Joseph Altman 2 Song Learning in Birds Offers a Model for Neuronal Replacement in Adult Brain................................................................... Fernando Nottebohm 3 47 Part II Basic Aspects of Neurogenesis 3 Fate Specification of Neural Stem Cells................................................. Masakazu Namihira and Kinichi Nakashima 87 4 Fractones: Home and Conductors of the Neural Stem Cell Niche........................................................................................ 109 Frederic Mercier, Jason Schnack, and Maureen Saint Georges Chaumet Part III Adult Neurogenesis in Non-mammalian Vertebrates 5 Adult Neurogenesis in Teleost Fish......................................................... 137 Günther K.H. Zupanc 6 Adult Neurogenesis in Reptiles............................................................... 169 Susana González-Granero, Melissa Lezameta, and José Manuel García-Verdugo Part IV Adult Neurogenesis in the Hippocampus 7 From Embryonic to Adult Neurogenesis in the Dentate Gyrus........... 193 Tatsunori Seki ix x Contents of Volume I 8 Activity-Dependent Regulation of the Early Phase of Adult Hippocampal Neurogenesis..................................................... 217 Tatsuhiro Hisatsune, Yoko Ide, and Rokuya Nochi 9 Integration of New Neurons into the Adult Hippocampus.................. 237 Wei Deng, Chunmei Zhao, and Fred H. Gage 10 Adult Neurogenesis in the Hippocampus: Lessons from Natural Populations......................................................... 257 Jan Martin Wojtowicz 11 Regulation of Adult Neurogenesis by Environment and Learning............................................................................................ 271 Gerd Kempermann Part V Adult Neurogenesis in the Olfactory Bulb 12 Epithelial Organization of Adult Neurogenic Germinal Niches.......... 287 Zaman Mirzadeh, Young-Goo Han, José Manuel García-Verdugo, and Arturo Alvarez-Buylla 13 Neurogenesis in the Adult Rabbit: From Olfactory System to Cerebellum.............................................................................. 319 Giovanna Ponti, Federico Luzzati, Paolo Peretto, and Luca Bonfanti 14 Neuronal Migration in the Adult Brain................................................. 337 Masato Sawada, Shi-hui Huang, Yuki Hirota, Naoko Kaneko, and Kazunobu Sawamoto 15 Development and Survival of Adult-Born Olfactory Neurons............. 357 Masahiro Yamaguchi 16 Wiring New Neurons with Old Circuits................................................. 371 Pierre-Marie Lledo 17 Control of Adult-Born Neuron Production by Converging GABA and Glutamate Signals...................................... 395 Jean-Claude Platel and Angélique Bordey Index.................................................................................................................. 407 Contributors Djoher Nora Abrous (Chapter 4) Inserm U862, Institut F. Magendie, University of Bordeaux 2, 146 Rue Leo-Saignat, 33077 Bordeaux Cedex, France Andréanne Bédard (Chapter 1) Centre de Recherche Université Laval Robert-Giffard, 2601, de la Canardière, Local F-6500a, Beauport, QC, Canada G1J 2G3 Patrick J. Bernier (Chapter 1) Centre de Recherche Université Laval Robert-Giffard, 2601, de la Canardière, Local F-6500a, Beauport, QC, Canada G1J 2G3 Ronald S. Duman (Chapter 5) Division of Molecular Psychiatry, Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, Yale University School of Medicine, New Haven, CT, USA Jason G. Emsley (Chapter 9) MGH-HMS Center for Nervous System Repair, Departments of Neurosurgery and Neurology, and Program in Neuroscience, Harvard Medical School and Nayef Al-Rodhan Laboratories, Massachusetts General Hospital and Department of Stem Cell and Regenerative Biology, and Harvard Stem Cell Institute, Harvard University, Boston, MA 02114, USA Nannan Guo (Chapter 6) Division of Developmental Neuroscience, Tohoku University Graduate School of Medicine, 2-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8575, Japan Seiji Hitoshi (Chapter 10) Division of Neurobiology and Bioinformatics, National Institute for Physiological Sciences, 5-1 Higashiyama Myodaiji, Okazaki, Aichi 444-8787, Japan and Department of Physiological Sciences, School of Life Sciences, Graduate University for Advanced Studies, Kanagawa 240-0193, Japan xi xii Contributors Sebastian Jessberger (Chapter 3) Department of Biology, Institute of Cell Biology, Swiss Federal Institute of Technology (ETH) Zürich, Schafmattstrasse 18, 8093 Zurich, Switzerland Tod Kippin (Chapter 10) Department of Psychology, University of California, Santa Barbara, CA, USA and The Neuroscience Research Institute, University of California, Santa Barbara, CA, USA Noriyuki Kishi (Chapter 9) MGH-HMS Center for Nervous System Repair, Departments of Neurosurgery and Neurology, and Program in Neuroscience, Harvard Medical School and Nayef Al-Rodhan Laboratories, Massachusetts General Hospital and Department of Stem Cell and Regenerative Biology, and Harvard Stem Cell Institute, Harvard University, Boston, MA 02114, USA Muriel Koehl (Chapter 4) Inserm U862, Institut F. Magendie, University of Bordeaux 2, 146 Rue Leo-Saignat, 33077 Bordeaux Cedex, France Zaal Kokaia (Chapter 7) Laboratory of Neural Stem Cell Biology, Section of Restorative Neurology, Stem Cell Institute, University Hospital, 221 84, Lund, Sweden and Lund Strategic Research Center for Stem Cell Biology and Cell Therapy, Lund, Sweden Michel Le Moal (Chapter 4) Inserm U862, Institut F. Magendie, University of Bordeaux 2, 146 Rue Leo-Saignat, 33077 Bordeaux Cedex, France Olle Lindvall (Chapter 7) Laboratory of Neurogenesis and Cell Therapy, Section of Restorative Neurology, Wallenberg Neuroscience Center, University Hospital, 221 84, Lund, Sweden and Lund Strategic Research Center for Stem Cell Biology and Cell Therapy, Lund, Sweden Jeffrey D. Macklis (Chapter 9) MGH-HMS Center for Nervous System Repair, Departments of Neurosurgery and Neurology, and Program in Neuroscience, Harvard Medical School and Nayef Al-Rodhan Laboratories, Massachusetts General Hospital and Department of Stem Cell and Regenerative Biology, and Harvard Stem Cell Institute, Harvard University, Boston, MA 02114, USA Contributors xiii Hideki Mochizuki (Chapter 2) Department of Neurology, School of Medicine, Kitasato University, 1-15-1 Kitasato, Sagamihara, Kanagawa 228-8555, Japan Shin Nakagawa (Chapter 5) Department of Psychiatry, Hokkaido University Graduate School of Medicine, Kita 15, Nishi 7, Kita-ku, Sapporo 060-8638, Japan Noriko Osumi (Chapter 6) Division of Developmental Neuroscience, Tohoku University Graduate School of Medicine, 2-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8575, Japan André Parent (Chapter 1) Centre de Recherche Université Laval Robert-Giffard, 2601, de la Canardière, Local F-6500a, Beauport, QC, Canada G1J 2G3 Jack M. Parent (Chapter 3) Department of Neurology, University of Michigan, 109 Zina Pitcher Place 5021 BSRB, Ann Arbor, MI 48109, USA U. Shivraj Sohur (Chapter 9) MGH-HMS Center for Nervous System Repair, Departments of Neurosurgery and Neurology, and Program in Neuroscience, Harvard Medical School and Nayef Al-Rodhan Laboratories, Massachusetts General Hospital and Department of Stem Cell and Regenerative Biology, and Harvard Stem Cell Institute, Harvard University, Boston, MA 02114, USA Derek van der Kooy (Chapter 10) Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada Tetsumori Yamashima (Chapter 8) Department of Restorative Neurosurgery, Kanazawa University Graduate School of Medical Science, Takara-machi 13-1, Kanazawa 920-8641, Japan Chapter 1 Neurogenesis in Monkey and Human Adult Brain A. Bédard et al.Andréanne Bédard, Patrick J. Bernier, and André Parent Abstract This paper summarizes the results of our studies on adult neurogenesis in some key structures of the monkey and human brain. These results stem from mul‑ tiple immunocytochemical investigations undertaken with antigens raised against various molecular markers of neurogenesis, either alone or in combination with the DNA synthesis indicator bromodeoxyuridine (BrdU). This approach has allowed us to provide the first detailed picture of the organization of the rostral migratory stream (RMS) in adult squirrel monkeys. Furthermore, studies on human postmortem tissue have revealed, for the first time, that the adult human olfactory bulb is the recipient of neuroblasts that migrate along the RMS and progressively develop a GABAergic or dopaminergic phenotype. A new migratory stream that provides newborn neurons to the amygdala and adjacent piriform cortex has also been visualized in squirrel monkeys. Finally, the striatum of normal adult squirrel monkeys was found to harbor newly generated cells that eventually become projection neurons. The recruitment of such striatal neurons was markedly increased in the presence of brain-derived neurotrophic factor (BDNF), a finding that raises hope for the development of novel therapeutic approaches for the treatment of certain neurodegenerative diseases. Abbreviations ac AdBDNF AMY BDNF BrdU c CB Anterior commissure Adenoviral vector overexpressing BDNF Amygdala Brain-derived neurotrophic factor Bromodeoxyuridine Caudal (posterior) Calbindin A. Bédard, P.J. Bernier, and A. Parent (*) Centre de Recherche Université Laval Robert-Giffard, 2601, de la Canardière, Local F-6500a, Beauport, QC, Canada G1J 2G3 e-mail: [email protected] T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_1, © Springer 2011 1 2 cc CD ChAT CMV CR CT DARPP-32 DCX EPL FL GAD GAD65/67 GCL GFAP GL HIPPO HL ic Ki-67 LV MAP-2 MCL NA NeuN NeuroD A. Bédard et al. Corpus callosum Caudate nucleus Choline acetyltransferase Cytomegalovirus Calretinin Cortex Dopamine- and cAMP-regulated phosphoprotein of 32 kDa Doublecortin External plexiform layer Fiber layer Glutamic acid decarboxylase GAD 65 and 67 isoforms Granular cell layer Glial fibrillary acidic protein Glomerular layer Hippocampus Horizontal limb Internal capsule Cell cycle-associated protein Lateral ventricle Microtubule-associated protein 2 Mitral cell layer Nucleus accumbens Neuronal nuclear protein Neuronal determination and differentiation transcription factor OB Olfactory bulb ONL Olfactory nerve layer OT Olfactory tubercle PCNA Proliferating cell nuclear antigen PIRI Piriform cortex PL Posterior limb PSA-NCAM Polysialylated neural cell adhesion PUT Putamen PV Parvalbumin r Rostral (anterior) RMS Rostral migratory stream SS Somatostatin SVZ Subventricular zone SVZa Anterior part of SVZ TH Tyrosine hydroxylase tLV Temporal horn of LV TS Temporal migratory stream TUC-4 Collapsin response mediator protein-4 TuJ1 b-Tubulin-III VL Vertical limb 1 Adult Neurogenesis in Primate Brain 3 1.1 Introduction The pioneering work of Altman, Kaplan and their collaborators initiated more than 40 years ago have seriously challenged the idea that brain neurogenesis stops at birth, a dogma that has prevailed since the end of the nineteenth century (Altman and Das 1965; Kaplan and Hinds 1977). It is now widely accepted that new neurons are pro‑ duced on a continuous basis in normal adult brain, with neural stem/progenitor cells residing in two major brain regions: the subventricular zone (SVZ) lining the lateral ventricles and the dentate gyrus (DG) of the hippocampus. Studies undertaken with bromodeoxyuridine (BrdU), which is incorporated into the DNA of mitotic cells, have shown that neuroblasts are produced throughout adult life in DG of rodents (Cameron et al. 1993; Kuhn et al. 1996; Seki 2002), monkeys (Gould et al. 1999a; Kornack and Rakic 1999) and humans (Eriksson et al. 1998). These neuroblasts pro‑ liferate locally and become incorporated into DG microcircuitry (Gould et al. 1999a). In contrast, neuroblasts produced in the SVZ undertake a rather long journey toward the olfactory bulb (OB) along the so-called rostral migratory stream (RMS) (Luskin 1993; Doestch and Alvarez-Buylla 1996; Kornack and Rakic 2001; Pencea et al. 2001a). At the olfactory bulb level, neuroblasts leave the RMS, migrate radially and differentiate into granular or periglomerular interneurons (Saghatelyan et al. 2005). Newborn neurons might also be produced in brain regions other than DG and SVZRMS-OB system (Gould 2007; Cameron and Dayer 2008). For example, studies using the antiapoptotic protein Bcl-2 as a marker of neuronal immaturity, either alone or in combination with BrdU, have indicated that newborn neurons migrate from the tem‑ poral horn of the lateral ventricle to the rostral portion of the temporal lobe in monkeys (Bernier and Parent 1998a,b; Bernier et al. 2002). The neocortex might also benefit from the addition of new neurons throughout adult life (Gould et al. 1999b; Cameron and Dayer 2008), but the neurons produced there appear to be transient (Gould et al. 2001; Gould 2007). Other possible sites of adult neurogenesis include the striatum, which is the main input station of the basal ganglia (Bédard et al. 2002a, 2006). The possibility of recruiting new neurons in various adult brain structures has a profound implication for our understanding of a wide variety of brain functions and dysfunctions, ranging from the functional basis of brain plasticity to the pathophysiology of neurogenerative diseases. It was therefore thought worthwhile reviewing our current knowledge of adult neurogenesis in the brain of monkeys and humans, with an emphasis on olfactory-related structures, the amygdala and surrounding piriform cortex and the striatum. 1.2 Materials and Methods 1.2.1 Tissue Preparation Our experiments on adult primate neurogenesis were performed on either monkeys or postmortem human brain tissue. Studies on nonhuman primates were done mainly on adult (4–6 years of age) squirrel monkeys (Saimiri sciureus). The monkeys 4 A. Bédard et al. were raised in a markedly enriched environment for about 3 years before being used for experimentation. They lived in a colony of 25–30 individuals housed in a single large animal facility room in which they could play with various toys and used a complex system of ropes and shelves to move around and rest. All the experimental procedures followed the guidelines of the Canadian Council on Animal Care and the Laval University Committee on Animal Use and Housing approved our experi‑ mental protocol. Some monkeys were sacrificed without any treatment, but the majority of them received BrdU injections before sacrifice. In most of these cases, the monkeys were first lightly anesthetized with isoflurane and then received an intravenous injection of BrdU twice a day for 3 consecutive days. The animals were allowed to survive for various times (1–35 days) after the last BrdU injection. After this last injection, animals were deeply anesthetized with a mixture of ketamine– xylazine and perfused transcardially with a saline solution, followed by 4% para‑ formaldehyde. The brains were then removed from the skull and sectioned at 40 mm with a freezing microtome. Studies involving human postmortem materials were limited to an analysis of olfactory structures. The olfactory bulbs, to which a short segment of the olfactory peduncle was often attached, came from ten individuals, aged between 19 and 69 years (mean = 46.2 years), without clinical or pathological signs of neurological or psychiatric disorders. The postmortem delay ranged from 4 to 24 h (mean = 12.6 h). The material was obtained from the brain bank that we have established at the Centre de Recherche Université Laval Robert-Giffard (CRULRG), and our brain banking and postmortem tissue handling procedures were approved by the Ethic Committee of the Centre Hospitalier Robert-Giffard and that of Université Laval. The olfactory bulbs were postfixed by immersion for 2 days in 4% paraformaldehyde and stored at 4°C in 0.1 M phosphate buffer (pH 7.4) with 15% sucrose and 0.1% sodium azide. They were then cut with a freezing microtome into 50-mm-thick sections along sagittal or horizontal planes. These sections were serially collected and kept frozen in a cryoprotecting solution until further assayed. 1.2.2 Immunohistochemistry Complete series of monkey and human brain sections were used to visualize various molecular markers of adult neurogenesis (Table 1.1) with standard immu‑ noprecipitate methods or with immunofluorescence procedures. In the first case, the immunoprecipitate was revealed with 3,3¢-diaminobenzidine (DAB) or nickelintensified DAB as the chromogen (see Bernier et al. 2002 for details). In the second case, double and triple immunostaining procedures with fluorescent chromogens were used to evaluate the coexpression of BrdU with various molecular markers of cytogenesis and astrocytic activity (Table 1.1). BrdU was usually revealed with Alexa 488-conjugated goat anti-rat IgG, while the molecular markers were visualized with Texas Red Streptavidine and/or Cy5-conjugated goat anti-rabbit IgG. Sections incubated without the primary antibodies remain unstained 1 Adult Neurogenesis in Primate Brain 5 Table 1.1 Information about antibodies used in our studies Antibodies Host Dilution Source Bcl-2 Mouse monoclonal 1:50 Sigma CB Mouse monoclonal 1:1,000 Sigma CR Rabbit polyclonal 1:1,500 Swant ChAT Goat polyclonal 1:100 Chemicon DARPP-35 Goat polyclonal 1:100 SantaCruz DCX Goat polyclonal 1:100 SantaCruz GAD65/67 GFAP Ki-67 Nestin Rabbit polyclonal Rabbit polyclonal Mouse monoclonal Rabbit polyclonal 1:500 1:80 1:100 1:200 Sigma Sigma NovaCastra Chemicon NeuroD PV PCNA PSA-NCAM Goat polyclonal Mouse monoclonal Mouse monoclonal Mouse monoclonal 1:100 1:2,000 1:3,000 1:200 SantaCruz Sigma Sigma Chemicon SS TH TUC-4 Rabbit polyclonal Rabbit polyclonal Rabbit polyclonal 1:400 1:500 1:500 R. Benoît Chemicon Chemicon Tuj1 Mouse monoclonal 1:400 Promega Description Antiapoptotic protein Calcium-binding protein Calcium-binding protein Cholinergic cell marker Striatal projection neuron marker Migrating neuroblast marker GABAergic cell marker Astrocyte marker Proliferating cell marker Progenitor/stem cell marker Early neuronal marker Calcium-binding protein Proliferating cell marker Migrating neuroblast marker Neuroactive peptide Dopamine cell marker Postmitotic granular neuroblast marker Early neuronal marker and served as controls. Fluorescent signals were imaged by using a Zeiss LSM 510 confocal laser-scanning microscope. The emission signals of Texas Red, Alexa 488 and Cy5 were assigned to red, green and blue, respectively (see Bédard et al. 2006 for details). 1.2.3 DiI and Adenovirus Injections Three squirrel monkeys, including one that was injected with BrdU, received a single intraventricular injection of 80 ml of 1,1¢-dioctadecyl-3,3,3¢,3¢-tetramethylindocarbocyanine (SP-DiIC18, or DiI) dissolved in dimethylformamide. The dye was injected with a Hamilton syringe in the left lateral ventricle using stereotaxic coor‑ dinates. The animals were perfused 3 weeks after the injection. Four other squirrel monkeys received a single stereotaxic intraventricular injection of 400 ml of adeno‑ viral vectors overexpressing brain-derived neurotrophic factor (BDNF). The construction, production, purification and titration of the Ad5-CMV-BDNF (AdBDNF) adenoviral vectors have been described previously (Gravel et al. 1997). AdLacZ vectors coinjected with AdBDNF vectors were used to monitor the diffusion of BDNF within the brain tissue. 6 A. Bédard et al. 1.3 Results 1.3.1 The RMS in Squirrel Monkeys The use of BrdU in conjunction with various molecular markers of cytogenesis and neurogenesis has allowed us to delineate the topographical organization of the SVZRMS in squirrel monkeys (Fig. 1.1a). The following markers revealed different aspects of this organization: (1) MAP-2 and NeuN, two markers of adult neurons; (2) TuJ1, a marker of early committed neurons; (3) GFAP, a marker for astrocytes; (4) Bcl-2, a protein known for its antiapoptotic activity; (5) PSA-NCAM, a faithful indicator of cell migration; and (6) TUC-4, a marker of postmitotic granule neuro‑ blasts (Bédard et al. 2002b). Particularly revealing were the single labeling experi‑ ments with TUC-4 (Fig. 1.1b), Bcl-2 (Fig. 1.1c–e), and TuJ1. They showed that the anterior part of the SVZ (SVZa) in squirrel monkeys is more densely populated, much thicker and more intensely immunoreactive than its posterior part. In squirrel monkeys, the SVZa caps the caudate nucleus as it protrudes medially into the lateral ventricle and varies markedly in thickness throughout its rostrocau‑ dal extent. Two major enlargements of the SVZa have been noted: one located at the rostral tip of the lateral ventricle, beneath the rostrum of the corpus callosum, and another one bordering the caudal tip of the caudate nucleus near the rostral pole of the thalamus (Fig. 1.1a). These two SVZ extensions merge ventrally in front of the anterior commissure, and it is from this point of convergence that the vertical limb of the RMS emerges. As it approaches the ventral surface of the brain, the vertical limb of the RMS sweeps rostrally to form the horizontal limb, which courses toward the olfactory bulb. Furthermore, we discovered a very thin extension of the RMS that originates from the dorsal part of the vertical limb and courses caudally toward the olfactory tubercle (Fig. 1.1a, b). We termed this caudal exten‑ sion the posterior limb of the RMS. In BrdU-injected monkeys, numerous BrdU-positive (+) nuclei were detected in all the various components of the SVZa-RMS. They were particularly abundant in the rostral enlargement of the SVZa, but also present along vertical and horizontal limbs of the RMS, as well as in the olfactory bulb itself. A smaller number of BrdU+ nuclei occurred along the posterior limb of the RMS and in the olfactory tubercle (Bédard et al. 2002b). The entire SVZa-RMS stained for Bcl-2, TuJ1 and TUC-4. The Bcl-2+ cells in the SVZa formed typical clusters that were particularly numerous in the rostral enlarge‑ ment of this structure. In the vertical and horizontal limbs of the RMS, most Bcl-2+ cells formed parallel chains that were aligned along the major axis of each segment. This chain-like pattern was less obvious in the posterior limb of the RMS, but isolated Bcl-2+ cells were nevertheless scattered throughout the length of this segment of the RMS, as well as in the olfactory tubercle itself. At the olfactory tubercle level, the Bcl-2+ cells appeared either as small granular neurons in the core of the island of Calleja or as slightly larger multipolar neurons distributed at the periphery of these islands (Fig. 1.1d, e). Some Bcl-2+ cells were also scattered within various sectors of olfactory bulb, including the periglomerular layer (Fig. 1.1c). 1 Adult Neurogenesis in Primate Brain 7 Fig. 1.1 Neurogenesis in various olfaction-related structures in adult squirrel monkeys. (a) Schematic drawing of a parasagittal section through the rostral basal forebrain showing the overall arrangement of the SVZa-RMS in the squirrel monkey. The rostral (r) and caudal (c) SVZa extensions converge (asterisk) in front of the anterior commissure (ac) to form the RMS. The RMS possesses a major branch, composed of a vertical limb (VL) and a horizontal (HL) limb, which courses rostrally toward the olfactory bulb (OB). It also comprises a smaller branch (PL, posterior limb) that runs caudally toward the olfactory tubercle (OT). (b) Parasagittal section immunostained for TUC-4 showing the bifurcation of the vertical limb (VL) of the RMS giving rise to the horizontal limb (HL) that courses toward the olfactory bulb and the posterior limb (PL) that runs toward the olfactory tubercle (OT). (c) Bcl-2+ cells in the periglomerular layer of the olfactory bulb. (d) Intense Bcl-2 immunoreactivity displayed by the islands of Calleja (arrows) that are scattered within the portion of the olfactory tubercle (OT) that borders medially the nucleus accumbens (NA), and in the subventricular zone (arrowhead) at the basis of the lateral ventricle (LV). (e) High-power view of the multitude of small and intensely stained neurons encountered in one of the island of Calleja (inset in d). Scale bars (b) 100 mm (c, e) 50 mm (d) 500 mm. Other abbreviations: cc corpus callosum, CD caudate nucleus, LV lateral ventricle, TH thalamus 8 A. Bédard et al. The immunostaining for TuJ1 and TUC-4 (Fig. 1.1b) displayed by the entire SVZaRMS system was strongly indicative of the presence of newborn neurons in that sector of the primate forebrain. This was confirmed by the fact that many of these TuJ1+ cells, which were morphologically similar to the TUC-4+ cells, had a BrdU+ nucleus. The bipolar TuJ1+ cells scattered along the RMS were embedded within a dense meshwork of astrocytic (GFAP+) cells that formed typical tubular structures (Bédard et al. 2002b). Also indicative of cell migration was the presence of PSA-NCAM immunostaining in the entire RMS. Furthermore, double immunostaining observations made in animals that survived 5 weeks after the last BrdU injection revealed the pres‑ ence of newborn (BrdU+) cells that expressed markers of mature neurons (NeuN or MAP-2) in virtually all the major components of the SVZa-RMS system. Such doublelabeled neurons were much less abundant in animals that survived less than 5 weeks after the last BrdU injection indicating that newborn cells in the SVZa-RMS can dif‑ ferentiate into mature neurons within a 5-week period (Bédard et al. 2002b). 1.3.2 Newly Generated Neurons in Human Olfactory Bulb Our analysis of human postmortem materials has revealed the presence of newborn neurons in RMS and OB, whose lamination was delineated with the help of hema‑ toxylin-eosin staining (Fig. 1.2a). Single labeling experiments revealed the presence of cells that stained for PSA-NCAM in the central portion of the olfactory peduncle and in the core of OB (Fig. 1.2b). Many of these PSA-NCAM+ cells were often organized in chains. Furthermore, numerous periglomerular cells were markedly enriched with the antiapoptotic protein Bcl-2, and mitotic cells immunostained for Ki-67 or PCNA were detected principally in the glomerular and granular layers of the human olfactory bulb (Bédard and Parent 2004). Immunofluorescence confocal microscopic examination has revealed numerous cells expressing the migrating neuroblast marker DCX scattered from the olfactory peduncle to the granular layer of OB. They formed a cell continuum that corresponds to the horizontal limb of the RMS, as identified in rodents and nonhuman primates. Furthermore, some of the DCX+ cells were also immunostained for TuJ1 in the granu‑ lar layer (Fig. 1.2c), which also harbored cells that coexpress the cell cycle marker PCNA and the neuroepithelial/stem cell marker Nestin. Other proliferative cells in the glomerular layer coexpressed Ki-67, a proliferating cell marker, and NeuroD, an early neuronal transcription factor required for neuronal differentiation. The presence of different chemical markers of mature neurons was used to docu‑ ment the neuronal differentiation of newborn cells in the granular and glomerular cell layers of the human OB. We found numerous cells expressing glutamic acid decarboxylase (GAD), the rate-limiting enzyme in the biosynthesis of GABA, throughout the granular and glomerular cell layers (Fig. 1.2d). In contrast, cells displaying tyrosine hydroxylase (TH), a reliable marker of dopaminergic neurons, were strictly confined to the glomerular layer (Fig. 1.2e). We also found numerous periglomerular neurons that expressed both TuJ1 and the calcium-binding protein calretinin (CR) and detected some periglomerular cells that express both TuJ1 and 1 Adult Neurogenesis in Primate Brain 9 Fig. 1.2 Neurogenesis in various olfaction-related structures in adult human. (a) Schematic drawing of a sagittal section through the human olfactory bulb showing the laminar organization of the structure. Note that olfactory connections have been simplified for a better understanding. (b) A fluorescent photomicrograph showing a typical PSA-NCAM+ cell that migrates along the RMS. (c) A putative newborn neuron in the granular layer that stains for DCX (red) and TuJ1 (green), two molecular markers that are expressed by developing neurons. (d) A putative immature periglomerular neuron (TuJ1+, in red) that expresses GAD65/67 (green), suggesting the presence of newborn GABAergic cells in the glomerular layer of the human olfactory bulb. (e) Putative newborn periglomerular cell (TuJ1+, red) that expresses tyrosine hydroxylase (TH, green). Scale bars (b–e) 20 mm 10 A. Bédard et al. TH, but we did not find any periglomerular cells that stained for both TH and CR. The glomerular layer also harbored cells displaying immunoreactivity for both TuJ1 and parvalbumin (PV), another calcium-binding protein. All PV+ neurons encountered in the glomerular layer in human OB were devoid of the aging pigment lipofuscin (Bédard and Parent 2004). 1.3.3 The Temporal Migratory Stream in Monkeys Our studies with BrdU have revealed a novel migratory stream in monkey brain. Numerous BrdU+ nuclei occurred along a more or less continuous pathway that extended from the rostral tip of the ventral portion of the temporal horn of the lateral ventricle (tLV) to the amygdala and piriform cortex (Fig. 1.3b), and which was termed the temporal stream (TS). Many of these BrdU+ nuclei displayed an elongated shape and most of them had their longest axis oriented parallel to the main axis of the TS (Bernier et al. 2002). As they approached the amygdala and piriform cortex, how‑ ever, the elongated BrdU+ nuclei were oriented in all directions. The BrdU+ nuclei abounded particularly within the basolateral sector of the amygdala, whereas they tended to form clusters within the piriform cortex. A few BrdU+ nuclei were also disclosed within the deep layers of the inferior temporal cortex. The neuronal nature of many BrdU+ cells encountered in the amygdala, the surrounding piriform cortex and the inferior temporal cortex was ensured by the fact these cells also expressed immunoreactivity for NeuN or MAP-2, two markers of mature neurons, or TuJ1, a marker of early committed neurons (Fig. 1.4). Numerous cells expressing Bcl-2 were scattered throughout the TS (Fig. 1.5a). They were small, round to oval, with only one or two processes, and formed typical chains or clusters scattered all along the TS. As the pathway reached the piriform cortex, the Bcl-2+ cells appeared as tightly packed clusters (Fig. 1.5b). In the amygdala, Bcl-2+ neurons displayed a typical bipolar appearance and many of them appeared linked to one another by their processes (Fig. 1.5c–e). The TS in squirrel monkeys was clearly delineated in material immunostained for PSA-NCAM. The PSA-NCAM+ cells were scattered all along TS, but appeared in the form of clusters of various sizes in the piriform cortex. Many chains of neu‑ roblasts in TS expressed TuJ1, and some of these TuJ1+ neurons appeared to have been newly generated because BrdUrd+/TuJ1+ cells were detected all along TS (Fig. 1.4g). The TS in squirrel monkeys was found to stain for TUC-4, a protein whose early expression, relative abundance in newborn neurons and the restriction in its expression to the period of initial neuronal differentiation makes it a specific marker of recently generated neurons in the adult brain. A thin array of TUC-4+ cells extended from the tLV to the sub-amygdalaloid region. Furthermore, small clusters of TUC-4+ cells with thick fibers fascicles oriented toward TS were seen in the piriform cortex (Bernier et al. 2002). In monkeys that received a single intraventricular injection of the highly lipo‑ philic dye DiI, a cohort of DiI+ cells could be traced from the tLV to the amygdala and piriform cortex, thus forming a continuous pathway that was identical to TS 1 Adult Neurogenesis in Primate Brain 11 Fig. 1.3 Neurogenesis in amygdala-related structures in squirrel monkeys. (a) Sagittal view of the staining observed following intraventricular injection of the carbocyanide dye DiI. Dots rep‑ resent BrdU+ cells found in amygdala (AMY), whereas asterisks indicate BrdU+ cells lying along the DiI+ pathway and its branches. For the sake of clarity, the numerous and locally generated BrdU+ cells that occur in the dentate gyrus of the hippocampus have not been indicated here. The inset depicts the germinal matrix extensions under amygdala (AMY) and hippocampus (HIPPO), as they can be seen on a sagittal section of the temporal lobe of a 26-day-old human embryo (Modified from Feess-Higgins and Larroche 1987). (b) Plotting of BrdU+ nuclei on sagittal sec‑ tions of adult monkeys sacrificed at three different times after the last BrdU injection. The red circles indicate BrdU+ nuclei that appear at day 1, 7 and 14 after the last BrdU injection. Scale bars (a) 250 mm. tLV indicates the tip of the temporal horn of the lateral ventricle identified above on the basis of BrdU staining (Fig. 1.3). Chains of DiI+ cells also occurred at the ventral border of the amygdala. The variation in the pattern of dis‑ tribution of the BrdU+ nuclei in the deep portion of monkey temporal lobe accord‑ ing to the survival time after the last BrdU injection is congruent with the idea that the newborn neurons detected in the amygdala and piriform cortex may have migrated from the SVZ that borders the tLV. In the animal that was allowed to survive only 1 day after a single injection, BrdU+ cells were largely confined to the border of the tLV, whereas in the animal that survived 7 days, the majority of BrdU+ cells lay along TS and a smaller number of BrdU+ cells occurred in the basolateral portion of the amygdala and in the piriform cortex, including the piri‑ form area, entorhinal cortex and periamygdaloid cortex (Fig. 1.3b). A few BrdU+ 12 A. Bédard et al. Fig. 1.4 Colocalization of neurogenesis markers in the anterior portion of the temporal lobe of adult squirrel monkeys. (a–f) Examples of BrdU+/NeuN+ cells encountered in the amygdala (a–c) and the inferior temporal cortex (d–f), which have incorporated BrdU and also express NeuN. In each group of fluorescent photomicrographs (a–c and d–f) the left panel shows a confocal micro‑ scope orthogonal view, whereas the right panels display separate labeling view. (g) A TuJ1+/BrdU+ cell in the temporal stream and (h) a MAP-2+/BrdU+ cell in amygdala. The various neuronal mark‑ ers are stained in red, whereas BrdU is in green. Scale bars (a, d) 25 mm; (g–h) 15 mm Fig. 1.5 The squirrel monkey temporal stream as seen on parasagittal sections immunostained for Bcl-2. (a) Photomontage showing typical Bcl-2 immunostaining that extends from the tip of the temporal horn of the lateral ventricle (tLV) to the amygdala (AMY) and piriform cortex (PIRI). (b) High power view of a cluster of Bcl-2+ cells (box in a) lying in superficial layers of piriform cortex. (c–e) Examples of the immature features displayed by Bcl-2+ neurons in amygdala of monkeys. (c) Low-power view of the intensely immunostained neurons lying at the basis of the amygdala (AMY), along the fiber layer (FL) that separates the amygdala from the adjoining entorhinal cortex (CT). (d, e) High-power view of two clusters of immunoreactive neurons, the exact location of which in the amygdala is indicated by insets in (c). Scale bars (a) 500 mm, (b) 20 mm, (c) 200 mm, (d, e) 50 mm 1 Adult Neurogenesis in Primate Brain 13 cells were also detected in the inferior temporal cortex from that time on. From 14 to 21 days postinjection, the BrdU+ cells were much more numerous in the amygdala and piriform cortex than in the TS. 1.3.4 Newly Generated Neurons in the Striatum of Monkeys The striatum appears to be another brain area in primates where adult neurogen‑ esis occurs. Indeed, a significant number of BrdU+ cells were detected in the striatum of normal squirrel monkeys 3 weeks following the last injection of the mitotic marker (Fig. 1.6a). These BrdU+ cells were more numerous in the caudate nucleus than in the putamen and more abundant in dorsomedial than ventrolateral part of both caudate nucleus and putamen. Their distribution displayed clear rostrocaudal and mediolateral decreasing gradients. Some BrdU+ cells were also present in the ventral striatum, which includes the nucleus accumbens, the most ventral portions of both caudate nucleus and putamen, and the deeper layers of the olfactory tubercle. Because the number of newborn cells in monkey striatum was rather small (10–50/section) under normal conditions, an adenoviral vector encoding BDNF was injected intraventricularly to enhance striatal neurogenesis and, hence, facili‑ tate the determination of the origin and the phenotype of newly generated striatal neurons (Fig. 1.7). In normal (non-AdBDNF-injected) adult monkeys that received BrdU injections, numerous BrdU+ cells were found in the SVZ, which borders the parenchyma of the head of the caudate nucleus, and BrdU+ cells were also seen in significant numbers in the striatum itself. In normal monkeys, there was about 324 BrdU+ cells/mm3 in the caudate nucleus compared to 220 BrdU+ cells/mm3 in the putamen, whereas the corresponding values for AdBDNF-injected monkeys were 3,500 and 3,180, respectively. The BrdU+ cells were distributed according to clear rostrocaudal and mediolateral-decreasing gradients. They were more numerous in the dorsomedial than in the ventrolateral sector of both caudate nucleus and puta‑ men. Their total number ranged from about 1,200 to 16,000 in each section that passed through the medial half of the striatum, and from 2,900 to 8,600 in each section though the lateral half of the structure. Numerous BrdU+ cells were found to express the immature neuronal marker TuJ1, whereas many other BrdU+ cells colocalized with the mature neuronal mark‑ ers MAP-2 and NeuN (Fig. 1.7b). The majority of these double-labeled neurons were located in the medial half of the striatum. The chemical phenotype of the newly generated neurons was determined on adjacent striatal sections of AdBDNFtreated monkeys immunostained for various molecular markers of striatal neurons and interneurons. Some BrdU+ cells were found to express calbindin-D28 kDa (CB), a typical marker of striatal projection neurons, and several BrdU+ cells were also immunostained for GAD65/67, a marker of GABAergic neurons, and for DARPP-32, a marker of striatal projection neurons. We have not seen BrdU+ cells expressing PV, CR, somatostatin (SS) or choline acetyl transferase (ChAT), markers 14 A. Bédard et al. Fig. 1.6 Newborn neurons in the striatum of adult squirrel monkeys. (a) Drawing of a parasagittal section through the striatum of a squirrel monkey showing the distribution and relative number of BrdU+ cells in that structure. Each symbol represents one labeled cell. The green dots represent BrdU+ nuclei, whereas the green dots surrounded by a red circle indicate double-labeled (BrdU+/ NeuN+) newborn neurons. (b) A confocal microscope orthogonal view of a double-labeled (BrdU+/NeuN+) newborn neuron in the head of the caudate nucleus. (c, d) Many BrdU+ cells pres‑ ent in the striatum appeared in pairs and were likely daughter cells of a single mitotic event. (e–g) Various aspects of the immunostaining for DCX and PSA-NCAM observed along the lateral ventricle in monkeys injected with AdBDNF. Low-power view of the DCX immunostaining present in the SVZ and adjacent caudate nucleus (e). High-power view of DCX immunostaining displayed by the SVZ (box area in e) (f). An intricate network of DCX+ fibers that emerge from the lateral ventricle border and extends deeply into the caudate nucleus is also present in this region. High-power view of a neuron displaying PSA-NCAM immunoreactivity along the lateral ventricle, near the SVZa (g). Its bipolar aspect as well as its leading process oriented away from the SVZ, suggest a migratory status. Scale bars (b–d, g) 20 mm, (e) 100 mm that label different types of striatal interneurons. These results reveal that a treat‑ ment with AdBDNF enhances the number of newborn neurons that differentiate principally into projection neurons in the ipsilateral striatum of adult monkeys. After adenoviral injection, we found many bipolar elongated cells oriented toward the caudate nucleus (and hence away from the SVZ) and expressing the migrating neuroblast markers DCX and PSA-NCAM (Fig. 1.6f, g). In AdBDNFinjected monkeys, the SVZ was strongly immunoreactive for DCX, a migrationassociated protein (Fig. 1.6e). The caudate nucleus of normal monkeys was devoid of cells displaying a migratory status, suggesting that the intraventricular injection 1 Adult Neurogenesis in Primate Brain 15 Fig. 1.7 Intraventricular injections of an adenoviral vector overexpressing BDNF increase the number of newly generated neurons in SVZ, caudate nucleus and putamen of squirrel monkeys. (a, b) Sagittal sections showing the distribution of BrdU+ cells (green) located in the SVZa of a control monkey (a) and in an AdBDNF-injected monkey (b). (c) Histogram showing the increased level of newly-generated cells (BrdU+) that coexpress the mature neuronal marker NeuN in AdBDNFinjected monkey (blue), as compared to control monkeys (red ). Scale bars (a, b) 100 mm of AdBDNF induces neuronal migration of SVZ progenitors into the striatal parenchyma. On the other hand, numerous BrdU+ nuclei arranged in pairs have been visualized in both controls and AdBDNF-treated monkeys (Fig. 1.6c, d). This finding suggests that parenchymal progenitors might be a source of newborn neurons in normal conditions, and that both parenchymal and SVZ progenitors can be increased in number in response to an overexpression of BDNF. These results reveal that the primate striatum harbors several neuronal progeni‑ tors that can differentiate into mature neurons in normal adult monkey. They further show that a single injection of AdBDNF leads to a significant increase in the total number of newly generated cells that differentiate into mature neurons and develop a medium-spiny projection phenotype, 3 weeks after BrdU injections. 1.4 Discussion 1.4.1 Neurogenesis in Adult Primate Olfactory Structures The idea that adult neurogenesis is a rodent idiosyncrasy has persisted for many years, but started to erode when mitotic activity was detected in the SVZ of adult primates, including humans (see Sect. 1.1). The presence of a migratory stream that appears to be homologous to the rodent RMS has been fully documented in catar‑ rhine (Old World) monkeys (Kornack and Rakic 2001; Pencea et al. 2001a). Our own studies have revealed that a RMS also exists in S. sciureus, a typical represen‑ tative of the platyrrhine (New World) monkeys. Since squirrel monkeys have a long evolutionary history independent of other New World monkeys, this finding indicates that the SVZa-RMS has been conserved throughout primate evolution. 16 A. Bédard et al. Such a selective preservation is surprising in the face of the marked involution of the olfactory structures that characterizes the microsmatic condition of all extant primates. It underlines the functional importance of the SVZa-RMS in primates and indicates that this system might have played a crucial role in primate evolution. The SVZa-RMS in squirrel monkeys appears to more extended that its homologue in macaque monkeys as it provides newborn neurons as far as the olfactory tubercle. These neurons were linked to similar cells that belong to a caudally oriented segment of the SVZa-RMS that we termed the posterior limb of the SVZa-RMS. Although less massive than the vertical and horizontal limbs, the posterior limb of the SVZaRMS might nevertheless play an important role in brain olfactory function by providing new neurons to the olfactory tubercle. Also of interest is the fact that the SVZa-RMS in squirrel monkeys displayed an intense immunostaining for the antiapoptotic protein Bcl-2. Such a finding is congruent with the notion that Bcl-2 protects neuroblasts that have not yet estab‑ lished proper axonal connections and facilitates their maturation and differentiation. Intense immunostaining for Bcl-2 also occurred in the SVZ, olfactory tubercle, amygdala and piriform cortex of squirrel monkeys, and evidence for adult neuro‑ genesis has been obtained for all these basal forebrain regions in S. sciureus (Bernier et al. 2002; Bédard et al. 2002b). Furthermore, Bcl-2 was found to be colocalized with nestin, PSA-NCAM and TuJ1 in the human SVZ, indicating that the latter structure is also mitotically active (Bernier et al. 2000; Curtis et al. 2007). Cells immunostained for Bcl-2 were scattered throughout the rostrocaudal extent of the SVZa-RMS, that is, from the rostral and caudal enlargements of the SVZa, caudally, to the periglomerular layer of OB, rostrally. Altogether, these data indicate that Bcl-2 is a reliable marker of adult neurogenesis in primates. They also suggest that this antiapoptotic protein might protect immature neuroblasts that migrate along the SVZa-RMS until their complete integration in the microcircuitry of OB and olfactory tubercle. Our analysis of postmortem tissue from normal human individuals have allowed us to provide the first evidence that the human OB is a site of active cell proliferation and a recipient for migrating neuroblasts that mature and express morphological and chemical phenotypes typical of granular and periglomerular interneurons (Bédard and Parent 2004). The coincident expression of Ki-67 and NeuroD indicates that cells at a very early stage in the generation of new neurons exist in the olfactory bulb of the adult human brain. Further evidence stems from the fact that numerous periglom‑ erular cells were markedly enriched in Bcl-2 in the human olfactory bulb, suggesting that this protein might protect immature neuroblasts until their full integration in the microcircuitry of the glomerular layer. Likewise, cells displaying DCX or PSANCAM immunostaining were detected in the human RMS. These cells were scattered from the olfactory peduncle to the inner portion of the human OB, including the granular and glomerular layers. Altogether, these findings reveal that continuous migration of neuronal precursors occurs from the olfactory peduncle to OB in adult human. Such a view was confirmed by a recent postmortem investigation similar to ours, but in which the chemical phenotype of newborn OB neurons was not determined (Curtis et al. 2007). The latter study also proposed that neuroblasts 1 Adult Neurogenesis in Primate Brain 17 migrate along a rostral extension of the lateral ventricle in human, a suggestion that was severely criticized because the data supporting it were gathered from the brain of individuals who suffered from hydrocephalus (Sanai et al. 2007). The granular and glomerular layers of human OB were enriched with cells that expressed GAD and TuJ1, a marker of differentiating neurons, whereas the glom‑ erular layer harbors a smaller number of TuJ1+/TH+ neurons. The GAD+ and TH+ cells located in the glomerular layer shared the typical morphological features of periglomerular neurons. Furthermore, Tuj1+ neurons that coexpress PV or CR were also detected in the glomerular layer of human OB and these neurons were devoid of the aging pigment lipofuscin, supporting their newborn origin (Bédard and Parent 2004). The expression of calcium-binding proteins in newly generated granular and periglomerular neurons suggests an important role of calcium regula‑ tion in neuronal differentiation process. Despite the marked involution of the olfactory structures that characterizes the primate microsmatic condition, the proliferation, migration and maturation of bul‑ bar interneurons appear to occur in all primates, including human. This phenome‑ non may ensure neuronal plasticity in a variety of primary and secondary structures involved in the processing of olfactory information. Olfactory dysfunction is asso‑ ciated with many neuropsychiatric and neurodegenerative disorders such as schizo‑ phrenia, Alzheimer’s, Huntington’s and Parkinson’s diseases (Steiner et al. 2006). Olfactory dysfunction associated with different neuropsychiatric illnesses may result from a derangement in the production of new neurons in the human olfactory bulb. Hence, detailed studies of the status of adult neurogenesis in the olfactory bulb of human patients who died from neurodegenerative or psychiatric illnesses might shed a new light on the pathogenesis of these crippling diseases. 1.4.2 Neurogenesis in Adult Primate Temporal Lobe During rat brain embryogenesis, an extension of the lateral ventricle containing the germinal matrix is present along the growing olfactory bulb and it is generally accepted that this extension becomes the substrate of the postnatal RMS (Altman and Das 1965). Interestingly, another germinal matrix extension of the lateral ven‑ tricle occurs behind and under the amygdala during human brain development (Feess-Higgins and Larroche 1987). The striking similarity between this extension in embryonic human brain (Fig. 1.3a, inset) and TS in adult monkeys described here strengthens the notion of neurogenic activity in this pathway and its principal recipient structures. Moreover, by analogy with what occurs at the RMS level, it may be presumed that this temporal lobe extension of the germinal matrix serves as a morphological substratum for the adult TS. Cells doubly labeled for BrdU and specific neuronal markers have been detected in the amygdala and piriform cortex of squirrel monkeys, and our quantitative esti‑ mates indicate that at least 27% of the newly generated cells that were scattered in the temporal lobe of adult primates differentiate into neurons. Although estimates 18 A. Bédard et al. of newborn neurons were obtained from the sampling of only the rostral part of the temporal lobe, the values nevertheless indicate that the rate of neurogenesis at this level is rather impressive. It is important to note that these findings were gathered from the brain of monkeys raised in colony and housed in large rooms that was designed to avoid stress, which is known to inhibit neurogenesis (Gould et al. 1998), and to provide a maximally enriched environment, a condition that is known to favor neurogenesis (Kempermann et al. 1997). The facts that thick layers of TuJ1+ cells occur at TS level and that BrdU+ nuclei are scattered along this pathway suggest that newborn neurons in amygdala and piriform cortex might originate from the SVZ surrounding the temporal tip of the lateral ventricle (tLV). Also worth noting is the fact that Bcl-2 labeled virtually all components of the deep portion of the temporal lobe where signs of adult neurogenesis have been detected in the present study. The abundance of BrdU+ cells in the SVZ adjoining the tLV in early survival periods, and the decrease in the number of these cells at the expense of those in the amygdala in longer survival periods supports the idea that newborn cells are generated in the SVZ and migrate afterward into the parenchyma of the amygdala. However, the possibility that newly generated cells in the amygdala and surrounding structures result from division of in situ progenitor cells cannot be excluded. Furthermore, longer survival periods after BrdU injections are needed to study the fate of the newborn neurons encountered in the amygdala and surrounding cortex. This type of information is essential to know if the new neurons that populate the amygdala and adjoining cortex in primates are expressed only transiently or if they become fully mature and completely integrated within the neuronal circuitry of the tem‑ poral lobe. Because parts of both amygdala and piriform cortex receive direct olfactory inputs, the implantation of newly generated neurons into these structures may par‑ allel the continuing addition of new neurons in OB and olfactory epithelium. These concomitant phenomena might confer stability and plasticity to the olfactory sys‑ tem and temporal lobe. As is the case for DG, the rate of postnatal neurogenesis in the amygdala and adjoining regions of the temporal lobe might be altered by vari‑ ous external conditions, such as stressful events (Gould et al. 1998). Such a phe‑ nomenon could help generate novel hypotheses about the pathogenesis of psychiatric diseases, such as mood/affect disorders and schizophrenia. 1.4.3 Neurogenesis in Adult Primate Striatum Our initial investigation in normal, adult, squirrel monkeys has shown that new cells are generated throughout adult life in primate striatum and that a subset of these proliferative cells develops a mature neuronal phenotype and survives at least 3 weeks (Bédard et al. 2002a). However, because the number of newborn striatal neurons is very low in adult monkeys under normal conditions (10–50 BrdU+ cells/section), we used an adenoviral vector encoding the BDNF gene to recruit a larger number of 1 Adult Neurogenesis in Primate Brain 19 neuronal progenitors in the SVZ and striatum (Pencea et al. 2001b), a strategy that greatly facilitated the phenotypic characterization of newborn striatal neurons. The abundance of BrdU+ cells noted in the SVZ of adBDNF-injected monkeys, together with the decreasing numerical gradient of such cells along the mediolateral axis of the striatum, raised the possibility that newborn striatal cells were generated in the SVZ and had migrated afterward into the striatal parenchyma. The presence of PSA-NCAM+ and DCX+ elongated bipolar SVZ cells, which are typically ori‑ ented toward the caudate nucleus, also supported the notion that the newborn stri‑ atal neurons derive, at least in part, from migratory SVZ progenitors. Striatal damage was shown to induce a migration of SVZ precursors toward the lesion, and their differentiation into medium-spiny neurons (Arvidsson et al. 2002). However, these findings did not rule out the possibility that division of progenitor cells intrin‑ sic to the striatum are a possible source of striatal newborn neurons. This view is supported by the fact that several BrdU+ cells occurred in close pairs in striatal parenchyma of normal and AdBDNF-injected monkeys, and were thus likely the daughters of single mitotic events. Other supporting evidence includes the harvest‑ ing of multipotent progenitor cells from striatal parenchyma (Reynolds et al. 1992) and the increased cellular proliferation resulting in the addition of new striatal neu‑ rons that results from intraventricular injections of epidermal growth factor in adult rodents (Craig et al. 1996). The striatum is mainly composed of GABAergic projection neurons of medium size that display dendrites covered with spines, hence their name medium-spiny neurons. These neurons express the calcium-binding protein CB as well as DARPP-32 and send their axons to the globus pallidus and the substantia nigra. Two weeks after adenoviral injection, several newborn (BrdU+) striatal neurons had matured (NeuN+) and differentiated into medium-spiny projection neurons, which expressed CB, GAD and DARPP-32. It is tempting to speculate that the new projection neurons detected in the primate striatum had reached their normal target, since retrograde celllabeling studies in rodents have revealed that newly generated medium-spiny neurons in adult striatum successfully project to the globus pallidus (Chmielnicki et al. 2004). It would thus be interesting to use a similar approach to evaluate the long-term survival and functional integration of newly generated striatal neurons in primates. Among the newborn neurons detected in the striatum of normal and AdBDNFinjected monkeys, none displayed the chemical phenotype of striatal interneurons, further suggesting that BDNF might act preferentially on the recruitment and/or the differentiation of medium-spiny projection neurons. On the basis of its cytological composition, the striatum strikingly differs from the majority of brain structures, such as OB and DG, where interneurons greatly outnumber projection neurons. The striatum also differs from OB and DG with respect to adult neurogenesis, which leads to the production of local circuit neurons in OB and DG and of projection neurons in the striatum (Mizuno et al. 1994; Ventimiglia et al. 1995; Bédard et al. 2006). This difference suggests that neurogenesis in the striatum of adult monkeys may obey different rules and/or may respond to different needs than neurogenesis that occurs in adult OB and the DG. 20 A. Bédard et al. Huntington’s disease is characterized by a progressive motor deficit that results from the selective degeneration of striatal medium-spiny projection neurons consequent to the accumulation of the mutant protein huntingtin. Since the accumulation of the mutant huntingtin protein is accompanied by a decrease of BDNF in the striatum, the neuronal toxicity and death of striatal medium-spiny neurons might be due to an insufficient neurotrophic support at the striatal level. Thus, in situ overproduction of various neurotrophic factors that specifically target neuronal progenitors could represent a possible strategy for the replacement of striatal projection neurons that have died in Huntington’s disease, and to improve motor and cognitive symptoms associated with this neurodegenerative disorder. However, the fact that many nonneuronal cells are also produced in the presence of growth factors might hamper the development of such therapeutic avenues. It is also unclear as to whether newly generated neurons will survive in an environment within which major neurodegenerative processes are still at play. Acknowledgments This study was supported by grant MT-8756 of the Canadian Institutes for Health Research (CIHR) to A. Parent. A. Bédard and P.J. Bernier were recipients of CIHR Studentships. References Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–336. Arvidsson A, Collin T, Kirik D et al (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970. Bédard A, Parent A (2004) Evidence of newly generated neurons in the human olfactory bulb. Dev Brain Res 151:159–168. Bédard A, Cossette M, Lévesque M et al (2002a) Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett 328:213–216. Bédard A, Lévesque M, Bernier PJ et al (2002b) The rostral migratory stream in adult squirrel monkeys: contribution of new neurons to the olfactory tubercle and involvement of the anti‑ apoptotic protein Bcl-2. Eur J Neurosci 16:1917–1924. Bédard A, Gravel C, Parent A (2006) Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res 170:501–512. Bernier PJ, Parent A (1998a) Bcl-2 protein as a marker of neuronal immaturity in postnatal pri‑ mate brain. J Neurosci 18:2486–2497. Bernier PJ, Parent A (1998b) The anti-apoptosis bcl-2 proto-oncogene is preferentially expressed in limbic structures of the primate brain. Neuroscience 82:635–640. Bernier PJ, Vinet J, Cossette M et al (2000) Characterization of the subventricular zone in the adult human brain: evidence for Bcl-2 involvement. Neurosci Res 37:67–78. Bernier PJ, Bédard A, Vinet J et al (2002) Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA 99:11464–11469. Cameron HA, Dayer AG (2008) New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry 63:650–655. Cameron HA, Woolley CS, McEwen BS et al (1993) Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56:337–344. Chmielnicki E, Benraiss A, Economides AN et al (2004) Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci 24: 2133–2142. 1 Adult Neurogenesis in Primate Brain 21 Craig CG, Tropepe V, Morshead CM et al (1996) In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci 16: 2649–2658. Curtis MA, Kam M, Nannmark U et al (2007) Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315:1243–1249. Doestch F, Alvarez-Buylla A (1996) Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA 93:14895–14900. Eriksson PS, Perfilieva E, Björk-Eriksson T et al (1998) Neurogenesis in the adult human hip‑ pocampus, Nat Med 4:1313–1317. Feess-Higgins A, Larroche JC (1987) Development of the human foetal brain, an anatomical atlas. Masson, Paris. Gould E (2007) How widespread is adult neurogenesis in mammals? Nat Rev Neurosci 8:481–488. Gould E, Tanapat P, McEwen BS et al (1998) Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 95:3168–3171. Gould E, Reeves AJ, Fallah M et al (1999a) Hippocampal neurogenesis in adult Old World pri‑ mates. Proc Natl Acad Sci USA 96:5263–5267. Gould E, Reeves AJ, Graziano MS et al (1999b) Neurogenesis in the neocortex of adult primates. Science 286:548–552. Gould E, Vail N, Wagers M et al (2001) Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA 98:10910–10917. Gravel C, Gotz R, Lorrain A et al (1997) Adenoviral gene transfer of ciliary neurotrophic factor and brain-derived neurotrophic factor leads to long-term survival of axotomized motor neu‑ rons. Nat Med 3:765–770. Kaplan MS, Hinds JW (1977) Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 197:1092–1094. Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. Kornack DR, Rakic P (1999) Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA 96:5768–5773. Kornack DR, Rakic P (2001) The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA 98:4752–4757. Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16:2027–2033. Luskin MB (1993) Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11:173–189. Mizuno K, Carnahan J, Nawa H (1994) Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol 165:243–256. Pencea V, Bingaman KD, Freedman LJ et al (2001a) Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol 172:1–16. Pencea V, Bingaman KD, Wiegand SJ et al (2001b) Infusion of brain-derived neurotrophic factor into lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus and hypothalamus. J Neurosci 21:6706–6717. Reynolds BA, Tetzlaff W, Weiss S (1992) A multipotent EGF-responsive striatal embryonic pro‑ genitor cell produces neurons and astrocytes. J Neurosci 12:4565–4574. Saghatelyan A, Roux P, Migliore M et al (2005) Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron 46:103–116 Sanai N, Berger MS, Garcia-Verdugo JM et al (2007) Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science 318:393. Seki T (2002) Hippocampal adult neurogenesis occurs in a microenvironment provided by PSANCAM-expressing immature neurons. J Neurosci Res 69:772–783. Steiner B, Wolf S, Kempermann G (2006) Adult neurogenesis and neurodegenerative disease. Regen Med 1:15–28. Ventimiglia R, Mather PE, Jones BE et al (1995) The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci 7:213–222. Chapter 2 Adult Neurogenesis in Parkinson’s Disease Hideki Mochizuki Abstract Parkinson’s disease (PD) is one of the most common neurodegenerative diseases. Its pathogenesis is based on diminution of neurons in the substantia nigra (SN) that under normal conditions acts as the source of dopamine in the nigros‑ triatal circuit. Although the etiology of PD remains poorly understood, recent understanding of the mechanisms of neurogenesis may reveal insights into the pathogenesis of PD and provide useful tools to treat PD. This review will focus on adult neurogenesis in SN, subventricular zone, striatum and olfactory bulb of PD and PD models. We also focus on adult neurogenesis in genetic PD models. The enhancement of progenitor cells may represent a potential new source of cells for replacement therapy in PD. In this review, possible treatments to enhance neu‑ rogenesis in PD models are also described. 2.1 Introduction Parkinson’s disease (PD) is the second most common neurodegenerative disorder affecting approximately 1% of people over the age of 65. Clinical symptoms of PD are resting tremor, rigidity, akinesia, and postural instability. Two major pathological hallmarks are noted: the selective loss of dopaminergic (DA) neurons in the sub‑ stantia nigra (SN) and the formation of intraneuronal protein inclusions, termed Lewy bodies, in the remaining DA neurons. The current standard PD treatment is oral administration of l-dopa, a precursor of dopamine biosynthesis, to comple‑ ment decreased dopamine levels in the striatum, with or without co-administration of dopamine agonists. However, it is symptomatic treatment not retarding nor halting the progression of the disease. A new treatment to inhibit or slow the progress of this disease is required. H. Mochizuki (*) Department of Neurology, School of Medicine, Kitasato University, 1-15-1 Kitasato, Sagamihara, Kanagawa 228-8555, Japan e-mail: [email protected] T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_2, © Springer 2011 23 24 H. Mochizuki On the other hand, increasing evidence points to the presence of adult neural stem cells (NSCs) in many areas of the mammalian brain (Doetsch et al. 1997; Eriksson et al. 1998). In the adult mammalian brain, the main areas of neurogenesis are the hippocampus and subventricular zone (SVZ) near the lateral ventricle. NSCs in the SVZ migrate into the olfactory bulb (OB) via the rostral migratory stream. Adult NSCs represent an attractive source for the production of specific types of neurons in neurodegenerative disorders and for the development of new regenerative gene therapies. This article will review adult neurogenesis in SN, SVZ, striatum (ST) and OB of PD and PD models that exemplify how adult neurogenesis responds to PD. 2.2 Adult Neurogenesis of SN in PD and PD Models 2.2.1 Adult Neurogenesis of SN in PD To identify proliferating cells in the adult brain, retroviral labeling or BrdU methods have been used, especially in animal models. On the other hand, intrinsic molecules unique to young neurons, such as nestin, polysialylated neural cell adhesion mol‑ ecule (PSA-NCAM) and doublecortin, can also be used to detect neurogenesis (Seki and Arai 1993; Fukuda et al. 2003). This is especially important in human subjects, where experimental markers like 5-bromodeoxyuridine (BrdU) or retroviral vectors are not applicable. However, these antibodies only provide adequate staining of high quality, free-floating sections fixed in buffered 4% formaldehyde solution rather than paraffin-embedded sections. Using these methods, we described a detailed examination of neurogenesis in the midbrains of PD autopsy cases (Yoshimi et al. 2005). In our manuscript, staining for intrinsic markers of neuro‑ genesis was attempted, but doublecortin was not very clear in the SN. Nestin gave intense staining of blood vessels but no staining of neural progenitors in the SN, which was evident in the hippocampus. There were no proliferating cells in SN of PD patients. On the other hand, a large number of PSA-NCAM-positive cells were detected in the SN pars reticulata (SNr) of some patients with PD compared to normal subjects (Fig. 2.1). In rats and a macaque monkey, the dopamine-depleted hemispheres showed more PSA-NCAM staining than the intact side. Furthermore, a small number of tyrosine hydroxylase (TH)-positive cells were PSA-NCAM-positive in these models. We speculate that the PSA-NCAM/TH double-positive cells identified in our study represent newly differentiated dopaminergic neurons in the adult SN. However, no PSA-NCAM and TH double-positive cells in PD patients were detected. The staining patterns of the SNr in humans suggested increases of PSA-NCAM-positive cells in some PD patients, but the results were not conclusive. Further studies and new techni‑ cal developments to detect newborn cells are warranted to examine adult neurogenesis in the midbrain of PD subjects. 2 Parkinson’s Disease a Control b 100 PSA Cells / section d 120 PD 60 SNr SNc 50 PSA Cells / section c 25 80 60 40 40 30 20 10 20 0 0 C PD C PD Fig. 2.1 Polysialic acid (PSA)-positive cells in the substantia nigra. Sections of humans are immunostained with monoclonal anti-PSA. (a, b) PSA-positive cells in human substantia nigra (arrows). SNr of a control (a) and Parkinson’s disease (b) brain. (c, d) Number of PSA-positive cells in the SNc (c) and SNr (d) of human hemisphere sections. The average of duplicated counting of each sample by blinded observer is shown. Some nigral samples of Parkinson’s disease showed the presence of large numbers of PSA-positive cells especially in the SNr 2.2.2 Adult Neurogenesis of SN in PD Models Whether dopaminergic neurogenesis occurs in the adult SN in PD brains or in PD animal models is still a matter of debate. The potential of neurogenesis in the SN has been studied by labeling proliferative neural precursor cells with BrdU (Kay and Blum 2000; Lie et al. 2002; Zhao et al. 2003; Frielingsdorf et al. 2004). Expecting compensatory recovery (Nakatomi et al. 2002; Zhu et al. 2004; Kawai et al. 2004), neurogenesis after dopaminergic cell deprivation has been examined 26 H. Mochizuki as well as in intact brains. Kay and Blum (2000) reported the presence of BrdU-positive proliferative cells in the SN: some of these cells were microglia, but none of them differentiated into dopaminergic neurons. In another study, neuronal progenitor cells isolated from the SN of rats could differentiate into neurons in the hippocampus but not in the midbrain (Lie et al. 2002). Dopaminergic neurons with BrdU-positive nuclei were found in the SN, which were considered to have migrated from the midbrain aqueduct (Zhao et al. 2003), although a different con‑ clusion was reported in another study (Frielingsdorf et al. 2004). The discrepancy between the two studies (Zhao et al. 2003; Frielingsdorf et al. 2004) was due to technical uncertainty in confocal microscopy to locate the BrdU-positive nuclei within the cytoplasm. On the other hand, it was reported recently that mature neurons become immunopositive for proliferative cell markers in some neurodegenerative conditions (Hoglinger et al. 2007). It is important to distinguish such cell death cascades from cell proliferation and neurogenesis in the adult brain. To identify living proliferating cells, retroviral labeling is more selective than BrdU (Lie et al. 2002; Gould and Gross 2002) because retroviral integration into DNA requires new DNA synthesis (Fig. 2.2). We have already shown efficient labeling of NSCs by retroviral infection, both in vitro and in vivo (Kaneko et al. 2001; Suzuki et al. 2002; Yamada et al. 2004; Tanaka et al. 2004). Enhanced green fluorescent protein (GFP) filled the cytoplasm of the cells and expressed a clear Golgi-like morphology of infected cells. Moreover, local injection in the brain tissue allowed a clear mapping of the migration route (Yamada et al. 2004; Suzuki and Goldman 2003) (Fig. 2.3). First, proliferating cells in SN were labeled efficiently with retroviral infection of GFP. Subsequent differentiation of labeled cells was examined, and many infected cells became microglia but none had differ‑ entiated into TH-positive neurons at 4 weeks postinfection, in both intact and MPTP-treated rodents. Second, PSA-NCAM-like immunoreactivity, indicative of newly differentiated neurons, was detected in the SN of rodents and primates. In rats and a macaque monkey, the dopamine-depleted hemispheres showed more PSA-NCAM staining than the intact side. A small number of TH-positive cells were Fig. 2.2 Improved retrovirus vector versus BrdU as a marker for neurogenesis: (a, b) differentiated cells by retrovirus injection; (c) BrdU positive cells by immunohistochemistry, It is easy to detect the detailed cell morphology by the retrovirus vector marking 2 Parkinson’s Disease 27 Fig. 2.3 Migration from SVZ to OB in rat retrovirus vector encoded green fluorescent protein are microinjected into the subventricular zone (red arrow). The proliferating cells in the subventricu‑ lar zone are detected as GFP positive cells: 1 week after infection, the GFP positive cells are present in the rostral migratory stream; 3 weeks after infection, the GFP positive cells are present in the olfactory bulb. Some GFP positive cells are also positive for MAP2 as a neuronal marker PSA-NCAM-positive (Yoshimi et al. 2005). We speculate that the PSA-NCAM/TH double-positive cells identified in our study represent newly differentiated dopamin‑ ergic neurons in the adult SN of PD models. In addition, Parish et al. describe the creation of a salamander 6-hydroxydopamine model of PD to examine midbrain DA regeneration (Parish et al. 2007). They demonstrate a robust and complete regeneration of the mesencephalic and diencephalic DA system after elimination of DA neurons. 2.3 Adult Neurogenesis of SVZ in PD and PD Models In the mammalian brain, the main areas of neurogenesis are the hippocampus and SVZ near the lateral ventricle. The NSCs in the SVZ migrate into the OB via the rostral migratory stream. It is well known that changes occurring in the SVZ 28 H. Mochizuki depend upon the pathological condition. Postmortem analysis of human brains suggested that stroke (Macas et al. 2006), Huntington’s disease (Curtis et al. 2003) and multiple sclerosis (Nait-Oumesmar et al. 2007) lead to an increase in precursor cell proliferation in the adult human SVZ. In this section, I focus on changes of adult SVZ neurogenesis in PD and PD models. 2.3.1 Adult Neurogenesis of SVZ in PD Using postmortem brains of PD patients, Hoglinger et al. reported changes in the SVZ of PD patients by immunohistochemistry (Hoglinger et al. 2004). The numbers of proliferating cells in the SVZ are reduced in postmortem brains of individuals with PD. They also found that dopaminergic fibers contact epidermal growth factor receptor (EGFR)-labeled cells in the normal human SVZ. They concluded that the generation of neural precursor cells is impaired in PD as a consequence of dop‑ aminergic denervation. 2.3.2 Adult Neurogenesis of SVZ in PD Models The neurogenetic potential of SVZ in PD models is a highly debated topic. Several groups have reported that experimental depletion of dopamine in rodents decreased precursor cell proliferation in the SVZ (Hoglinger et al. 2004; Baker et al. 2004). These results mainly rely on PCNA staining using immunohistochemistry. Hoglinger et al. has also shown that there are dopaminergic afferents in the adult mammalian SVZ (Baker et al. 2004). D1L receptors were present in the cytoplasm of type C (transit amplifying) cells and in the plasma membranes of type A (neuro‑ blast) cells. D2L receptors were most abundant in C-cells. They concluded that there was dopaminergic regulation of neural precursor cell proliferation in the adult brain, and that dopaminergic denervation may be responsible for the reduction in neural precursors. On the other hand, some groups have reported increased proliferation of neural precursors in the SVZ of PD models. Liu et al. (2006) unilaterally lesioned the nigrostriatal pathway by injection of 6-hydroxydopamine (6-OHDA), and then BrdU was injected (ip). They showed that the BrdU-positive cells increased signifi‑ cantly in the SVZ ipsilateral to the lesion. The differences in these results in each group could be from differences in the toxin treatment to create the PD models or differences in the methods to detect proliferating cells. We found alterations of the differentiation-related molecular expression in SVZ after acute or chronic MPTP treatment (Oizumi et al. 2008). We examined the relationship between proliferation and differentiation of NSCs in SVZ of both acute and chronic PD models. Only acute MPTP treatment significantly increased the areas of glial fibrillary acidic protein (GFAP)-expressing cells and decreased the areas of PSA-NCAM-expressing 2 Parkinson’s Disease 29 cells in the SVZ. In the case of caspase-11 knockout mice, MPTP did not induce alterations in the areas of GFAP-expressing cells and PSA-NCAM-expressing cells. Our results suggest that neuroinflammation related to the caspase-11 cascade in the striatum also regulates differentiation of NSCs in the SVZ of a mouse model of PD. Regarding SVZ cell death in PD models, He et al. reported the details of the changes in the SVZ after exposure of MPTP in mice (He et al. 2006). They detected apoptotic cells in the SVZ that peaked 24 h after MPTP treatment. In this study, the majority of cells undergoing apoptosis in the SVZ were identified as migrating neuroblast (type A cells). MPTP may also directly interact with cells in the SVZ in addition to regulation by the dopaminergic fibers from SN. Problems of evaluating SVZ changes include difficulties in identifying and counting cell numbers, because these cells are very dense in a narrow space. New methods are needed for detecting significant changes of cell densities over the entire area of immunostained sections. 2.4 Adult Neurogenesis of OB in PD and PD Models Olfactory deficits are an early and common symptom in PD (Ponsen et al. 2004). In the OB, TH-positive cells are present in the glomerular layer (GL), which is the most superficial layer in the OB. As most newborn SVZ cells migrate to the OB to form new neurons, the changes of neurogenesis in OB could be important for the pathogenesis of olfactory deficits as PD symptoms. 2.4.1 Adult Neurogenesis of OB in PD Olfactory stimulation clearly established the presence of olfactory impairment in PD (Ponsen et al. 2004; Barz et al. 1997). This olfactory deficit is so reliable that it is used in the diagnosis of idiopathic PD (Hawkes and Shephard 1998). On an ana‑ tomical level, work by Hawkes and colleagues (Pearce et al. 1995; Hawkes et al. 1997) indicated significant neuronal loss in the anterior olfactory nucleus (AON) obtained from postmortem of patients with IPD. In addition, recent data suggest that neurodegeneration at early PD stages involves the olfactory system, including the OB (Del Tredici et al. 2002; Braak et al. 2003). 2.4.2 Adult Neurogenesis of OB in PD Models Several groups have created PD models and examined neurogenesis in the OB. Hoglinger et al. examined newborn neurons in the OB of mice with four intraperitoneal injections of 10 mg/kg of MPTP by BrdU treatment (Hoglinger et al. 2004). They reported that fewer BrdU positive cells had migrated to the OB in MPTP-lesioned 30 H. Mochizuki than in control mice, and fewer newborn neurons had integrated into the OB, as assessed by the colocalization of BrdU with the neuronal marker neuronal nuclear antigen (NeuN) by confocal microscopy. They suggested that chronic impairment of neural (neuronal or glial) regeneration might also contribute to olfactory impair‑ ment in PD. In 6-OHDA models, Winner et al. also examined the details of OB neurogenesis after treating animals with BrdU (Winner et al. 2006). They found that transient decreases in the granule cell layer contrasted with a sustained increase of newly generated neurons in the GL. They concluded that the loss of dopaminergic input to the SVZ led to a distinct cell fate decision towards stimulation of dopaminergic neurogenesis in the OB GL. We also reported enhanced neurogenesis in the OB after dopaminergic neuron loss by MPTP (Yamada et al. 2004) (Fig. 2.3). Neurogenesis was confirmed mainly by the uptake of BrdU, a marker of proliferating cells, but methodological problems related to BrdU labeling might result in inaccurate findings with respect to specificity, toxicity and incorporation into normal/lesioned brain. For a better identification of neurogenesis, we used a modified retroviral vector reported previously. First, we investigated the population dynamics of newly generated neurons in different regions of OB including the GL, the most superficial layer of OB. Quantification of neurogenesis in OB revealed by our retroviral vector was substantially similar to that found by BrdU-based methods. Next, we investigated the influence of dopamin‑ ergic neuron loss induced by MPTP, a selective toxin for dopaminergic neurons, on dopaminergic neurogenesis in the OB. One week after MPTP intoxication, neuro‑ genesis of dopaminergic neurons in the OB increased by threefold while there was minimal influence on nondopaminergic neurogenesis. These results indicate profiles of selective neurogenesis in OB in response to various lesions. In addition, adult neurogenesis may be enhanced as a repair system in the TH-positive cells of the OB after MPTP administration in PD models (Hayakawa et al. 2007). The isolation of NSCs from the OB after MPTP administration has helped to establish the cellular basis of neurogenesis and supports a role for the transplant-mediated treatment of PD. 2.5 Adult Neurogenesis in Genetic PD Models Mutation or duplication in the a-synuclein gene is a cause of familial PD. Transgenic mice expressing wild-type (WT) mouse and mutant (mut) human a-synuclein serves as a good model of PD. Using these mice, Winner et al. have shown that accumula‑ tion of WT and mut-a-synuclein in the CNS of tg mice results in reduced neuro‑ genesis in the OB and hippocampus (Winner et al. 2004, 2008). The studies in a-synuclein-overexpressing mouse embryonic stem (ES) cells and in young–adult a-synuclein tg mice by Crews et al. offer a clue suggesting that accumulation of a-synuclein might impair neurogenesis by reducing NPC survival via downregulation of Notch-1 expression (Crews et al. 2008). A recent report also 2 Parkinson’s Disease 31 suggests that Notch activity supports the survival of both progenitors and newly differentiating cells in the developing nervous system (Mason et al. 2006). These results suggest that accumulation of a-synuclein might impair survival of NPCs by interfering with the Notch signaling pathway. We investigated neurosphere formation in vitro and migration of NSCs in vivo after transduction of an a-synuclein-encoding retroviral vector (alpha-syn) to char‑ acterize the function of a-synuclein in NSCs. Overexpression of alpha-syn caused less effective formation of neurospheres and induced morphological changes. Fluorescence-activated cell sorting showed diminished NSC cell cycle progression induced by overexpression of a-synuclein. Intriguingly, suppression of NSC migra‑ tion along the rostral migratory stream was observed when the a-synuclein-encoding vector was directly injected into the SVZ of mice in vivo. These results indicate that the accumulation of a-synuclein in NSCs led to a cell-autonomous influence on the generation of neural progenitors, suggesting that effective elimination of toxic species of a-synuclein could pave the way to halt and possibly reverse the clinical symp‑ toms of patients with a-synucleinopathy (Tani et al. 2010). 2.6 Therapeutic Window for Adult Neurogenesis in PD The enhancement of progenitor cells may represent a potential new source of cells for replacement therapy in PD. The possible treatment to enhance neurogenesis in PD models is discussed below. Dopamine D2L (D2, D3 and D4 receptors) agonists were reported to enhance the damaged SVZ in PD models (Hoglinger et al. 2004). Especially, Van kampen and colleagues reported that D3 receptor stimulation promoted proliferation in the SVZ (Van Kampen et al. 2004) and SN (Van Kampen and Robertson 2005) in adult rat. The same group examined the cell proliferative, neurogenic, and behavioral effects of a dopamine D3 receptor agonist in a 6-hydroxydopamine model of PD (Van Kampen and Eckman 2006). They observed a significant induction of cell proliferation in the SNc with a time-dependent adoption of a neuronal dopaminergic phenotype in many of these cells. The dopamine D(3) receptor may play a key role in SVZ neurogenesis, as a particularly strong expression of D(3) receptor mRNA occurs in the proliferative SVZ during pre‑ natal and early postnatal ontogeny. Winner et al. reported that treatment with the oral dopamine receptor agonist pramipexole (PPX) selectively increases adult neurogenesis in the SVZ-OB system by increasing proliferation and cell survival of newly generated neurons. They also demonstrated that D2 and D3 receptors are present on adult rat SVZ-derived neural progenitors in vitro, and PPX specifically increased mRNA levels of EGFR and paired box gene 6 (Pax6) (Winner et al. 2009). However, several researchers have reported that dopamine antagonist antipsychotic drugs also enhance neurogenesis (Kippin et al. 2005; Dawirs et al. 1998). In addition, Milosevic J et al. reported that Dopamine D2/D3 receptor stimulation fails to promote dopaminergic neurogenesis 32 H. Mochizuki of murine and human midbrain-derived neural precursor cells by microarray data analysis and quantitative RT-PCR (Milosevic et al. 2007). In this area, the function of specific dopamine receptor agonists in neurogenesis is still debated. 2.6.1 Neurotrophic Factors Neural progenitor cells respond to several neurotrophic factors that affect cell proliferation, migration and maturation. In PD models, Copper et al. found that intrastriatal transforming growth factor alpha delivery induced proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons (Cooper and Isacson 2004). Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) also induce striatal neurogenesis in PD models (Mohapel et al. 2005). The effects of i.c.v. infused PDGF-BB and BDNF on cell genesis, as assessed with BrdU incorporation, were studied in adult rats with unilateral 6-hydroxydopamine lesions. It is well known that the most potent neurotrophic factor for dopamine neurons described so far is the glial-cell-line-derived neurotrophic factor (GDNF) (Gash et al. 1996). Although Chen et al. (2005) indicated a GDNF-mediated increase in cell proliferation, providing further evidence for the restorative actions of this growth factor, they failed to demonstrate that the TH-positive cells were newly generated. Fibroblast growth factor 2 also enhances striatal and nigral neurogenesis in the acute MPTP model of PD as examined by incorporation of BrdU in cells expressing an immature neuronal marker (Peng et al. 2008). Yang et al. investigated the potential role of ciliary neurotrophic factor (CNTF), because it is predominantly produced in the nervous system and is an endogenous regulator of neurogenesis (Yang et al. 2008). They reported that nigrostriatal denervation did not affect SVZ proliferation in CNTF−/− mice, suggesting that the dopaminergic innervation normally regulates neurogenesis through CNTF. 2.6.2 G-CSF Granulocyte colony-stimulating factor (G-CSF) is a member of the cytokine family of growth factors and is clinically used for the treatment of neutropenia. G-CSF stimulates the proliferation, survival, and maturation of cells committed to the neutrophilic granulocyte (NG) lineage by binding to a specific G-CSF receptor (G-CSFR) (Hartung 1998). In addition to its use for the treatment of different types of neutropenia in humans, G-CSF also has trophic effects on neuronal cells; several recent reports have described the neuroprotective effects of G-CSF in stroke (Schabitz et al. 2003; Komine-Kobayashi et al. 2006). One of the main mechanisms of action for this effect is activation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway through intracellular signaling from G-CSF-G-CSFR binding in cerebral ischemia (Komine-Kobayashi et al. 2006; 2 Parkinson’s Disease 33 Jung et al. 2006). Furthermore, recent studies suggest that G-CSF triggers neurogenesis in an animal model of neurological disorders (Sehara et al. 2007; Tsai et al. 2007). G-CSF could induce the differentiation of NSCs in vitro, and this effect paralleled the in vivo finding that G-CSF also induced neurogenesis and subsequently enhanced functional recovery after cortical cerebral ischemia (Schneider et al. 2005). In PD models, several reports indicate that G-CSF rescues dopaminergic cell loss (Meuer et al. 2006; Cao et al. 2006; Huang et al. 2007). In our experiment, we examined whether G-CSF can protect dopaminergic neurons against MPTP-induced cell death in a mouse model of PD. G-CSF significantly prevented MPTP-induced loss of TH-positive neurons (p < 0.05), increased Bcl-2 protein and decreased Bax protein expression. We now plan to examine the relationship between G-CSF treatment and changes in neurogenesis in PD models. 2.7 Conclusion Dopamine is an important molecule in neurogenesis. Therefore, many investigators now focus on neurogenesis in PD. However, controversy persists regarding neuro‑ genesis in SN and/or in PD models. These problems should be resolved by progress in techniques to identify neurogenesis in vivo. Another goal is to identify new treatments or drugs to upregulate or enhance neurogenesis of SVZ, ST or SN in PD. We are now assaying for several drugs which possess such effects to rescue the degeneration in PD as a replacement therapy. Another concern is potential side effects of drugs to enhance neurogenesis in PD models. Excessive enhancement of neurogenesis could cause the problems of tumor formation after exposure to such drugs. Newborn neurons need to migrate and differentiate and need to work function‑ ally at the appropriate areas. Some papers have indicated functional recovery in PD models but this may not indicate the function of new neurons. Cell numbers are controlled by an intricate balance of cell death and cell proliferation (Hipfner and Cohen 2004). Deregulation of the apoptotic/proliferative balance leads to impaired development and many diseases including neurodegenerative disorders, viral infec‑ tions, and tumors. Newborn neurons may help to keep the balance, but they also need appropriate cell death signals. To be or not to be? It could be an eternal theme in human beings. Acknowledgment This study was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO). References Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci 2004;20:575–579. Barz S, Hummel T, Pauli E, Majer M, Lang CJ, Kobal G. Chemosensory event-related potentials in response to trigeminal and olfactory stimulation in idiopathic Parkinson’s disease. Neurology 1997;49:1424–1431. 34 H. Mochizuki Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211. Cao XQ, Arai H, Ren YR, et al. Recombinant human granulocyte colony-stimulating factor protects against MPTP-induced dopaminergic cell death in mice by altering Bcl-2/Bax expression levels. J Neurochem 2006;99:861–867. Chen Y, Ai Y, Slevin JR, Maley BE, Gash DM. Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Exp Neurol 2005;196:87–95. Cooper O, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinson’s disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J Neurosci 2004;24:8924–8931. Crews L, Mizuno H, Desplats P, et al. Alpha-synuclein alters Notch-1 expression and neurogen‑ esis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci 2008;28:4250–4260. Curtis MA, Penney EB, Pearson AG, et al. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci USA 2003;100:9023–9027. Dawirs RR, Hildebrandt K, Teuchert-Noodt G. Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm 1998;105:317–327. Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 2002;61:413–426. Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 1997;17:5046–5061. Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998;4:1313–1317. Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci USA 2004;101:10177–10182. Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopula‑ tions of nestin-positive cells in adult mouse dentate gyrus. J Neurosci 2003;23:9357–9366. Gash DM, Zhang Z, Ovadia A, et al. Functional recovery in Parkinsonian monkeys treated with GDNF. Nature 1996;380:252–255. Gould E, Gross CG. Neurogenesis in adult mammals: some progress and problems. J Neurosci 2002;22:619–623. Hartung T. Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol 1998;5:221–225. Hawkes CH, Shephard BC. Olfactory evoked responses and identification tests in neurological disease. Ann N Y Acad Sci 1998;855:608–615. Hawkes CH, Shephard BC, Daniel SE. Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1997;62:436–446. Hayakawa H, Hayashita-Kinoh H, Nihira T, Seki T, Mizuno Y, Mochizuki H. The isolation of neural stem cells from the olfactory bulb of Parkinson’s disease model. Neurosci Res 2007;57:393–398. He XJ, Nakayama H, Dong M, et al. Evidence of apoptosis in the subventricular zone and rostral migratory stream in the MPTP mouse model of Parkinson disease. J Neuropathol Exp Neurol 2006;65:873–882. Hipfner DR, Cohen SM. Connecting proliferation and apoptosis in development and disease. Nat Rev Mol Cell Biol 2004;5:805–815. Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 2004;7:726–735. Hoglinger GU, Breunig JJ, Depboylu C, et al. The pRb/E2F cell-cycle pathway mediates cell death in Parkinson’s disease. Proc Natl Acad Sci USA 2007;104:3585–3590. Huang HY, Lin SZ, Kuo JS, Chen WF, Wang MJ. G-CSF protects dopaminergic neurons from 6-OHDA-induced toxicity via the ERK pathway. Neurobiol Aging 2007;28:1258–1269. 2 Parkinson’s Disease 35 Jung KH, Chu K, Lee ST, et al. Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain Res 2006;1073–1074:190–201. Kaneko S, Onodera M, Fujiki Y, Nagasawa T, Nakauchi H. Simplified retroviral vector gcsap with murine stem cell virus long terminal repeat allows high and continued expression of enhanced green fluorescent protein by human hematopoietic progenitors engrafted in nonobese diabetic/ severe combined immunodeficient mice. Hum Gene Ther 2001;12:35–44. Kawai T, Takagi N, Miyake-Takagi K, Okuyama N, Mochizuki N, Takeo S. Characterization of BrdU-positive neurons induced by transient global ischemia in adult hippocampus. J Cereb Blood Flow Metab 2004;24:548–555. Kay JN, Blum M. Differential response of ventral midbrain and striatal progenitor cells to lesions of the nigrostriatal dopaminergic projection. Dev Neurosci 2000;22:56–67. Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci 2005;25:5815–5823. Komine-Kobayashi M, Zhang N, Liu M, et al. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab 2006;26:402–413. Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci 2002;22:6639–6649. Liu BF, Gao EJ, Zeng XZ, et al. Proliferation of neural precursors in the subventricular zone after chemical lesions of the nigrostriatal pathway in rat brain. Brain Res 2006;1106:30–39. Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci 2006;26:13114–13119. Mason HA, Rakowiecki SM, Gridley T, Fishell G. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev Neurosci 2006;28:49–57. Meuer K, Pitzer C, Teismann P, et al. Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson’s disease. J Neurochem 2006;97:675–686. Milosevic J, Schwarz SC, Maisel M, et al. Dopamine D2/D3 receptor stimulation fails to promote dopaminergic neurogenesis of murine and human midbrain-derived neural precursor cells in vitro. Stem Cells Dev 2007;16:625–635. Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience 2005;132:767–776. Nait-Oumesmar B, Picard-Riera N, Kerninon C, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci USA 2007;104:4694–4699. Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002;110:429–441. Oizumi H, Hayashita-Kinoh H, Hayakawa H, et al. Alteration in the differentiation-related molecular expression in the subventricular zone in a mouse model of Parkinson’s disease. Neurosci Res 2008;60:15–21. Parish CL, Beljajeva A, Arenas E, Simon A. Midbrain dopaminergic neurogenesis and behavioural recovery in a salamander lesion-induced regeneration model. Development 2007;134:2881–2887. Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson’s disease. Mov Disord 1995;10:283–287. Peng J, Xie L, Jin K, Greenberg DA, Andersen JK. Fibroblast growth factor 2 enhances striatal and nigral neurogenesis in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neuroscience 2008;153:664–670. Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol 2004;56:173–181. Schabitz WR, Kollmar R, Schwaninger M, et al. Neuroprotective effect of granulocyte colonystimulating factor after focal cerebral ischemia. Stroke 2003;34:745–751. Schneider A, Kruger C, Steigleder T, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 2005;115:2083–2098. 36 H. Mochizuki Sehara Y, Hayashi T, Deguchi K, et al. Potentiation of neurogenesis and angiogenesis by G-CSF after focal cerebral ischemia in rats. Brain Res 2007;1151:142–149. Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci 1993; 13:2351–2358. Suzuki SO, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci 2003;23:4240–4250. Suzuki A, Obi K, Urabe T, et al. Feasibility of ex vivo gene therapy for neurological disorders using the new retroviral vector GCDNsap packaged in the vesicular stomatitis virus G protein. J Neurochem 2002;82:953–960. Tanaka R, Yamashiro K, Mochizuki H, et al. Neurogenesis after transient global ischemia in the adult hippocampus visualized by improved retroviral vector. Stroke 2004;35:1454–1459. Tani M, Hayakawa H, Yasuda T, Nihira T, Hattori N, Mizuno Y, Mochizuki H. Ectopic expression of alpha-synuclein affects the migration of neural stem cells in mouse subventricular zone. J Neurochem. 2010;115:854–863. Tsai KJ, Tsai YC, Shen CK. G-CSF rescues the memory impairment of animal models of Alzheimer’s disease. J Exp Med 2007;204:1273–1280. Van Kampen JM, Eckman CB. Dopamine D3 receptor agonist delivery to a model of Parkinson’s disease restores the nigrostriatal pathway and improves locomotor behavior. J Neurosci 2006;26:7272–7280. Van Kampen JM, Robertson HA. A possible role for dopamine D3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience 2005;136:381–386. Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur J Neurosci 2004;19:2377–2387. Winner B, Lie DC, Rockenstein E, et al. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol 2004;63:1155–1166. Winner B, Geyer M, Couillard-Despres S, et al. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp Neurol 2006;197:113–121. Winner B, Rockenstein E, Lie DC, et al. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol Aging 2008;29:913–925. Winner B, Desplats P, Hagl C, et al. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp Neurol 2009;219:543–552. Yamada M, Onodera M, Mizuno Y, Mochizuki H. Neurogenesis in olfactory bulb identified by retroviral labeling in normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated adult mice. Neuroscience 2004;124:173–181. Yang P, Arnold SA, Habas A, Hetman M, Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci 2008;28:2231–2241. Yoshimi K, Ren YR, Seki T, et al. Possibility for neurogenesis in substantia nigra of Parkinsonian brain. Ann Neurol 2005;58:31–40. Zhao M, Momma S, Delfani K, et al. Evidence for neurogenesis in the adult mammalian substan‑ tia nigra. Proc Natl Acad Sci USA 2003;100:7925–7930. Zhu DY, Lau L, Liu SH, Wei JS, Lu YM. Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 2004;101:9453–9457. Chapter 3 Adult Neurogenesis in Epilepsy Sebastian Jessberger and Jack M. Parent Abstract Epilepsy is a devastating illness affecting about 50 million people worldwide that disturbs multiple aspects of brain integrity and function. Epileptic seizures may not only lead to degeneration of affected areas but also strongly induces the birth of new neurons generated by adult neural stem cells (NSCs) in the two neurogenic areas of the adult brain, the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) lining the lateral ventricles. In this chapter, we focus on mesial temporal lobe epilepsy (mTLE), which is the most common and often intractable form of acquired epilepsy. We will review and discuss recent data derived from rodent models of mTLE and human samples analyzing the effects of epileptic activity on NSC activity, neuronal differentiation and integration, and the potential role that newborn neurons may play in the epileptic disease process. 3.1 Introduction Epilepsy is a diverse brain disorder causing spontaneous recurrent seizures that are induced by aberrant firing of neuronal networks, which eventually lead to abnormal behavior or sensations. In the United States alone, more than two million people are diagnosed with epilepsy (Engel, 1996). Epilepsies have many possible causes and are generally grouped into idiopathic or primary epilepsies with inherited mutations (or presumed mutations) in distinct sets of genes, and symptomatic or secondary epilepsies that eventually develop as a consequence of structural brain abnormalities S. Jessberger (*) Department of Biology, Institute of Cell Biology, Swiss Federal Institute of Technology (ETH) Zürich, Schafmattstrasse 18, 8093 Zurich, Switzerland e-mail: [email protected] J.M. Parent (*) Department of Neurology, University of Michigan, 109 Zina Pitcher Place 5021 BSRB, Ann Arbor, MI 48109, USA e-mail: [email protected] T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_3, © Springer 2011 37 38 S. Jessberger and J.M. Parent from causes such as tumors, traumatic brain injury, stroke, infection, or developmental malformations. In the following, we will focus on mesial temporal lobe epilepsy (mTLE), which is the most common and often intractable form of acquired epilepsies. The initiating site of focal seizures, referred to as a “seizure focus,” in patients suffering from mTLE is typically the hippocampal formation or neighboring struc‑ tures of the mesial temporal lobe. Seizures in mTLE patients are most commonly partial seizures, with seizure activity restricted to the temporal lobe. However, mTLE-associated seizures may also secondarily generalize, thus extending potential damage to the nervous system. Treatment of mTLE and control of seizure activity is challenging and requires a high degree of patient compliance. Most mTLE patients are still treated with anti-epileptic drugs (AEDs) that aim to inhibit the excess excitation of neuronal networks. AEDs are a diverse group of drugs but unfortunately most of them are associated with limiting cognitive or other adverse effects, calling for improved and novel treatment strategies. Even with adequate control of clinical seizures, the syndrome of mTLE often leads to substantial impairment of life quality and also commonly affects multiple cognitive domains in the course of the disease (Helmstaedter, 2002; Elger et al., 2004; Blume, 2006; von Lehe et al., 2006). In addition, many mTLE syndromes become refractory to AED treatment, leaving the surgical excision of the epileptogenic focus as the only treatment option (Wiebe et al., 2001). In cases of mTLE that require surgery, the hippocampus and other mesial temporal structures are excised. Together with post‑ mortem material, surgical specimens are used to characterize the structural altera‑ tions induced by mTLE. Most hippocampi of mTLE patients show substantial structural abnormalities that include loss or thinning of the pyramidal cell layer, astrogliosis, and structural reorganization of the dentate gyrus circuitry including mossy fiber sprouting and dispersion of the granule cell layer (Sutula et al., 1989; Ben-Ari, 2001). The underlying cause of epilepsy-associated structural alterations remains largely unclear. Rodent models of mTLE were developed to study the dynamics and molecular changes associated with epilepsy. The difficulty has been to develop a valid animal model for such a diverse syndrome. Interestingly, most mTLE patients report a history of a “precipitating” insult, such as complicated febrile seizures, leading to the development of epilepsy after a latent period in later childhood or adolescence. This feature of mTLE disease progression has led to the development of the status epilepticus (SE) models, which are now most commonly used to study epileptogenic mechanisms in mTLE. In these models, a prolonged seizure induced by chemoconvulsants (typically kainic acid or pilocarpine) or extrinsic electrical stimulation leads to an initial SE. After a latent period lasting days to weeks, spon‑ taneous recurrent seizures develop along with hippocampal structural changes that resemble in many aspects the human pathology (reviewed in (Buckmaster, 2004). A series of papers in the late 1990s using rodent models of mTLE showed that seizures not only affect existing, mature structures of the hippocampal formation but also lead to alterations of the neural stem cell (NSC) niche within the adult rodent dentate gyrus (Bengzon et al., 1997; Parent et al., 1997; Scott et al., 1998). The dramatic effects of seizures on the number and integration pattern of newborn 3 Adult Neurogenesis in Epilepsy 39 neurons in the adult hippocampus added a completely new level of complexity to the understanding of the causes and consequences of mTLE. In the remaining discussion, we will focus on the consequences of seizure activity on proliferation of NSCs, the maturation and integration of newborn neurons, and the functional role that seizure-induced neurogenesis might play in the epileptic disease process. 3.1.1 Cell Proliferation After SE Under control conditions, several types of progenitors divide in the subgranular zone of the adult dentate gyrus (Seri et al., 2001; Kronenberg et al., 2003; Encinas et al., 2006; Suh et al., 2007) that continuously generate new neurons throughout life (Gage, 2002). The number of newborn neurons is not fixed but instead dynami‑ cally regulated by a variety of factors (e.g., Kempermann et al., 1997; Gould et al., 1999; Van Praag et al., 1999), suggesting a tight regulatory control of the number of dividing cells in the adult dentate gyrus. Judged by Ki67 expression or shortpulse BrdU labeling, prolonged seizure activity dramatically increases the amount of cell proliferation in the dentate gyrus that reaches its maximum several days after SE (Parent et al., 1997; Gray and Sundstrom, 1998; Jessberger et al., 2005) (Fig. 3.1a, b). Furthermore, the number of dividing cells in the second neurogenic Fig. 3.1 Pilocarpine-induced SE increases dentate gyrus cell proliferation and neural progenitors. Immunoreactivity for BrdU (a, b) or green fluorescent protein (GFP; c, d) in the dentate gyri of adult nestin-GFP mice 14 days after pilocarpine-induced SE (Seizure, b, d) or saline treatment (Control, a, c). These mice have the nestin CNS-specific second intronic enhancer directing GFP expression in neural progenitors. BrdU labeling and GFP expression are increased markedly in the inner granule cell layer of the pilocarpine-treated mouse (b, d). Also, note hilar ectopic GFPlabeled cells in the animal experiencing SE (arrows in d). BrdU was given on day 7 after pilocarpine or saline treatment. Scale bar 100 mm 40 S. Jessberger and J.M. Parent region of the adult brain, the subventricular zone (SVZ) of the lateral ventricles, is strongly upregulated after SE (Parent et al., 2002). Which cell type mediates this dramatic increase in dividing precursors? In the dentate area, the early proliferative response is associated with an increase of divisions of radial glia-like Type 1 cells (Huttmann et al., 2003) that show under normal conditions only very limited or no proliferative activity (Kronenberg et al., 2003; Seri et al., 2004; Suh et al., 2007) (Fig. 3.1c, d). Several days after the initial SE, the number of cycling cells that express the microtubuli-associated protein doublecortin (DCX) is strongly increased (Jessberger et al., 2005) (Fig. 3.3a, b). DCX-expressing progenitors might represent committed neuroblasts, suggesting the potential aberrant proliferation of late stages of neural progenitors induced by SE. However, it remains poorly understood whether prolonged seizure activity recruits quiescent progenitors or instead enhances the division of the population of normally cycling cells that were active even before the initial SE. Supporting the latter possibility, BrdU labeling prior to SE showed that most of the proliferating cells that respond to seizure activity were already mitotically active prior to the insult (Parent et al., 1999). Other studies support the recruitment of additional precursors in response to SE (Huttmann et al., 2003). Importantly, the number of dividing progenitors is only transiently increased as cell proliferation returns to control levels approximately 4 weeks after SE (Parent et al., 1997; Jessberger et al., 2007a). Recent studies suggest that at late time points after SE the number of dividing cells might be dramatically reduced compared to control animals (Hattiangady et al., 2004), suggesting that structural alterations associated with epilepsy might chronically impair NSC function in the epileptic hippocampus. Alternatively, SE might “exhaust” the NSC pool leading to a loss of available progenitors. However, there are also recent studies using different mTLE models that found no decrease of neurogenesis even in the chronic phase of the disease, suggesting that the rodent model used represents a critical factor (Bonde et al., 2006; Jessberger et al., 2007a). Interestingly, the severity and also the duration of seizures do not seem to be a major determinant in the proliferative response of precursors in the dentate gyrus. In fact, even single discharges strongly induce cell proliferation (Bengzon et al., 1997), suggesting that one wave of excitation is sufficient to induce cell proliferation. However ,and in contrast to the proliferative response of dentate gyrus NSCs, the survival of seizure-generated granule cells (see below) is associated with seizure severity (Mohapel et al., 2004), which seems to be mediated by the inflammatory response of the epileptic hippocampus (Ekdahl et al., 2003). What are the molecular mechanisms that lead to enhanced proliferation of dividing cells after SE? Even under normal conditions, the interplay between pre-existing neural networks and the activity of NSCs is poorly understood. However, recent evidence suggests that NSCs are capable of “sensing” electrical activity (Deisseroth et al., 2004), which might involve activation of glutamate and GABA receptors expressed by dentate NSCs (Gould et al., 1994; Tozuka et al., 2005). NSC prolifera‑ tion could also be affected by elevated expression of trophic factors such as brainderived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) 3 Adult Neurogenesis in Epilepsy 41 by surrounding tissue (Isackson et al., 1991; Gall, 1993; Newton et al., 2003; Warner-Schmidt and Duman, 2007). Changes in histone acetylation induced by seizures might be an additional mechanism involved in seizure-induced cell prolif‑ eration (Huang et al., 2002; Jessberger et al., 2007b). Given the diverse effects of SE on multiple structural and molecular levels in the epileptic hippocampus (Elliott and Lowenstein, 2004), it seems most plausible that a combination of mechanisms is responsible for seizure-induced cell proliferation. 3.1.2 Effects of SE on Maturation and Integration of Newborn Granule Cells Under normal conditions, only a subset (25–40%) of immature neurons survives an activity-dependent selection process and becomes lastingly integrated into the dentate circuitry (Kempermann et al., 2003; Tashiro et al., 2006). Thus, heightened proliferation of precursors after SE does not necessarily translate into increased numbers of newborn neurons. However, the increase in NSC proliferation is, in most rodent models, closely reflected by a robust increase in survival of newborn granule cells, even though the survival appears to be associated with seizure severity (Mohapel et al., 2004). Approximately 75–90% of all newborn cells express neuronal markers characteristic of granule cells 4 weeks after labeling dividing cells with BrdU or retroviral reporter vectors, which is comparable to the rate of neuronal differentiation in control animals (Parent et al., 1997; Jessberger et al., 2005, 2007b). Importantly, seizure-generated neurons show striking differences in neuronal differentiation, migration, morphological maturation, and functional integration compared to new neurons that were born under control conditions. These differ‑ ences might be the key to understanding the role of adult neurogenesis in the context of epilepsy. New granule cells that are born under normal conditions undergo distinct maturation steps during which they show enhanced excitability (Schmidt-Hieber et al., 2004) before they become indistinguishable from cells born during embryonic or early postnatal development (Laplagne et al., 2006; Ge et al., 2007). The speed of neuronal maturation and synaptic integration is precisely controlled. For example, the first dendritic spines are observed approximately 16 days after cells are born (Zhao et al., 2006; Toni et al., 2007). Interestingly, seizure activity seems to accelerate the speed of neuronal maturation and integration (Overstreet-Wadiche et al., 2006). The underlying cause for that acceleration is not fully understood but it seems plausible to speculate that higher levels of synaptic activity as well as alterations in growth factor expression in the dentate gyrus of rodents that have experienced seizures might contribute to the faster maturation process. In addition to faster maturation, a subset of granule cells born after SE also extends abnormal dendritic processes toward the hilus (Fig. 3.2), called hilar basal dendrites (HBDs) (Ribak et al., 2000; Dashtipour et al., 2003; Shapiro et al., 2005; Shapiro and Ribak, 2006). Under normal conditions, basal dendrites are only 42 S. Jessberger and J.M. Parent Fig. 3.2 Neurogenesis following KA-induced seizures is largely aberrant. Four-week-old newborn granule cells that were labeled with a retrovirus expressing GFP after stereotactic injection of the virus into the adult dentate gyrus. Note the typical highly polarized morphology with a single apical dendrite (arrows in left panel ) oriented toward the molecular layer (left panel ). In striking contrast, KA-induced seizures lead in addition to the apical dendrite (arrows in right panel ) to the extension of a basal dendrite (arrowheads in right panel ). Aberrant hilar basal dendrites are covered with spines (blue arrows in inset) and synaptically integrate into the dentate circuitry. The 4-week-old example shown in the right panel was born 1 week after the initial SE. GCL granule cell layer, ML molecular layer. Scale bar 20 mm transiently formed and do not become synaptically integrated (Seress and Pokorny, 1981). In contrast, SE leads to the rapid development of synapse formation onto HBDs (Shapiro et al., 2007). As cells extending HBDs mature, they form dendritic spines (Fig. 3.2b), indicating aberrant synaptic integration into the hilar region of the dentate gyrus (Jessberger et al., 2007a; Walter et al., 2007). HBDs persist for long durations after SE and even a fraction of the cells that were born 2 months after the initial SE extend abnormal dendrites (Jessberger et al., 2007a). Interestingly, there seems to be a critical period during which newborn granule cells are suscep‑ tible to form aberrant dendritic processes, as cells born prior to SE do not appear to extend HBDs (even though differences between species and TLE models might exist) (Jakubs et al., 2006; Jessberger et al., 2007a; Walter et al., 2007). The signals 3 Adult Neurogenesis in Epilepsy 43 responsible for the formation of HBDs or why cells are vulnerable only during a certain developmental stage are not understood. Importantly, previous studies showed that the aberrant circuitry formed by HBDs might be a critical component in the formation of a hyperexcitable state in the hippocampus after SE (Austin and Buckmaster, 2004; Patel et al., 2004). But not only dendrites show abnormal growth after SE. In addition, axonal processes extending from dentate granule cells (called mossy fibers) show a dramatic sprouting within the hilus but also into the inner molecular layer that might also contribute to a hyperexcitable network. Early studies had already shown that newborn neurons do not substantially contribute to mossy fiber sprouting (Parent et al., 1999). Indeed, only neurons born at least 4 weeks prior to SE grow aberrant axons into the inner molecular layer, whereas granule cells born after SE showed no substantial signs of abnormal axonal growth (Jessberger et al., 2007a). Apparently, cell intrinsic properties that are yet to be identified prevent axonal sprouting of granule cells born after SE whereas aberrant dendritic growth only occurs in cells born after SE. The identification of the underlying mechanisms of aberrant neurite extension after SE might not only be helpful to understand seizureassociated structural alterations but might also help us better understand basic mechanisms of dendritic and axonal pathfinding and growth. SE also leads to the ectopic migration of a substantial number of newborn granule cells into the hilus, which under normal conditions only occurs rarely (Parent et al., 1997; Scharfman et al., 2000, 2007) (Figs. 3.1, 3.3, and 3.4). Despite their abnormal location, ectopic granule cells synaptically integrate into the dentate-CA3 circuitry and become partially synchronized with pyramidal cells in area CA3 (Scharfman et al., 2000, 2002; Pierce et al., 2007). Given this integration pattern, ectopic gran‑ ule cells are suspected to contribute importantly to the formation of a recurrent excitatory network after SE (Parent and Lowenstein, 2002; Scharfman and Gray, 2007). Why SE causes ectopic migration of newborn granule cells away from the granule cell layer into the hilus is not well understood. Interestingly, cell death in the hilar region, which is observed in many rodent models of mTLE, does not seem to be a major factor regulating aberrant migration (Jiao and Nadler, 2007) even though the frequency of seizures correlates well with the number of ectopic cells (McCloskey et al., 2006). SE seems to induce abnormal chain migration of granule cell progenitors towards the hilus (Parent et al., 2006) (Figs. 3.3 and 3.4). Interestingly, SE dramatically alters reelin signaling (Rice and Curran, 2001). Reelin is a glycoprotein secreted by Cajal Retzius cells during embryonic develop‑ ment, and probably by GABAergic interneurons during postnatal life, that appears to be involved in granule cell migration in the healthy but also epileptic dentate gyrus (Gong et al., 2007). Furthermore, altered reelin signaling has been implicated in the development of granule cell dispersion, a widening of the granule cell layer, which is commonly observed in human samples of mTLE and some rodent models (Haas et al., 2002; Heinrich et al., 2006). Again, the identification of the molecular mechanisms underlying ectopic migration of dentate gyrus granule cells after SE might not only be interesting from a disease perspective but might also help us to understand basic mechanisms of neuronal migration in the adult brain. 44 S. Jessberger and J.M. Parent Fig. 3.3 Ectopic granule cells in experimental and human TLE. (a, b) Doublecortin (DCX) immunoreactivity in adult rat dentate gyrus 14 days after saline treatment (Control, a) or pilocarpine-induced SE (b) shows chains of immature neurons extending into the hilus of the epileptic rat (arrows in b) but not the control. The dashed white line denotes the hilar (h)/granule cell layer (gcl) border. (c, d) NeuN immunoreactivity in dentate gyri from a human autopsy control (c) and a patient with mTLE (d) shows gcl dispersion and hilar ectopic granule-like neurons (arrows in d) only in the patient with mTLE who had mesial temporal sclerosis (MTS). Arrowheads (c) point to larger, NeuN-immunoreactive hilar neurons in the control tissue that are absent in the mTLE subject. ml, molecular layer. Scale bar (a, b) 25 mm, (c, d) 75 mm 3.1.3 Functional Significance of Adult Neurogenesis Seizure activity in rodent models of mTLE dramatically stimulates the generation of newborn neurons. However, several highly controversial issues with unanswered questions remain. For example, what is the situation in mTLE patients compared to rodent models? Second, is increased neurogenesis an attempt of the injured brain to repair itself? Finally, do some or most seizure-generated neurons contribute to the disease process and further damage the epileptic brain? In contrast to rodent models of mTLE in which elevated levels of cell proliferation are undisputable, the situation in humans remains much less clear. Recent data failed to provide evidence of increased hippocampal neurogenesis in samples of adult mTLE patients (Fahrner et al., 2007) even though the numbers of neural progenitors seemed to be increased (Blumcke et al., 2001; Crespel et al., 2005). 3 Adult Neurogenesis in Epilepsy 45 Fig. 3.4 Aberrant integration of adult-born neurons after SE alters dentate gyrus circuitry. Under control conditions, neurogenic precursors in the adult hippocampus are restricted to the subgranular zone (SGZ) of the dentate gyrus (red cells). Dividing cells mainly give rise to glutamatergic granule cells that extend dendrites into the molecular layer and send their axons toward area CA3 (green cell). In experimental mTLE (bottom panel), seizures increase the number of proliferating cells (red cells). Furthermore, SE alters the integration of differentiating neurons. A substantial number of granule cells extend persistent HBDs that synaptically integrate and thus might change the functional connectivity of the dentate circuitry. In addition, neuroblasts show a chain-like migration toward the hilus, leading to the ectopic localization of newborn granule cells in the hilus. Ectopic granule cells appear to be synchronized with pyramidal cells in area CA3 and might thus form a recurrent excitatory network contributing to the process of epileptogenesis. ML molecular layer, GCL granule cell layer, SGZ subgranular zone In contrast, other groups found enhanced cell proliferation early in the disease process (Takei et al., 2007). Several reasons complicate the conclusive interpreta‑ tion of available data. First, all analyses rely completely on endogenous markers of cell proliferation (such as Ki67) or neuroblasts (such as DCX) as no tissue with BrdU labeling is available. Furthermore, the time point of the disease process, typi‑ cally late stages, during which the human samples were derived might be an impor‑ tant factor, because in rodent models of mTLE the severity of seizures and the duration after the first seizure influences the total number of newborn neurons (Hattiangady et al., 2004; Mohapel et al., 2004). It seems plausible to speculate that at least some of the structural alterations in human mTLE samples instead represent the end point of the disease process. Neurogenesis in human mTLE samples could be significantly different at early disease stages where material for experimental analyses is rare. 46 S. Jessberger and J.M. Parent Interestingly, a substantial fraction of granule cells in the dentate gyrus of humans and primates extends HBDs (approximately between 10 and 25%, respectively) (Seress, 1992), which is in striking contrast to rodent granule cells that hardly form HBDs under normal conditions (Jessberger et al., 2007a; Walter et al., 2007). Thus, synaptic coupling of HBDs with hilar structures might be a normal feature of the human dentate area. However, there is evidence that the number of granule cells extending HBDs in humans is actually increased in tissue from epileptic patients (Franck et al., 1995; von Campe et al., 1997), suggesting that, despite the occurrence of HBDs in the normal human hippocampus, seizures might affect the synaptic connectivity through additional extension of HBDs from newborn neurons. Without a doubt, the simple transfer of data obtained using rodent models to the human situation in mTLE is not free of problems. Therefore, conclusions derived from animal experiments have to be drawn cautiously (Scharfman and Gray, 2007). A potential contribution of seizure-induced neurogenesis to the cause or progression of mTLE in humans is unclear. The potential function and significance of seizure-induced neurogenesis remains controversial in the context of rodent models of mTLE. In addition to epileptogenesis, changes in neuronal birth may be implicated in a variety of mTLE comorbidities such as cognitive impairment and depression (Helmstaedter, 2002). On the other hand, increased neurogenesis after SE might be a regenerative attempt of the brain to balance excess excitation or to repair itself. What is the evidence that favors a role for seizure-generated neurons as part of the disease or in contrast as an endogenous repair mechanism after SE? As outlined above, SE leads not only to many more newborn neurons but also induces structural alterations of newborn granule cells (Fig. 3.4). Thus, ectopic or aberrant new granule cells could alter the connectivity of the hippocampal circuitry. Aberrant neurogenesis could contribute to the process of epileptogenesis (Dalby and Mody, 2001; Magloczky and Freund, 2005) by establishing recurrent excitatory networks (Parent, 2002; Scharfman et al., 2007; Scharfman and Gray, 2007; Scharfman and Hen, 2007). Seizure-generated granule cells show two features that support an epileptogenic role. First, those with HBDs receive excitatory input from mossy fibers (Shapiro and Ribak, 2006; Jessberger et al., 2007a). Similarly, ectopic granule cells are abnormally synchronized with spontaneous, rhythmic bursts of CA3 pyramidal neurons (Scharfman et al., 2000). Indeed, several studies found reduced levels of spontaneous recurrent seizures when aberrant neurogenesis was blocked in rodent SE models (Jung et al., 2004, 2006). In contrast, there is also recent evidence that the properties of cells born after SE might change in a manner that compensates for loss of inhibition (Jakubs et al., 2006). Jakubs and colleagues found that seizure-generated granule cells with a regular morphology and located within the granule cell layer had reduced excit‑ ability compared to cells born in running rats, suggesting that seizure-induced neurogenesis might be an attempt to counterbalance excess excitation. Supporting this assumption, brain irradiation suppressing neurogenesis was found to enhance excitability in a kindling model of epilepsy, suggesting that neurogenesis might in fact reduce the levels of excitability in the epileptic dentate gyrus (Raedt et al., 2007). The mechanisms by which new granule cells might reduce overall levels of 3 Adult Neurogenesis in Epilepsy 47 excitability remain unclear and the findings require validation in additional epilepsy models. Thus, apparently contradictory studies exist at this point in the literature. Clearly, the answer to the question of what role seizure-generated neurons play in the context of epileptogenesis requires more specific and controllable methods to inhibit seizure-induced neurogenesis, and this approach should be taken using multiple rodent models of mTLE. In addition to a role of seizure-induced neurogenesis in epileptogenesis, new neurons might alter the connectivity of the dentate gyrus circuitry resulting in an impairment of normal synaptic transmission. This is especially interesting given the fact that mTLE has a high comorbidity with cognitive impairment (Helmstaedter et al., 2003; Elger et al., 2004). Supporting an involvement of new neurons in the context of SE-associated deficits in cognition, reduced levels of seizure-induced neurogenesis rescued hippocampal function after SE (Jessberger et al., 2007b; Pekcec et al., 2008). However, there is no doubt that these data are far from conclusive as even under normal conditions the functional significance of adult hippocampal neu‑ rogenesis remains unclear and controversial (Shors et al., 2001, 2002; Santarelli et al., 2003; Snyder et al., 2005; Meshi et al., 2006; Saxe et al., 2006, 2007; Winocur et al., 2006). In addition, altered neurogenesis after SE might also contribute to later effects of the disease. Interestingly, a relatively high number of mTLE patients develop major depression. As outlined above, neurogenesis might be reduced in the chronic phase of the epilepsy (Hattiangady et al., 2004). Because reduced levels of newborn neurons in the dentate gyrus are associated with depression (Santarelli et al., 2003), exhaustion of the stem cell compartment might link epilepsy with the develop‑ ment of affective syndromes. Currently, however, the link between altered neurogenesis and depression remains largely speculative. 3.2 Summary The dramatic alteration of hippocampal neurogenesis in animal models of mTLE challenges our conceptual understanding of the cause and consequences of epilepsy. Even though many features of new granule cells born after SE are abnormal, such as extension of HBDs and ectopic migration into the hilus, the link between epilep‑ togenesis or seizure-associated cognitive impairment with aberrant neurogenesis remains unproven. The main question is whether new neurons are good or bad for the epileptic brain. In fact, seizure-generated granule cells might play a dual role depending on the progression of the disease state and the underlying cause. To solve this problem, we will need a better basic characterization of the structural and electrical alterations of seizure-induced neurogenesis. Furthermore, we will have to identify the cellular and molecular mechanisms underlying seizure-induced neuro‑ genesis that will potentially also bring us one step further toward understanding general aspects of life-long plasticity in the adult brain. In any case, seizure-induced neurogenesis might turn out to be a promising target in the treatment of human epilepsy and its comorbidities. 48 S. Jessberger and J.M. Parent References Austin JE, Buckmaster PS (2004) Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J Comp Neurol 476:205–218. Ben-Ari Y (2001) Cell death and synaptic reorganizations produced by seizures. Epilepsia 42:5–7. Bengzon J, Kokaia Z, Elmér E, Nanobashvili A, Kokaia M, Lindvall O (1997) Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci USA 94:10432–10437. Blumcke I, Schewe JC, Normann S, Brustle O, Schramm J, Elger CE, Wiestler OD (2001) Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus 11:311–321. Blume WT (2006) The progression of epilepsy. Epilepsia 47 Suppl 1:71–78. Bonde S, Ekdahl CT, Lindvall O (2006) Long-term neuronal replacement in adult rat hippocampus after status epilepticus despite chronic inflammation. Eur J Neurosci 23:965–974. Buckmaster PS (2004) Laboratory animal models of temporal lobe epilepsy. Comp Med 54:473–485. Crespel A, Rigau V, Coubes P, Rousset MC, de Bock F, Okano H, Baldy-Moulinier M, Bockaert J, Lerner-Natoli M (2005) Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis 19:436–450. Dalby NO, Mody I (2001) The process of epileptogenesis: a pathophysiological approach. Curr Opin Neurol 14:187–192. Dashtipour K, Wong AM, Obenaus A, Spigelman I, Ribak CE (2003) Temporal profile of hilar basal dendrite formation on dentate granule cells after status epilepticus. Epilepsy Res 54:141–151. Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC (2004) Excitationneurogenesis coupling in adult neural stem/progenitor cells. Neuron 42:535–552. Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003) Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA 100:13632–13637. Elger CE, Helmstaedter C, Kurthen M (2004) Chronic epilepsy and cognition. Lancet Neurol 3:663–672. Elliott RC, Lowenstein DH (2004) Gene expression profiling of seizure disorders. Neurochem Res 29:1083–1092. Encinas JM, Vaahtokari A, Enikolopov G (2006) Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA 103:8233–8238. Engel J, Jr. (1996) Surgery for seizures. N Engl J Med 334:647–652. Fahrner A, Kann G, Flubacher A, Heinrich C, Freiman TM, Zentner J, Frotscher M, Haas CA (2007) Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol 203:320–332. Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA (1995) Physiologic and morphologic char‑ acteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia 36:543–558. Gage FH (2002) Neurogenesis in the adult brain. J Neurosci 22:612–613. Gall CM (1993) Seizure-induced changes in neurotrophin expression: implications for epilepsy. Exp Neurol 124:150–166. Ge S, Yang CH, Hsu KS, Ming GL, Song H (2007) A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54:559–566. Gong C, Wang TW, Huang HS, Parent JM (2007) Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci 27:1803–1811. Gould E, Cameron HA, McEwen BS (1994) Blockade of NMDA receptors increases cell death and birth in the developing rat dentate gyrus. J Comp Neurol 340:551–565. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260–265. 3 Adult Neurogenesis in Epilepsy 49 Gray WP, Sundstrom LE (1998) Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res 790:52–59. Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, Pollak S, Zentner J, Frotscher M (2002) Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci 22:5797–5802. Hattiangady B, Rao MS, Shetty AK (2004) Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis 17:473–490. Heinrich C, Nitta N, Flubacher A, Muller M, Fahrner A, Kirsch M, Freiman T, Suzuki F, Depaulis A, Frotscher M, Haas CA (2006) Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci 26:4701–4713. Helmstaedter C (2002) Effects of chronic epilepsy on declarative memory systems. Prog Brain Res 135:439–453. Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE (2003) Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol 54:425–432. Huang Y, Doherty JJ, Dingledine R (2002) Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci 22:8422–8428. Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhauser C, Gray WP (2003) Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci 18:2769–2778. Isackson PJ, Huntsman MM, Murray KD, Gall CM (1991) BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron 6:937–948. Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O (2006) Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron 52:1047–1059. Jessberger S, Romer B, Babu H, Kempermann G (2005) Seizures induce proliferation and disper‑ sion of doublecortin-positive hippocampal progenitor cells. Exp Neurol 196:342–351. Jessberger S, Zhao C, Toni N, Clemenson GD, Jr., Li Y, Gage FH (2007a) Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci 27:9400–9407. Jessberger S, Nakashima K, Clemenson GD, Jr., Mejia E, Mathews E, Ure K, Ogawa S, Sinton CM, Gage FH, Hsieh J (2007b) Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci 27:5967–5975. Jiao Y, Nadler JV (2007) Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol 205:569–582. Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK (2004) Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpineinduced status epilepticus. Eur J Neurosci 19:3219–3226. Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK (2006) Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis 23:237–246. Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH (2003) Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130:391–399. Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467:455–463. 50 S. Jessberger and J.M. Parent Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF (2006) Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol 4:e409. Magloczky Z, Freund TF (2005) Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci 28:334–340. McCloskey DP, Hintz TM, Pierce JP, Scharfman HE (2006) Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci 24:2203–2210. Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R (2006) Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci 9:729–731. Mohapel P, Ekdahl CT, Lindvall O (2004) Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol Dis 15:196–205. Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS (2003) Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci 23:10841–10851. Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL (2006) Seizures accelerate functional integration of adult-generated granule cells. J Neurosci 26:4095–4103. Parent JM (2002) The role of seizure-induced neurogenesis in epileptogenesis and brain repair. Epilepsy Res 50:179–189. Parent JM, Lowenstein DH (2002) Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog Brain Res 135:121–131. Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997) Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorga‑ nization in the adult rat hippocampus. J Neurosci 17:3727–3738. Parent JM, Tada E, Fike JR, Lowenstein DH (1999) Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci 19:4508–4519. Parent JM, Valentin VV, Lowenstein DH (2002) Prolonged seizures increase proliferating neuro‑ blasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci 22:3174–3188. Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH (2006) Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol 59:81–91. Patel LS, Wenzel HJ, Schwartzkroin PA (2004) Physiological and morphological characterization of dentate granule cells in the p35 knock-out mouse hippocampus: evidence for an epileptic circuit. J Neurosci 24:9005–9014. Pekcec A, Fuest C, Muhlenhoff M, Gerardy-Schahn R, Potschka H (2008) Targeting epilepto‑ genesis-associated induction of neurogenesis by enzymatic depolysialylation of NCAM counteracts spatial learning dysfunction but fails to impact epilepsy development. J Neurochem 105:389–400 Pierce JP, Punsoni M, McCloskey DP, Scharfman HE (2007) Mossy cell axon synaptic contacts on ectopic granule cells that are born following pilocarpine-induced seizures. Neurosci Lett 422:136–140. Raedt R, Boon P, Persson A, Alborn AM, Boterberg T, Van Dycke A, Linder B, De Smedt T, Wadman WJ, Ben-Menachem E, Eriksson PS (2007) Radiation of the rat brain suppresses seizure-induced neurogenesis and transiently enhances excitability during kindling acquisition. Epilepsia 48:1952–1963. Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV (2000) Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol 428:240–253. Rice DS, Curran T (2001) Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci 24:1005–1039. Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. 3 Adult Neurogenesis in Epilepsy 51 Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 103:17501–17506. Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R (2007) Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci USA 104:4642–4646. Scharfman HE, Gray WP (2007) Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia 48 Suppl 2:33–41. Scharfman HE, Hen R (2007) Neuroscience. Is more neurogenesis always better? Science 315:336–338. Scharfman HE, Goodman JH, Sollas AL (2000) Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implica‑ tions of seizure-induced neurogenesis. J Neurosci 20:6144–6158. Scharfman HE, Sollas AL, Goodman JH (2002) Spontaneous recurrent seizures after pilocarpineinduced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience 111:71–81. Scharfman H, Goodman J, McCloskey D (2007) Ectopic granule cells of the rat dentate gyrus. Dev Neurosci 29:14–27. Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187. Scott BW, Wang S, Burnham WM, De Boni U, Wojtowicz JM (1998) Kindling-induced neurogenesis in the dentate gyrus of the rat. Neurosci Lett 248:73–76. Seress L (1992) Morphological variability and developmental aspects of monkey and human granule cells: differences between the rodent and primate dentate gyrus. Epilepsy Res Suppl 7:3–28. Seress L, Pokorny J (1981) Structure of the granular layer of the rat dentate gyrus. A light micro‑ scopic and Golgi study. J Anat 133:181–195. Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21:7153–7160. Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A (2004) Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol 478:359–378. Shapiro LA, Ribak CE (2006) Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res 69:53–66. Shapiro LA, Korn MJ, Ribak CE (2005) Newly generated dentate granule cells from epileptic rats exhibit elongated hilar basal dendrites that align along GFAP-immunolabeled processes. Neuroscience 136:823–831. Shapiro LA, Figueroa-Aragon S, Ribak CE (2007) Newly generated granule cells show rapid neuroplastic changes in the adult rat dentate gyrus during the first five days following pilocarpine-induced seizures. Eur J Neurosci 26:583–592. Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376. Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E (2002) Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12:578–584. Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM (2005) A role for adult neurogenesis in spatial long-term memory. Neuroscience 130:843–852. Suh K, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH (2007) In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1:515–528. Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L (1989) Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 26:321–330. Takei H, Wilfong A, Yoshor D, Armstrong DL, Bhattacharjee MB (2007) Evidence of increased cell proliferation in the hippocampus in children with Ammon’s horn sclerosis. Pathol Int 57:76–81. 52 S. Jessberger and J.M. Parent Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH (2006) NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442:929–933. Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH (2007) Synapse formation on neurons born in the adult hippocampus. Nat Neurosci 10:727–734. Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T (2005) GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47:803–815. Van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci USA 96:13427–13431. von Campe G, Spencer DD, de Lanerolle NC (1997) Morphology of dentate granule cells in the human epileptogenic hippocampus. Hippocampus 7:472–488. von Lehe M, Lutz M, Kral T, Schramm J, Elger CE, Clusmann H (2006) Correlation of healthrelated quality of life after surgery for mesial temporal lobe epilepsy with two seizure outcome scales. Epilepsy Behav 9:73–82. Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC (2007) Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci 27:7541–7552. Warner-Schmidt JL, Duman RS (2007) VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA 104:4647–4652. Wiebe S, Blume WT, Girvin JP, Eliasziw M (2001) A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345:311–318. Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S (2006) Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16:296–304. Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26:3–11. Chapter 4 Stress Disorders Muriel Koehl, Michel Le Moal, and Djoher Nora Abrous Abstract Recent years have produced rapid and enormous growth in our understanding of the mechanisms regulating adult neurogenesis. Emerging fields of research now aim at clarifying the relationships between neurogenesis and dis‑ eases. This paper deals with neurogenesis and stress-related disorders. After a brief overview of the stress response, we first provide a comprehensive analysis of the literature on the effects of stress on neurogenesis and the potential mechanisms underlying these effects. This analysis clearly indicates that, whatever the nature, intensity and duration of the stressor, stress decreases cell proliferation. Comparing different stress paradigms further indicates that these effects are transient when stress is performed during adulthood, while it has a much greater and longer last‑ ing impact when applied during developmental periods. When uncontrolled or repeated, stressors are risk factors for physiological and psychiatric disorders such as depression, pathological aging, drug misuse, anxiety and post-traumatic stress disorder. Studies linking these pathologies and neurogenesis constitute an impor‑ tant field of research, but advances are hampered by the lack of adequate animal models. The challenge for future research will certainly be to develop these animal models in order to envision therapeutic strategies aimed at stimulating plasticity in the stressed brain and preventing the alteration of affective and cognitive function that may appear in some individuals. Studying and understanding the biological and psychological effects of stress are very important in the field of health psychology as research has shown a direct relationship between stress and diseases, such as depression, post-traumatic stress disorder (PTSD), anxiety disorders, pathological aging, drug addiction, etc. In this chapter, we will concentrate on the relationships between these stress-related M. Koehl, M. Le Moal, and D.N. Abrous (*) Inserm U862, Institut F. Magendie, University of Bordeaux 2, 146 Rue Leo-Saignat, 33077 Bordeaux Cedex, France e-mail: [email protected] T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_4, © Springer 2011 53 54 M. Koehl et al. disorders and adult neurogenesis. In the first part, we will briefly review the concept of stress, its theories, its models and its neurobiology. In the second part, we will propose a thorough analysis of the literature on the effects of stress on neurogenesis, and in the third part, we will analyze how neurogenesis can take part in stress pathologies. 4.1 The Concept of Stress Maintaining the body steady state, or homeostasis, is essential for life. This state is constantly challenged by intrinsic or extrinsic adverse forces, whether real or per‑ ceived, the stressors. Stress is thus defined as a state of threatened homeostasis or disharmony and is counteracted by a complex repertoire of physiological and behavioral responses that allow the organism to adapt to everyday challenges. 4.1.1 Stress, Homeostasis, and Allostasis 4.1.1.1 Historical Perspective Claude Bernard was the first to suggest that the “internal milieu” of the body is critically important for maintaining stable states within the body. As early as 1859, he argued that two different environments impact on the organism: a “general milieu,” which is the outside world, and an “internal milieu” where the elements of the living body find an optimal climate for operation. The central theses of his theory were that the body was required to respond to outside stimuli by bodily adjustments, and that this local constancy made the organism somewhat indepen‑ dent of exterior challenges (Bernard 1865). He considered that normal and patho‑ logical states were part of the same process, the pathological state resulting from a failure of the organism to maintain constancy. Walter Cannon later coined this concept homeostasis (Cannon 1929), which can be defined as “preserving constancy in the internal environment” or “remaining stable by staying the same.” He also added the intervention of the adrenal-medullasympathetic system as an essential homeostatic system that serves to restore dis‑ turbed homeostasis and to promote survival of the organism. Hans Selye later popularized the term “stress.” He elaborated his theory after he observed that expo‑ sure to diverse noxious agents elicited a common response, which he named the pathological triad, i.e., adrenal enlargement, gastrointestinal ulceration, and thymi‑ colymphatic involution (Selye 1936). He thus defined stress as the nonspecific response of the body to any demand, emphasizing that the same pathological triad, what he called the stress syndrome, would result from exposure to any stressor. He also introduced the hypothalamo–pituitary–adrenal (HPA) axis as a key effector of the stress response. Following his theory of stress, many controversies emerged, 4 Stress Disorders 55 that still exist, and several attempts have been made to streamline the different stress definitions (for review, see Le Moal 2007). For instance, Chrousos and Gold defined stress as a state of disharmony or of threatened homeostasis, evoking physiologically and behaviorally adaptive responses that can be specific to the stressor. If generalized, nonspecific or stereotypic, they produce a “nonspecific” stress syndrome when the threat to homeostasis exceeds a threshold (Chrousos and Gold 1992). More recently, McEwen introduced in stress research the concept of allostasis (McEwen 1998), originally proposed by Sterling and Eyer (1988). Allostasis can be defined as the process of achieving stability through physiological or behavioral change, or “remaining stable by being variable.” In contrast to homeostatic mecha‑ nisms, allostatic regulations are broader and do not depend on set-point mechanisms, signals are not constant, and anticipation of need is an important element. While homeostasis is a loco-local process that refers only to the systems that are essential for life (glucose levels, body temperature, tension, pH, etc.), allostasis is a multisys‑ tem process referring to the higher level systems that maintain the homeostatic systems in balance as environment and life history change. It thus refers to the active process of adaptation by production of adrenal steroids, catecholamines, cytokines, etc. If allostatic responses are efficient, adaptation occurs and the organism is protected from damage. In situations where allostatic responses are prolonged, inad‑ equate, overstimulated by repeated “hits” from multiple stressors, or if a lack of adaptation occurs, allostatic load results in maladaptation and damage to various organs, and pathologies develop (McEwen and Wingfield 2003). Within the framework of allostasis, one theory deserves attention as it empha‑ sizes the role of cognitive appraisal. Indeed, stress occurs within a particular rela‑ tionship between an individual and a specific environment (Folkman and Lazarus 1984). Thus, we have to consider the impact of many intrapsychic (personality) and interpersonal (social context and status) variables on the stress process, which can be considered as a person–environment transaction. These variables all feed into the individual’s “meaning-systems,” which in turn form part of the stress appraisal process. Within this theory, cognition is central to the process of “primary appraisal,” in which events are evaluated in terms of impact and meaning with respect to the individual’s goals and beliefs. Cognition is also involved in “second‑ ary appraisal,” which concerns evaluation of the available options for dealing with the perceived demands (coping). The cognitive–motivational–relational theory of Lazarus (Fig. 4.1) (Lazarus 1991) does three important things: it highlights the complexity of the stress process; it locates the process within the individual rather than in the environment; and it explicitly incorporates mental activity as a driving force in the stress process. By placing the individual and mental activity as the centerpiece of the stress response, this theory raises the issue of individual differences in stress responsiveness. Within this framework, genetic and epigenetic factors interplay to determine behavioral and brain responses to stress. Indeed, although genetic makeup accounts for a signifi‑ cant part of the variability in the activity of the stress system (Bouchard 1994; Plomin et al. 1994), adult patterns of stress reactivity can be permanently imprinted 56 M. Koehl et al. stressor Primary appraisal : is the stressor negative? Yes No No stress Secondary appraisal : can I control the situation? If coping ressources are adequate, then consider options: problem-focused or emotion-focused coping strategies Stress response Physiological component: arousal, hormone secretion Emotional component: anxiety, fear, grief, …. Behavioral component: problem-focused coping strategies aimed at reducing or eliminating the source of stress emotion-focused coping strategies aimed at reducing the emotional impact of the stressor Fig. 4.1 Schematic of the cognitive–motivational–relational theory of Lazarus by early influences. Thus, the intrauterine period, infancy, childhood, and adoles‑ cence are periods of increased plasticity for the stress system (Cicchetti and Cannon 1999; Nowakowski and Hayes 1999), and environmental challenges during these critical periods have profound effects on its later function, especially as it relates to pathophysiology. Another consequence of the central role of mental activity in the outcome of stressor exposure is that many of the behavioral consequences of stress can be modulated by stressor controllability/predictability. Thus, only exposure to an uncontrollable stressor, and not to a controllable stressor, results in a wide variety of behavioral outcomes, such as learned helplessness (Maier and Watkins 2005). 4.1.1.2 Models of “Stress” Several models have been developed to understand the neuronal changes that medi‑ ate stress effects, as well as their associated pathologies. These models can be clas‑ sified in relation to the level of arousal they induce. Indeed, according to Hennessy and Levine, and as stated by Koob (1999), “the construct of stress may represent the extreme pathologic continuum of overactivation of the body’s normal activa‑ tional or emotional systems, and thus is linked to the construct of arousal.” Thus, stressors can be grossly categorized as inducing physical stress, psychological 4 Stress Disorders 57 stress, and/or arousal. Models of physical stress consist in submitting animals to immobilization, electrical shock, pain, heat/cold, toxins, etc. Their main conse‑ quences in terms of pathology are the pathological triad defined by Selye. Adding a psychological dimension, psychosocial stressors involve a defeat (a frustration), classically evaluated in the dominant/subordinate or the resident/intruder paradigm, a fear (of a predator) or a conflict. Finally, stress can also result from enhanced arousal obtained by exposing animals to novelty, uncertainty, hunger and thirst. Modeling this enhanced arousal in animals is problematic since, after repeated application of a given stressor, an opponent process takes place. To overcome this problem, several models of multiple unpredictable chronic mild stress (UCMS), combining immobilization (cold), forced swim (cold), crowding, isolation, food and/or water deprivation, subordination stress, vibration, reversed light/dark cycle, and continuous overnight illumination, have been developed (Ducottet et al. 2003; Herman et al. 1995; Willner et al. 1987; Willner 2005). The emerging concept of individual differences in both stress responsiveness and stress appraisal has added a new dimension to these models which start to take into account personal factors influencing the stress response such as (1) experiential variables (previous stressor experiences, early life events, (2) personal characteris‑ tics, i.e., coping skills, personality, and (3) social context (for review, see Anisman and Matheson 2005). 4.1.2 The Stress Response: Neuroanatomy and Physiology 4.1.2.1 Neuroendocrine Effectors of the Stress Response Although the entire central nervous system is involved in the maintenance of the internal homeostasis and participates in the organization of the stress response, specific areas have critical roles in these mechanisms. Two distinct but interrelated systems are at play: the sympathetic/adrenomedullary system (SAM) and the hypothalamic–pituitary–adrenocortical (HPA) axis (Fig. 4.2). Briefly, SAM pro‑ vides a rapidly responsive mechanism to control a wide range of functions, includ‑ ing cardiovascular, respiratory, gastrointestinal, renal, and endocrine ones (Gilbey and Michael Spyer 1993). Its activation leads to the release of catecholamines, predominantly epinephrine (EPI) but also some norepinephrine (NE), by the medulla of the adrenal gland. EPI and NE play multiple roles in fight/flight reac‑ tions mainly by ensuring blood supply to the brain and muscles. However, neither EPI nor NE crosses the blood–brain barrier (BBB), but their peripheral action is paralleled in the brain by NE produced by the locus coeruleus (LC). The role of this brain locus in response to stress is mainly to support vigilance and arousal and nar‑ rowing attention, along with participation in processes that activate the HPA axis. The activation of the HPA axis leads to the release of glucocorticoids (GCs, cortisol in humans, corticosterone in most rodents) from the cortex of the adrenal gland. These hormones cross the BBB and can thus target the brain. Their production 58 M. Koehl et al. Threatening Signals Cognitive appraisal Thalamus Primary sensory cortex Orbitofrontal cortex Anterior cingulate LC BNST NE Amygdala - Raphe Nucleus 5-HT Hippocampus N. Accumbens, Subst. Nigra Gaba - DA Hypothalamus CRH, AVP - Pituitary gland Brain stem nuclei ACTH Spinal cord Adrenal gland Ach, NE Glucocorticoids NE, EPI Metabolic/Physiological/behavioral responses Fig. 4.2 Simplified neurobiological organization of the stress system: corticolimbic structures (anterior cingulate cortex and orbitofrontal cortex) relay information to subcortical structures. They are reciprocally interconnected with each other and with the amygdala, which itself projects to the hippocampus and the BNST. The BNST provides pathways into the paraventricular nucleus of the hypothalamus which releases CRH and AVP while the hippocampus and cortical areas maintain feedback control of the PVN 4 Stress Disorders 59 takes time (~25 min to peak levels) and their impact continues for long periods (several hours). The activating pathway whereby the brain translates stimuli into an integrated response at the hypothalamic level involves neuronal inputs from amin‑ ergic and cholinergic nuclei that converge into the parvocellular neurons of the paraventricular nucleus (PVN) in the hypothalamus (Owens and Nemeroff 1991). These neurons express two secretagogues of the HPA axis, corticotrophin-releasing hormone (CRH) and arginine-vasopressin (AVP). Both are released from the median eminence into the hypophysal portal system to reach the anterior pituitary where they stimulate the proopiomelanocortin (POMC) producing cells to release adrenocorticotropin hormone (ACTH). While the binding of CRH on CRH-R1 receptors of the corticotrophs is permissive for the secretion of ACTH, AVP acts as a potent synergistic factor of CRH with little ACTH secretagogue activity by itself (Abou-Samra et al. 1987; Gillies et al. 1982). Once released into the general blood‑ stream, ACTH reaches the adrenal cortex where it causes steroidogenesis and increase of plasma GC levels, the final effectors of the HPA axis. These hormones are pleiotropic and exert their effects through ubiquitously distributed intracellular receptors. They have a strong catabolic action and thus increase lipolysis as well as glycogenolysis, which increase blood glucose levels. Together, these actions assist the organism under stressful conditions by increasing the availability of energy sub‑ strates. GR also suppress immunological responses, thus reducing the occurrence of inflammation at a time when mobility may be important (Munck et al. 1984). However, continued or repeated exposure to elevated GC levels can present a serious risk for the organism (Sapolsky 1996). Therefore, inhibition of steroid secre‑ tion in order to limit the duration of tissue exposure to GC, thus minimizing the cata‑ bolic, lipogenic, immunosuppressive effects of these hormones, is an important feature of the system. The termination of stress response operates through feedback mechanisms involving binding of circulating GC with at least two distinct types of corticosteroid receptors (de Kloet and Reul 1987; Reul and de Kloet 1986). The type I or mineralocortioid receptor (MR) responds to low levels of corticosterone due to its high affinity for GCs. The type II or GC receptor binds corticosterone with lower affinity. Because of their differential affinities for GCs, MRs are almost fully occu‑ pied by basal levels of GCs while GRs are occupied only at the peak of the circadian cycle or under stress conditions. Thus, GRs mediate most of the stress effects of GCs whereas MRs tend to mediate most basal effects. Importantly, these basal effects play a permissive role in stress by allowing NE and EPI from SAM activation to have maximal impacts on their target tissue, allowing effective fight/flight responses (de Kloet 2000). 4.1.2.2 Controlling the Stress Response Many systems are involved in controlling the stress response, thus insuring stress adaptation (for review, see Joca et al. 2007). Briefly, reacting to stressors, especially in the case of psychological ones, first requires appraisal. This involves corticolim‑ bic structures such as the cingulate cortex and the orbitofrontal cortex, which are 60 M. Koehl et al. reciprocally interconnected. These structures relay information to subcortical structures such as the amygdala. This brain locus sustains fear-related behaviors, and its acti‑ vation is important for emotional analysis of all the relevant stored information for any given stressor. Indeed, strong emotions lead to “flash-bulb” memories, e.g., remembering the location and events associated with a very positive or very nega‑ tive life event. Both catecholamines acting via b-adrenergic receptors (Cahill et al. 1994) and GC hormones play an important role in establishing these long-lasting memories (McGaugh 2000). They also involve the hippocampus that helps us remember “where we were and what we were doing” at the time the amygdala is turned on (Roozendaal 2000). Thus, EPI and GC promote the memory of events and situations that in the future may be dangerous. In the long run, however, when chronicity installs, stress hormones can contribute to impairments in cognitive functions and promote damage to the hippocampus (Sapolsky 1992). The sensitiv‑ ity of the hippocampus to stress-induced damage certainly relies on its richness in corticosteroid receptors, since as such it is the preferred brain target of these hor‑ mones that play a highly significant role in GC feedback (Herman et al. 1989, 1990; Jacobson and Sapolsky 1991). However, this role has been recently disputed, and it has been suggested that the hippocampus is not necessary for tonic inhibition of adrenocortical activity, which implies that the HPA axis also receives efficient negative feedback inhibition from other brain systems (Tuvnes et al. 2003). For instance, high densities of GC receptors are present in several extrahippocampal structures (e.g., the prefrontal cortex, the lateral septum, the bed nucleus of the stria terminalis, the PVN) that provide additional negative feedback inhibition of the HPA axis (Herman and Cullinan 1997). Finally the mesocorticolimbic dopaminer‑ gic (DA) system deserves attention as its projection to the prefrontal cortex is thought to centrally suppress the response of the stress system and would be impli‑ cated in anticipatory phenomena and cognitive functions (Roth et al. 1988). The mesoaccumbens projection plays a pivotal role in motivational/reinforcement/ reward phenomena and in the formation of the central DA “reward” system (Koob and Le Moal 2001). 4.1.3 Conclusion In conclusion, the concept of stress is complex and depends upon several genetic and environmental factors that modulate its outcome. In consequence, a plethora of animal models have been developed to understand the neuronal changes that mediate stress effects. Alterations of adult neurogenesis are part of these neuronal changes, which could lead to pathologies. In the following section, we will review the impact of stress on cell proliferation, survival and differentiation, whatever the nature, intensity, or duration of the stressor (see also Table 4.1). Considering the factors involved in sustaining and restraining the stress response (see above), we will also review papers linking these factors with altered neuro‑ genesis in response to stressors. 45 min 3 h 45 min 45 min 50 min 20 min 1 h 4°C + 30 m 25°C 1 × Formalin IS IS IS Restraint Restraint Restraint Restraint Restraint + FS Foot-shock Foot-shock Foot-shock Pain Restraint 3 h Restraint Rat Rat Rat Rat Rat Rat Mice Rat Rat Mice Rat Rat Rat SD Wistar SD SD Wistar SD C57Bl/6J SD SD C57Bl/6J SD SD SD M M M M M M M M M M M M M ~240 g 3 M 3 M 7–8 W 10 W ~240 g ~28 g 3 M ~300 g – 7–8 W ~325 g Cells born1W before IS: 0 BrdU ↓ BrdU ↓ BrdU (SVZ: 0) 0 Ki-67 – – – – 0 BrdU ↓ BrdU (SVZ: 0) ↓ BrdU (SVZ: 0) ↓ BrdU (hilus too) ↓ BrdU – ↓ BrdU (SVZ: 0) ↑ BrdU (SVZ: 0) ↓ BrdU – – – 0 BrdU 0 Ki67 %BrdU-NeuN: 0 %BrdU-GFAP: 0 – – – – – – – – Table 4.1 Influence of stressful experience on cell proliferation, cell survival and cell differentiation within the neurogenic zones of the adult brain Paradigm Condition Species Strain Sex Age/weight Proliferation Survival Differentiation Acute physical stress Restraint 2 or 6 h Rat SD M ~270 g 0 BrdU – – (continued) Pham et al. (2003) Rosenbrock et al. (2005) Duric and McCarson (2006) Bain et al. (2004) Vollmayr et al. (2003) Koo and Duman (2008) Lagace et al. (2006) Heine et al. (2004) Duric and McCarson (2006) Pham et al. (2005) Dagyte et al. (2009) Koo and Duman (2008) References Rat Foot-shock 14 D (6 h/D) 10 D (45 m/D) 1 W (4 h/D) 14 D (6 h/D) 21 D (6h/D) CRS CRS CRS CRS CRS 6 W (6 h/D) IS or ES IS or ES Chronic physical stress CRS 3 W (6 h/D) Foot-shock SD Rat Rat Rat Rat Rat Wistar SD SD SD SD SD Rat Rat SD Rat Wistar SD Rat IS Strain SD Species Rat Table 4.1 (continued) Paradigm Condition Foot-shock IS ES Foot-shock IS ES Foot-shock IS M M M M M M MF M M M Sex M ~210 g ~210 g ~210 g 7–8 W ~325 g ~270 g ~385 g ~300 g ~300 g ~300 g ~280 g Age/weight ~200 g – – 7 D: ↓ BrdU 21 D: ↓ BrdU – ↓ Ki67 ↓BrdU (SVZ: 0) ↓ BrdU 5 W: ↓ BrdU Cells born 3 W before: 0 BrdU Last 3 W: ↓ BrdU ↓ BrdU ↓ BrdU – Cells born 1 W before training: 0 BrdU Survival 4 w: 0 BrdU 4 w: 0 BrdU 4 w: ↓ BrdU 4 w: 0 BrdU – ↓ BrdU 0 BrdU Proliferation ↓ BrdU 0 BrdU ↓ BrdU 0 BrdU 7 D later: ↓ BrdU 1&14 D later: 0 BrdU – %BrdU-NeuN: ↓ %BrdU-S100: 0 %BrdU alone: ↑ – – – %BrdU-NeuN:0 %BrdU-GFAP: 0 %BrdU-NeuN: ↓ %BrdU-GFAP: ↓ – – – Differentiation %BrdU-NeuNS100b: 0 %BrdU-NeuN: 0 %BrdU-GFAP: 0 – Rosenbrock et al. (2005) Duric and McCarson (2006) Luo et al. (2005) Xu et al. (2006) Veena et al. (2009) Pham et al. (2003) LopezFernandez et al. (2007) Shors et al. (2007) References Malberg and Duman (2003) Bland et al. (2006) Vollmayr et al. (2003) IS for 2 W Foot-shock Acute psychosocial stress Dominant/ subordinate Resident/ intruder Resident/ intruder IS for 3 W Foot-shock 6–9 W M Rat SD 3 Y M ~350 g 7 M–2.5 Y M 3 M ~300 g F M ~385 g M Holtzman Wistar SD 3 M 7–8 W Age/weight 8 W M F M F M F F M Sex M Tree – shrew Marmoset – Rat Rat IS vs ES for 7 D Rat Foot-shock Grouped Wistar IS for 1 W Individual Grouped IS for 3 W Individual Foot-shock Rat SD 3 CFA over 3 W Rat Strain CD1 Pain Species Mice Condition 3 W Paradigm Forced swim 1 W post stress: ↓ BrdU 3 W post stress: ↓ BrdU – ↓ BrdU 0 BrdU – – 0 BrdU 2 W: ↓ BrdU 2 W: ↑ BrdU 2 W : 0 BrdU 2W : 0 BrdU – Survival 4 W: SVZ: ↓ BrdU 1 W: BO: ↓ BrdU – ↓ BrdU LH:↓ BrdU NLH: 0 BrdU BrdU IS < BrdU ES BrdU IS = BrdU ES ↓ Ki-67 0 BrdU 0 BrdU Proliferation SVZ & OB: ↓ BrdU ↓BrdU (SVZ: 0) %BrdU-NeuN: 0 – – – – – – Differentiation – (continued) Gould et al. (1997) Gould et al. (1998) Thomas et al. (2007) Dagyte et al. (2009) Chen et al. (2006) Shors et al. (2007) References Hitoshi et al. (2007) Duric and McCarson (2006) Westenbroek et al. (2004) 1h 1 h 20 min 5 min 25°C TMT TMT TMT FS Chronic psychosocial stress Dominant/ 35 D subordinate Resident/ 7 D intruder Resident/ 18 D intruder Resident/ 5 W intruder Resident/ 7 D intruder Rat 1h TMT Wistar Wistar C57BL Rat Mice – LAL SAL SD SD SD Tree shrew Tree shrew Rat Mice Rat Rat Rat SD Rat SD Strain CFW Species Mice Table 4.1 (continued) Paradigm Condition Resident/ 1 or several intruder defeats TMT 1h M M 7 W ~190 g 3 M 12 ± 4 M M M 5–30 M 14 W M M ~225 g ~325 g F M M ~275 g ~400 g ~240 g Age/weight 9 W M M M Sex M – ↓ BrdU 18 D: ↓ BrdU 3 W: ↓ Brdu (SVZ: 0) ↓ BrdU ↓ BrdU (SVZ: 0) – – ↓ BrdU 0 BrdU – – ↓ BrdU 0 BrdU ↓ BrdU – 0 BrdU 0 BrdU ↓ BrdU – 1W: ↓ BrdU 3 W: 0 BrdU – ↓ BrdU ↓ BrdU Survival – Proliferation 0 BrdU %BrdU-NeuN: 0 %BrdU-GFAP: 0 %BrdU-NeuN: 0 %BrdU-GFAP: 0 – – – – – %BrdU-NeuN/ NSE: 0 %BrdU-GFAP: 0 – %BrdU-Tuj1: 0 %BrdU-GFAP: 0 – Differentiation – Simon et al. (2005) Czeh et al. (2001) Czeh et al. (2002) Czeh et al. (2007) Mitra et al. (2006) Hill et al. (2006) Thomas et al. (2006) Veenema et al. (2007) References Yap et al. (2006) Tanapat et al. (2001) Holmes and Galea (2002) Falconer and Galea (2003) Rat 1 W 6 M Isolation Rat 8 D partial SD Rotating drum 1 D SD SF 4, 7 D treadmill Rat 24 h total SD SD Rat SD Wistar Wistar SD 24 h 72 h PF over water 12 h handling SD Rat 96 h treadmill SD SD SD Rat 96 h treadmill SD SD C57BL6/ SJL SD Strain CFW Rat Rat Sleep alteration SD 56 h disk-overwater Mice Species Mice Condition 10 daily defeats Paradigm Resident/ intruder Isolation M M M M M M M M + F M Sex M ~310 g 350 ~225 g ~300 g ~310 g ~325 g ~275 g 6 M ~275 g Age/weight 9 W – ↓ BrdU ↓ BrdU & Ki67 0 BrdU& Ki67 ↓ Ki67 ns ↓ Ki67 ↑ BrdU (SVZ: 0) 0 BrdU ↓ BrdU (SVZ: 0) – – nt 1 W: ↓ BrdU 3 W: ↓ BrdU 2 W & 30 D: ↑ BrdU Survival rate: 0 Cells born 5 D before SD: 0BrdU 3 W: ↓ BrdU Anterior: 0 BrdU – Posterior: ↓ BrdU Total: 0 BrdU Rostral: ↓ BrdU – – Survival – 0 BrdU & Ki67 Proliferation ↓ BrdU GuzmanMarin et al. (2003) GuzmanMarin et al. (2005) Mirescu et al. (2006) Tung et al. (2005) References Yap et al. (2006) Stranahan et al. (2006) Dong et al. (2004) 3 W: %BrdUNeuN: ↓ %BrdU-S100b: 0 (continued) GuzmanMarin et al. (2007) %BrdU-NeuN:↓ %BrdU-Dcx: 0 %BrdU-S100b: 0 nt %BrdU-Tuj1: 0 %BrdU-NeuN: 0 %BrdU-Calbindin: 0 Grassi et al. %BrdU-GFAP: 0 (2006) %BrdU-NeuN: 0 Roman et al. (2005) %BrdU-GFAP: 0 – – – – Differentiation – 19 D 20 D 4 W 3 W 24 D 5 W 5 W 3 W 4 W 5 W 1–5 W CMS CMS CMS CMS CMS CMS CMS CMS CMS CMS Mice 3 or 7 W CMS Rat Rat Mice Mice Rat Mice Mice Rat Rat Rat Rat Wistar Rat SD SD C57Bl/6J ICR Kun-ming C57BL6 X 129/Sv SD Wistar Wistar SD SD Balbc Strain Species Table 4.1 (continued) Paradigm Condition Chronic mild stress CMS 3 W M M M M M M M M M M M M Sex ~230 g ~240 g ~28 g 4–5 W ~260 g 18–20 g ? ~220 g 350 g ~200 g ~165 g 17–32 g 17 W Age/weight – – 3 W: ↓ BrdU ↓ BrdU ↓ BrdU ↓ BrdU 0 BrdU ↓ BrdU 2 W: ↓ BrdU 2 W: ↓ BrdU 1 W: ↓ BrdU 25 D: ↓ BrdU Survival rate: 0 2 W: ↓ BrdU 3 W: ↓ BrdU 5 W: ↓ BrdU 21D: ↓ BrdU – – – Ventral: ↓ BrdU Dorsal: 0 BrdU Total: 0 BrdU 0 KI67 – %BrdU-PSANCAM:0 %BrdU-Tuj1:0 %BrdU-Dcx: ↓ %BrdU-Dcx: ↓ %BrdU-NeuN: 0 – – – – %BrdU-NeuN: 0 %BrdU-GFAP: 0 %BrdUCalbindin: 0 %BrdU-GFAP: 0 – ↓ BrdU 4 W: ↓ BrdU (SVZ: 0) ↓ BrdU (SVZ: 0) %BrdU-NeuN : 0 19 D: ↓ BrdU (hilus 0) 3 W: 0 BrdU ↓ BrdU & Ki67 Differentiation 0 BrdU Survival Proliferation Wang et al. (2008) Koo and Duman (2008) Zhou et al. (2007) Oomen et al. (2007) Li et al. (2006) Goshen et al. (2008) Liu et al. (2008) Xu et al. (2007) Jayatissa et al. (2006) Heine et al. (2004) Alonso et al. (2004) Lee et al. (2006) References Condition 6 W Perinatal stress PS GD 14–21 restraint 3 × 45 m/D PS GD 14–21 restraint 3 × 45 m/D PS GD 14–21 restraint 3 × 45 m/D PS GD 14–21 restraint 3 × 45 m/D PS GD 14–21 restraint 3 × 45 m/D PS GD 4–21 restraint 6 h/D PS GD 13–17 restraint 3 × 45 m/D PS GD 5–20 defeat /restraint Paradigm CMS SD SD SD SD SD Wistar HAB M Wistar LAB M Rat Rat Rat Rat Rat Rat M M F F M F M Wistar Rat M F Sex M SD Strain C57Bl/6J Balb/cJ DBA/2J C57Bl/6J Balb/cJ DBA/2J Rat Species Mice 7 W ~ED15 2 M 1,3, 10 M 22 M 24 M 21–23 W 4 M 26 M 1, 3, 4, 10, 22 M Age/weight 9 W – ↓ Ki67 ↓ Ki67 – 6 W: ↓ BrdU 6 W: 0 BrdU – 2 W: ns ↓ BrdU (continued) Lucassen et al. (2009) Kawamura et al. (2006) Koo et al. (2003) %BrdUCalbindin: 0 4 W: ↓ BrdU ↓ BrdU Darnaudery et al. (2006) Mandyam et al. (2008) Lemaire et al. (2006) Lemaire et al. (2000) References Mineur et al. (2007) Koehl et al. (2009) – – %BrdU-NeuN: 0 %BrdU-S100: 0 %BrdU-NeuN: 0 %BrdU-GFAP: 0 Differentiation %BrdU-NeuN: ↓ %BrdU-NeuN: ↓ %BrdU-NeuN: ↓ %BrdU-NeuN: ↓ %BrdU-NeuN: ↓ %BrdU-NeuN: ↓ 0 BrdU ↓ BrdU – 3W: ↓ BrdU Survival rate: 0 ↓ BrdU & Ki67 ↓ BrdU & Ki67 0 BrdU 2W: ↓ BrdU Survival rate: 0 Survival 3 W: ↓ BrdU (SVZ: ↓) ↓ BrdU (SVZ: ↓) ↓ BrdU (SVZ: ↓) 0 BrdU (SVZ: 0) ↓ BrdU (SVZ: ↓) ↓ BrdU (SVZ: ↓) Rostral: ↓ BrdU Proliferation – PND 1–14 PND 2–14 PND 14–21 PND 14–21 MD (24 h) MS (180 m) MS (180 m) MS MS Rat Rat Rat Rat SD SD SD SD Wistar Macaca Mulatta Monkey Rat Strain SD Species Rat ? ? M M F M M+F Sex ? PND21 PND21 3 & 7 M PND 4 PND 4 2 M 2.5 – 3Y Age/weight E15–E21 %BrdU-NeuN: 0 3W: ↓ BrdU 2.5 W: 0 BrdU – – – ↓ BrdU ↓ BrdU – – %BrdU-GFAP: 0 %BrdU-GFAP: 0 %BrdU-Tuj1: 0 %BrdU-NeuN: 0 %BrdU-GFAP: 0 – Differentiation – Survival 3 W: ↓ BrdU 0 KI67 3 W: 0 BrdU 0 KI67 3 W: 0 BrdU ↓ BrdU (SVZ : 0 ) 1 W: ↓ BrdU 3 W: 0 BrdU – Proliferation – Greisen et al. (2005) Park et al. (2002) Lee et al. (2001) Oomen et al. (2009) Mirescu et al. (2004) References Kim et al. (2006) Coe et al. (2003) Effects are given by default for the GCL of the dentate gyrus unless otherwise stated. The absence of changes is symbolized by 0 CRS Chronic restraint stress, CMS chronic mild stress, FS forced swim, FT foot-shock, IS inescapable shock, ES escapable shock, CGL granule cell layer, SVZ subventricu‑ lar zone, P-M-Cx primary motor cortex, PCx prefrontal cortex, PS prenatal stress, MS maternal separation, MD maternal deprivation, SD sleep deprivation, SF sleep fragmentation, SR sleep restriction, SW stress at PND21, 0 no effect, Ns nonstatistically significant, TMT trimethyl thiazoline, Offs offspring, M male, F female, CFA chronic Freund’s adjuvant, LH Learned helpless, NLH nonlearned helpless, SAL short latency to attack, LAL long latency to attack, HAB high anxiety-related behavior, LAB low anxiety-related behavior, Nt not tested, PF platform, GD gestational day, ED embryonic day, PND postnatal day, PS prenatal stress GD 145–157 Acoustic startle PND 3 Table 4.1 (continued) Paradigm Condition PS GD 15–21 noise 1 h 95dB/D PS GD 50–92 4 Stress Disorders 69 4.2 Stress and Neurogenesis 4.2.1 Effects of Stress on Adult Neurogenesis 4.2.1.1 Impact of Physical Stressors Acute physical stress consisting in restraining animals has been reported to exert discrepant effects on cell proliferation in rats. Indeed it was found to be either ineffective (Duric and McCarson 2006; Pham et al. 2003; Rosenbrock et al. 2005) or to decrease cell proliferation in the dentate gyrus (DG) (Bain et al. 2004; Koo and Duman 2008; Lagace et al. 2006; Vollmayr et al. 2003). This discrepancy also emerges in mice studies, since one study reported that acute restraint stress increases cell proliferation in the DG (Bain et al. 2004), while the other reported an opposite effect (Koo and Duman 2008), suggesting important strain and species differences in the outcome of stressor exposure. A more intense stress consisting in “acute” inescapable foot-shock (IS) has been shown to have no effect (Dagyte et al. 2009), or to decrease cell proliferation in an immediate (Bland et al. 2006; Shors et al. 2007) or delayed (Vollmayr et al. 2003; Malberg and Duman 2003) fashion. These changes were transient as they were no longer observed 2 weeks later (Vollmayr et al. 2003). Interestingly, a sexual difference in the outcome of IS was observed, cell proliferation being unchanged in female rats (Shors et al. 2007). Only three studies have examined the effect of acute stress, in particular the effect of foot-shock, on cell survival and fate. On the one hand, the number of cells generated 9 days after exposure to shock was not modified after a 1-month delay, suggesting a compensatory increased survival (Malberg and Duman 2003). On the other hand, the number of cells generated at the time of IS delivery was still decreased after a 1-month survival delay indicating a lack of effect on cell survival (Bland et al. 2006). Finally, the survival of cells generated 1 week before shock delivery was not influenced either (Lopez-Fernandez et al. 2007; Pham et al. 2005). Whatever the condition neuronal differentiation was unaffected (Bland et al. 2006; Malberg and Duman 2003). Chronic restraint stress for up to 3 weeks, as well as chronic pain induced by formalin, decreased (by 20–50%) cell genesis in the DG of male rats (Duric and McCarson 2006; Pham et al. 2003; Rosenbrock et al. 2005; Luo et al. 2005; Xu et al. 2006). The survival (as well as phenotypic differentiation) of cells generated before a chronic restraint stress was not influenced by 3 weeks of restraint (Pham et al. 2003). However, when the survival of cells generated after 3 weeks of stress was examined after 3 additional weeks of stress, or after 2 weeks of standard hous‑ ing, then the number of bromodeoxyuridine (BrdU)-labeled cells was lower in comparison to control rats (Pham et al. 2003; Veena et al. 2009) and neuronal differentiation was decreased while the percentage of cells that remained undiffer‑ entiated increased (Veena et al. 2009). The difference in BrdU cell numbers after a survival period is probably due to an initial difference in cell proliferation indicating 70 M. Koehl et al. that cell survival is not influenced by stress. Opposite conclusions have been drawn after chronic foot-shock (Westenbroek et al. 2004). Thus, IS for 3 weeks reduced the number of cells generated during the first week of the stress procedure in the DG of male rats housed singly. These changes reflect changes in cell survival since cell proliferation was not modified during the first week of stress. In this case, cell differentiation was not influenced by stress. Interestingly, the same study showed that IS induced an increase in the number of the 3-week-old cells in the DG of female rats housed singly. More recently, 3 weeks of chronic foot-shock were found to cause a temporary suppression of cell proliferation, and while it did not affect the survival of cells born before the period of stress, neurogenesis levels estimated by the number of doublecortin (DCX) immunoreactive cells, was decreased at the end of the procedure (Dagyte et al. 2009). Although most studies have focused on the DG, a few reports have evaluated the impact of chronic stress on the subventricular zone-olfactory bulb (SVZ-OB) system. A reduction of the number of BrdU-IR cells has been reported in the SVZ and the olfactory bulb after 3 weeks of chronic forced swim (Hitoshi et al. 2007), whereas chronic restraint was found inefficient on cell proliferation in the SVZ (Duric and McCarson 2006). 4.2.1.2 Impact of Psychological Stressors Historically, the first stressor that has been tested on neurogenesis was an acute social stress. Using first the dominant/subordinate paradigm in tree shrews and then the resident/intruder paradigm in monkeys, it has been shown that the stress of subordination quickly decreases cell proliferation in the DG (Gould et al. 1997, 1998). In contrast, an equivalent stressor does not modify cell prolifera‑ tion in rodents (Mitra et al. 2006; Yap et al. 2006; Thomas et al. 2007). In rats, short-term and long-term cell survival were, however, decreased, by 70 and 26%, respectively (Thomas et al. 2007). Together with the fact that stress did not influence cell differentiation, an overall decrease in neurogenesis was obtained in this later study. The predator odor paradigm is classically used to model a predator stress. In particular, trimethyl thiazoline (TMT), the major component of fox feces, a natural predator of the rat, is commonly used to induce stress and produce fearful responses (freezing, decreased exploratory behavior, and diminished grooming) and defensive behavior directed toward the stressor (TMT containing vial). Thus, exposure to TMT for 1 h suppresses cell genesis (and cell death) in the DG of male rats (Holmes and Galea 2002; Falconer and Galea 2003; Hill et al. 2006; Tanapat et al. 2001). This effect seems to require a minimum exposure time since a 20-min exposure was found ineffective on cell genesis (Thomas et al. 2006). Sex differences were also reported in this stress model since cell genesis remains unchanged in female rats in response to a 1-h TMT exposure (Falconer and Galea 2003). An aspect that deserves mention is that TMT-induced suppression of cell genesis in males is tran‑ sient: it lasts for 1 week and recovery is observed 3 weeks after labeling the cells 4 Stress Disorders 71 (Tanapat et al. 2001). This suggests a potential compensatory increase in cell survival in stressed animals. Such a transient effect has also been reported with a combination of physical (restraint) and psychological (forced swim) stressor, since 1 day after stressor exposure, cell proliferation, which was decreased, is normalized to control values (Heine et al. 2004). When the stress of subordination is repeated in rodents, decreases in both hip‑ pocampal cell proliferation (by »30%) and cell survival (by »35–55%) were observed (Yap et al. 2006; Czeh et al. 2002, 2007). The cells differentiate into neurons and overall this leads to a net decrease in neurogenesis (Czeh et al. 2007). No effect of stress was observed in the SVZ (Czeh et al. 2007). Similar decreases were observed in tree shrews with a 33% reduction of cell genesis in 11-month-old animals exposed to chronic social conflict (Czeh et al. 2001), and a 76% decrease in 21- to 30-month-old animals (Simon et al. 2005), which underlines that age is a factor of vulnerability to stress. In social animals such as rodents, social isolation is a stressor. Although a 1-week isolation does not affect cell proliferation in the DG (Stranahan et al. 2006), chronic isolation for 1 month decreases the number of immature DCX neurons (Menachem-Zidon et al. 2008). After 6 months, cell proliferation is still reduced (Dong et al. 2004). 4.2.1.3 Sleep Deprivation Sleep is important for survival, and sleep deprivation induces physiological changes similar to those seen after stress (McEwen 2006). For this reason, the consequences of sleep alteration have been examined on neurogenesis. Several paradigms of sleep fragmentation or deprivation, whether partial or total, have been used: a treadmill (Guzman-Marin et al. 2003, 2005, 2007), a disk-over water (Tung et al. 2005), a small platform over water (Mirescu et al. 2006), a slowly rotating drum (Roman et al. 2005), or gentle handling (Grassi et al. 2006), this last method being restricted to periods of sleep deprivation not exceeding 12 h. It was found that prolonged sleep loss or restriction reduces cell proliferation in the GCL and not the SVZ (GuzmanMarin et al. 2003, 2007; Tung et al. 2005; Mirescu et al. 2006; Roman et al. 2005). This effect lasted for 3 weeks, a time during which the majority of cells differenti‑ ate into neurons (Mirescu et al. 2006). Neuronal differentiation was found to be reduced after 3 weeks of sleep loss (Guzman-Marin et al. 2005) or after 1–7 days of sleep fragmentation (Guzman-Marin et al. 2007). When shorter periods of sleep deprivation are applied (12–24 h), no change (Mirescu et al. 2006) or a decrease (Roman et al. 2005) in cell proliferation is observed. After an initial decrease in proliferation, the number of cells in animals submitted to prolonged sleep depriva‑ tion normalizes to control values. This recovery is followed by a transient increase in cell proliferation (Mirescu et al. 2006). This is at variance with the increase in cell proliferation and neurogenesis that has been described after a one-night sleep deprivation (Grassi et al. 2006). 72 M. Koehl et al. 4.2.1.4 Unpredictable Chronic Mild Stress UCMS results from the combination of different physical (restraint, forced swim, vibration), psychological (subordination stress, crowding/isolation), or arousal (changes in sleep cycle, food and/or water deprivation; uncertainty resulting form the unpredictability of the stressors) variables. UCMS procedure for 3–7 weeks has been shown to decrease cell proliferation in the DG of mice (Alonso et al. 2004; Goshen et al. 2008; Li et al. 2006; Zhou et al. 2007) and rats (Heine et al. 2004; Liu et al. 2008; Xu et al. 2007), an effect not reproduced in all studies (Lee et al. 2006; Oomen et al. 2007; Wang et al. 2008) or only in the ventral portion of the DG (Jayatissa et al. 2006). A recovery of cell proliferation was observed 3 weeks after the cessation of the CMS (Heine et al. 2004). The number of cells that survived under a 3-week stress period has been shown to be decreased (Zhou et al. 2007; Liu et al. 2008; Lee et al. 2006; Oomen et al. 2007; Wang et al. 2008) or not affected (Heine et al. 2004) in comparison to control animals. Overall, when the number of surviving cells was compared to the number of proliferating cells (survival rate), it was concluded that CMS decreases (Lee et al. 2006; Wang et al. 2008), increases (Heine et al. 2004) or does not affect (Zhou et al. 2007; Liu et al. 2008) cell sur‑ vival. In most cases, however, since neuronal differentiation is unchanged, neuro‑ genesis was found to be decreased. One study, however, found an effect of CMS on neuronal differentiation in three strains of mice, whether males or females (Mineur et al. 2007), the net effect also being a decrease in neurogenesis. 4.2.2 Genetic and Environmental Factors Modulating the Effects of Stress 4.2.2.1 Genetic Control of Stress Effects As we mentioned previously, genetic and epigenetic factors interplay to determine the body’s stress response. For example, it is well known that individuals are dif‑ ferentially susceptible to the same environmental challenges depending on their genotype. In mice, C57BL/6J appear more resilient to the behavioral effect of stress when compared to BALB/cJ (Anisman and Matheson 2005). Given the existence of strain differences in neurogenesis, the question as to whether stress-related alterations in neurogenesis depend upon the genotype has been recently examined. It was shown that UCMS decreases the number of 6-week-old cells in the DG and the SVZ of male and female mice of three different strains (C57BL/6J, BALB/cJ DBA/2J), with the exception, however, of C57BL/6J females that were totally resis‑ tant to the effect of stress (Mineur et al. 2007). Given that the number of 3-week-old surviving BrdU-IR cells in male mice followed this pattern: C57BL/6J>BALB/ cJ>DBA/2J, the amplitude of stress was higher in the C57BL/6J when compared to BALB/cJ and DBA/2J strains. 4 Stress Disorders 73 As far as personality traits are involved, it has been shown that forced swim stress decreased cell proliferation in mice selected for a long latency to attack (LAL) a male resident in comparison to mice selected for a short latency to attack (SLA) (Veenema et al. 2007), and that IS decreases cell genesis only in animals developing learned helplessness (Chen et al. 2006). In this latter case, however, the weight of nature or nurture, i.e., the weight of genetic versus environmental shaping factors, remains undetermined. 4.2.2.2 Environmental Control of Stress Effects Social context can modulate the effects of stress on neurogenesis. Indeed, social housing abrogates the stress-induced changes in the number of cells generated dur‑ ing the stress procedure (Westenbroek et al. 2004). Thus, a difference in social experience might also explain some of the controversy in the observed effects of stress on neurogenesis. Furthermore, stressor controllability also plays an important role. Exposure to an escapable shock (ES), in contrast to an inescapable shock (IS), does not decrease cell proliferation in the DG when compared to unstressed control rats (Bland et al. 2006), and repeated IS decreases cell proliferation in male rats when compared to repeated ES exposure (Shors et al. 2007). It is well documented that stress has a greater impact when performed during perinatal periods. The perinatal period can be broken down into a prenatal period (until birth) and a postnatal period (from birth to weaning). Stress during the prenatal period consists in stressing the pregnant mothers and evaluating the consequence on their progeny. This stress can take several forms (restraint, noise exposure, intruder exposure), but similar effects on the progeny have been reported. Thus, gestational stress was found to decrease hippocampal cell prolif‑ eration from adolescence to senescence in male rodents (Kim et al. 2006; Koo et al. 2003; Lemaire et al. 2000, 2006). Interestingly, results obtained in female offspring indicate that, although prenatal stress also decreases cell proliferation, this effect is age-dependent. Thus, prenatal stress had no effect in young females (Zuena et al. 2008), while it decreased cell proliferation in older ones (Koehl et al. 2009; Mandyam et al. 2008). One study reported that old prenatally-stressed females presented levels of proliferation similar to controls, but the experimental paradigm involved a chronic ICV saline infusion that may have masked the effects of prenatal stress (Darnaudery et al. 2006). Altogether, these results thus suggest a heightened sensitivity of males to the deleterious effects of prenatal stress, at least in rodents. Cell survival rate and neuronal differentiation were found to be insensitive to early life stress (Koo et al. 2003; Lemaire et al. 2000, 2006), and overall, a decrease in neurogenesis has thus been consistently reported in rodents (Koo et al. 2003; Lemaire et al. 2000, 2006; Koehl et al. 2009; Lucassen et al. 2009) as well as in monkeys (Coe et al. 2003). Importantly, prenatal stress–induced alteration in neu‑ rogenesis was counteracted by a form of environmental enrichment, i.e., postnatal handling (Lemaire et al. 2006). 74 M. Koehl et al. In a recent study, the importance of individual variability in vulnerability to prenatal stress was investigated by comparing its consequences on rats selectively bred for high and low anxiety-related behavior, HAB and LAB rats, respectively. As expected, the impact of prenatal stress was found to be highly individual-dependent as it decreased neurogenesis only in HAB rats (Lucassen et al. 2009), indicating that the rate of neurogenesis is determined by the interaction between a genetic predis‑ position for anxiety and environmental factors. Stress during the postnatal period is usually obtained by maternal deprivation or maternal separation (MS), i.e., a separation of the dam–pup dyad for extended (24 h) or short (180 min) periods of time, respectively. Maternal separation per‑ formed during the stress hyporesponsive period (first 2 weeks of life) for 180 min/ day, downregulates hippocampal cell proliferation (Mirescu et al. 2004). After 1 week, MS animals exhibited fewer surviving cells whereas by 3 weeks they did no longer differ from control rats. This suggests that the decrease in proliferation and short-term survival is compensated by an increased survival of 3-week-old cells (Mirescu et al. 2004). In another study, the number of newlyborn cells was not modified in 3- and 7-month-old maternally-separated animals certainly because animals were sacrificed 2.5 weeks after BrdU pulse (Greisen et al. 2005). Models of maternal deprivation yielded contradictory results. Thus, on the one hand, mater‑ nal deprivation performed from the second week of life onward was associated with a decrease in cell genesis 1 week later (Lee et al. 2001; Park et al. 2002), an effect consistent with those of maternal separation. On the other hand, a recent study showed that a 24-h deprivation performed on the third day of life did not affect cell survival or proliferation rate measured 17 days later, but did increase neurogenesis assessed by the number of DCX positive cells in males (Oomen et al. 2009), suggesting that maternal deprivation impacts only a subset of immature cells. Interestingly, the opposite effect was reported in females (Oomen et al. 2009). Such sex differences have also been observed in response to prenatal stress where a heightened sensitivity of males was reported. Taken together, these studies under‑ lie that gonadal hormones may contribute to the effects of early life stress on hippocampal neuroplasticity. Nutrition might also be an important factor modulating the effects of stress. In support of this, it has been shown that the effect of postnatal stress on cell genesis in the hilus depends upon prenatal protein nutrition. Indeed, in well-nourished animals, a brief MS during the first week of life reduces cell proliferation. In contrast, in malnourished animals, neurogenesis was not affected by stress (King et al. 2004). 4.2.3 Potential Mechanisms Historically, stress has been defined biologically by various physiological changes, among which is the activation of the HPA axis that leads to the release of corticos‑ terone (see above). Because corticosterone is well known to decrease hippocampal 4 Stress Disorders 75 neurogenesis (for review see Abrous et al. 2005), its involvement in the effects of stress on neurogenesis has been studied. Tanapat was the first to show that the TMT-induced reduction in cell proliferation can be prevented by the blockade of stress-induced increase in corticosterone, obtained by adrenalectomy plus corticos‑ terone treatment aimed at restoring basal diurnal levels of the hormone (Tanapat et al. 2001). Since maternal separation is also known to increase the activity of the HPA axis (Sanchez et al. 2001), the involvement of corticosterone has also been investigated in this model. It was shown that an MS-induced decrease in cell pro‑ liferation is abrogated by the blockade of a stress-induced increase in corticosterone (Mirescu et al. 2004). Interestingly, high levels of corticosterone given to the dams (for 26 days from the day after delivery) mimic the effect of MS on neurogenesis. Indeed, the number of proliferating cells in 21-day-old male (but not female) off‑ spring from dams treated with corticosterone is decreased in comparison to control dams. After 3 weeks of survival, this difference was no longer observed suggesting a compensatory increase in cell survival (Brummelte et al. 2006). The same team later demonstrated that the MS-induced decrease in cell proliferation was depen‑ dent on corticosterone, whereas the delayed overshoot of cell proliferation observed after 1 week of recovery was not (Mirescu et al. 2006). A specific antagonist of the GR (mifepristone) was found to normalize the reduction in neurogenesis observed after 3 weeks of CMS (Oomen et al. 2007). A direct effect on GRs is possible since most newly-born neurons express this type of receptor (Garcia et al. 2004). Furthermore, individual differences in HPA axis reactivity and in GR hippocampal expression may be responsible for differences in acute stress-induced decrease in cell proliferation between Short (SAL) and Long (LAL) Attack Latency mice (Veenema et al. 2007). The activity of the HPA axis is known to be regulated by CRH and vasopressin, the roles of which have been evaluated in the CMS paradigm. Thus, chronic block‑ ade of CRF1 and V1B receptors (by SSR125543A and SSR149415, respectively) starting 3 weeks after the beginning of CMS, reverses the stress-induced reduction of neurogenesis (Alonso et al. 2004). Altogether, these studies place the HPA axis as the most likely mediator of the effects of stress on neurogenesis. However, other candidates have been tested. Thus, since stressful events are known to activate the release of endogenous opioids, their role has been evaluated in one study where administration of naltrexone, an opioid antagonist, did not attenuate the effect of TMT on cell proliferation (Holmes and Galea 2002), thus suggesting that opioids may not be involved. The endocannabinoid system is a buffer system in the stress response (Tasker 2004). For example, enhancement of endocannabinoid neurotransmission, by inhibiting endocannabinoid uptake with AM404, inhibits the stress-induced activa‑ tion of the HPA axis (Patel et al. 2004). For this reason, its protective effects on TMT-induced suppression of cell genesis have been examined, and it was found that pretreatment with AM404 reversed the effect of stress, certainly by increasing endocannabinoid activity (Hill et al. 2006). Exposure to stressful events is well known to increase the hippocampal levels of the proinflammatory cytokine interleukin-1 (IL-1), the signaling of which is 76 M. Koehl et al. mediated by the IL-1 receptor type I. This family is composed of three members: IL-1a and IL-1b, the signaling of which is mediated by the IL-1 receptor type I (IL-1R1), and IL1-receptor antagonist (IL-1ra), acting as a competitive antagonist. It has been shown that the effects of acute stress or CMS on neurogenesis are reversed by the genetic impairment of IL-1R1 (Koo and Duman 2008; Goshen et al. 2008). Furthermore, infusion of IL-1ra blocks the acute effect of stress (Koo and Duman 2008). In the same study, it was shown that IL-1ra treatment for 3 weeks increased the number of 2-week-old surviving cells and the number of Dcx-labeled cells compared to CMS animals. Furthermore, the effect of chronic isolation on neurogenesis is blocked by intrahippocampal transplantation of cells overexpress‑ ing IL-1ra (Menachem-Zidon et al. 2008). Although an in vitro study has suggested that IL-1b modulates neurogenesis directly through the IL-1R1 receptors localized on the progenitor cells via a reduction of cell cycling (Koo and Duman 2008), an indirect action through corticosterone can also be at play (Goshen et al. 2008). The role of neuronal nitric oxide synthase (nNOS) has also been evaluated given that nNOS inhibits neurogenesis (Abrous et al. 2005). It was shown that CMSinduced decrease in cell proliferation could be reversed by a treatment with 7-nitroindazole, a selective inhibitor of nNOS (Zhou et al. 2007). Further studies are, however, needed to confirm the involvement of the NO system. Given that stress is known to be a risk factor for the development of depression, the effects of various antidepressants have been evaluated. Treatment for 1 week with fluoxetine, a serotonin-selective reuptake inhibitor, prevents MS-induced decrease in cell proliferation observed in weanlings (Lee et al. 2001). Consistently, chronic administration of the atypical antidepressant tianeptine blocks the effects of subordination stress on neurogenesis in the adult tree shrews (Czeh et al. 2001), and the tricyclic AD, clomipramine, prevents the CMS-induced decreased in cell pro‑ liferation and cell survival (Liu et al. 2008). Signals regulating neurogenesis may be synthesized peripherally and released in neurogenic sites by blood vessels. For this reason, the role of the cerebral vasculature has gained importance as adult neurogenesis occurs within an “angiogenic niche” (Palmer et al. 2000). An alteration in the vascular microenvironment following stress has been proposed to be responsible for a disruption in neurogenesis (Heine et al. 2005). Indeed, chronic stress decreases the number of proliferating cells associated with the microvasculature. This was associated with a decrease in the expression of the angiogenic factor vascular endothelial growth factor (VEGF) and its receptor (VEGF-R2 = Flk1) in the GCL. A recovery was observed after 3 weeks. Anecdotally, acupuncture (HT7) (Park et al. 2002) treatment for 1 week also prevents the effects of MS. 4.2.4 Conclusion Overall, the following conclusion can be drawn. (1) Independently of the stressor, stress decreases cell proliferation, except in a very few studies. These discrepancies can 4 Stress Disorders 77 be explained in terms of strength of the stressor and vulnerability of the stressed animals: tree shrews appear extremely sensitive compared to rodents and in the rodent group, rats and mice respond differently. (2) These effects of stress, when performed during adulthood, are transient and reversible, and an overshoot has even been observed in one case. (3) Stress has a greater impact when performed during developmental periods, leading to long-lasting alterations of neurogenesis. (4) A few studies have reported a negative effect of stress on the survival rate or have suggested a compensatory increased survival. (5) Cell differentiation does not seem to be influenced by stress except in a few cases. (6) Studies have been performed mainly in male rats; when females are included, they show differences in stress sensitivity. (7) Most studies have been performed in rats, and mice are largely understudied albeit the importance of studying gene × environment interactions. And (8) the discrepant effects of stress between studies might depend upon housing condition, nutrition, personality, etc. Differences in these variables may buffer the effects of stress on neurogenesis. 4.3 Stress Disorders and Neurogenesis When uncontrolled or repeated, stressors induce long-lasting states of distress and vulnerable phenotypes which reflect aberrant stress systems activity and altered limbic functions. These aberrant activities are risk factors for physiological and psychiatric disorders such as depression, pathological aging, drug misuse, and PTSD. Studies linking these pathologies and neurogenesis constitute an important field of research, but for many of them advances are hampered by the lack of adequate animal models. Since depression is the topic of another chapter of this book, the implication of neurogenesis in this mood disorder will not be reviewed here. 4.3.1 Aging 4.3.1.1 Pathological Aging: The Glucocorticoid Cascade Hypothesis In the context of adult neurogenesis, and apart from depression, one of the most studied stress-related disorders is aging. Indeed, aging is considered as a time of impaired capacity to respond to stress (Sapolsky 1992). It has been extensively discussed that an overactivation of the stress response leading to a hypersecretion of glucocorticoids is damaging for the brain, and the hippocampus in particular. This has led to the glucocorticoid cascade hypothesis according to which an exces‑ sive release of glucocorticoids throughout the life of an individual can lead to memory disorders by endangering the hippocampus (Sapolsky 1992). However, memory alteration is extremely variable within a population: some old animals 78 M. Koehl et al. show a clear impairment of spatial memory while others exhibit memory capacities similar to those of younger individuals (Rapp and Amaral 1992). These individual differences have been linked to the basal activity and responsiveness of the HPA axis and to neuronal loss in the hippocampus (Issa et al. 1990; Landfield et al. 1981; Landfield 1988). Indeed, pathological aging, characterized by the appearance of spatial memory deficits (measured in the water maze), is associated with HPA upregulation and hippocampal neuron loss. 4.3.1.2 Aging, Neurogenesis and Memory Aging, like stress, is a potent negative “regulator” of hippocampal neurogenesis (for extensive review, see Drapeau and Abrous 2008; Klempin and Kempermann 2007). Given the existence of interindividual differences in the effects of aging (see above), we first examined whether the ability to perform hippocampus-related functions in aged rats was related to the capacity to generate new neurons. In basal conditions (of the learning phase), we have demonstrated in senescent rats that spatial reference memory abilities (measured in the water maze) are positively correlated with the number of proliferating cells, surviving cells and new neurons evaluated 3 weeks after training. In other words, animals with preserved spatial memory (aged-unimpaired, AU) exhibit a higher level of proliferating cells, of 1-month-old surviving cells, and of new neurons compared to animals displaying spatial memory impairments (aged-impaired, AI). A similar correlation was observed in some (Driscoll et al. 2006) but not all studies (Merrill et al. 2003; Bizon and Gallagher 2003; for discussion, see Drapeau and Abrous 2008). Age-related memory deficits result not only from an alteration of the new neuronal pool but also from changes in the dynamics of hippocampal neurogenesis. As recently reviewed (Drapeau and Abrous 2008), we have shown that in aged rats spatial memory abilities critically depend upon a dynamic homeostatic regulation of neurogenesis. Indeed, spatial learning (in the water maze) influences the fate of the newly-born cells according to their birth-date and to individual memory abili‑ ties (Drapeau et al. 2007). In AU rats, learning increases the survival of cells gener‑ ated at least 1 week before the learning episode, whereas it decreases survival of cells produced during the early phase of learning. In AI rats, cell survival was not influenced by learning. In fact, in these rats, an abnormal delayed rebound in cell proliferation was observed 2 weeks after the completion of spatial training, an effect consistent with a previous study (Bizon et al. 2004). Thus, a homeostatic regulation of neurogenesis observed in some aged individuals, by sculpting the networks, is likely to be crucial for hippocampal functioning. 4.3.1.3 Mechanisms Linking Aging, Neurogenesis and Memory Given the role of the HPA axis in pathological aging (see above), we and others hypothesized that chronically elevated corticosterone levels are responsible for the 4 Stress Disorders 79 reduced neurogenesis observed in the aging DG. We found a quantitative relationship between a reservoir of new neurons, spatial memory abilities, and the magnitude of HPA axis activity in old animals. Indeed, animals with the heaviest adrenal glands, indicative of chronic HPA axis hyperactivity, exhibit the worst spatial memory abilities in the water maze and the lowest number of proliferating cells or 3-weekold surviving cells (Montaron et al. 2006). This raises the possibility that hyperac‑ tivity of the HPA axis produces spatial memory deficits by decreasing hippocampal neurogenesis. To test the hypothesis that exposure to high levels of corticosterone throughout life is responsible for age-related memory disorders and decline in neurogenesis, the secretion of corticosterone was reduced from midlife onward by adrenalectomy. Under these circumstances, lowering corticosterone secretion reduced the decline in neurogenesis observed in old rats and prevented age-related memory disorders (Montaron et al. 2006). These results demonstrate that agerelated loss of new neurons is not an irreversible process. They also suggest that interindividual variation in functional expression of neurogenesis during aging results from a differential vulnerability to cope with stress. 4.3.2 Drug Addiction 4.3.2.1 Substance Abuse and Addiction Addiction is the endpoint of a continuum of intoxication ranging from experimental– social–circumstantial use, to more intensified and impulsive self-administration and ending in a chronic relapsing disorder, addiction per se. Needless to say, the percentage of circumstantial users who will succumb and become dependent is relatively small. From a social consumption to a compulsive one, different structures and neuronal systems are recruited (Koob and Le Moal 1997, 2001; Le Moal and Koob 2007). It is now thought that, besides specific characteristics for each drug used, a common clinical syndrome is shared by all addictions. This syndrome is classically character‑ ized by: (1) a loss of control and of self-regulation abilities which reflects compro‑ mised executive functions in the orbitofrontal cortical system, (2) a compulsive drug use corresponding functionally to enhanced reward–stimulus associations, and to a compromised ventral striatal-pallidal-thalamic-cortical circuitry, and (3) a breakdown of hedonic homeostasis reflecting alterations of the extended amygdala system (Koob and Le Moal 2006). It is now recognized that the course of the process depends upon individual characteristics, in particular for 80% of the patients if not more, upon psychopathological comorbidities such as depression, anxiety, and personality disor‑ ders for which the hippocampus is part of the neuropathology (for review, Goodman 2008). The role of the hippocampus in the development of addiction has been further emphasized by several authors due to its involvement in the memories, the stimulus– reward learning, the stimulus–context and drug-taking associations that are responsible for the maintenance of drug reinstatement and relapse (Koob and Le Moal 2001; Everitt and Wolf 2002; Goldstein and Volkow 2002; See et al. 2003). 80 M. Koehl et al. 4.3.2.2 Stress and Substance Abuse There is currently a great deal of interest in the importance of stress as a risk factor for substance abuse and addiction. Indeed, stressful life events, psychological and physical stressors that threaten homeostasis, may produce surges in alertness, arousal, vigilance, and increase cognitive processing, thereby facilitating addiction. Thus, exposure to stressful experiences has been shown to be a powerful mediator of the response of an individual to drugs and preclinical studies indicate that stress can potentiate the rewarding properties of addictive drugs (Shaham and Stewart 1994), increase the rate of drug self-administration (Goeders and Guerin 1994; Piazza and Le Moal 1998), and reinstate drug-seeking behavior (Shaham et al. 2000). These effects are certainly mediated by the action of stress hormones on DA neurons and limbic regions, hippocampus included (Piazza et al. 1991, 1993; Piazza and Le Moal 1996, 1997) and by the recruitment of the CRF system to progress from an impulsive to a compulsive state (Koob and Le Moal 2008). 4.3.2.3 Substance Abuse and the Hippocampus Recent discoveries have led to the hypothesis that addictive drugs are implicated in the regulation of neurogenesis (Canales 2007; Crews and Nixon 2003; Eisch and Harburg 2006; Nixon 2006; Powrozek et al. 2004). Moreover, given that the DG is quintessentially a neuroplastic region endowed of memory functions (Abrous et al. 2005; Christie and Cameron 2006; Ming and Song 2005), it was of interest to con‑ sider whether the DG was also involved in the pathological memories underlying drug addiction, along with the amygdala and dorsal striatum (Koob and Le Moal 2006). The hippocampus appears necessary for each aspect of drug-related memo‑ ries. Thus, stimulation of the hippocampus at theta frequency causes reinstatement of cocaine consumption in abstinent rats (Vorel et al. 2001), while its functional inactivation attenuates drug taking and reinstatement (Fuchs et al. 2005; Rogers and See 2007; Sun and Rebec 2003), suggesting an encoded association between con‑ text and drug experience. Using a conditioned place preference paradigm, it has been shown that acquisition and expression of place preference was blocked by lesion or inactivation of the hippocampus (Meyers et al. 2006). Imaging data (fMRI, PET) strengthen these animal studies. They show that the hippocampus is part of a network including the orbitofrontal cortex, extended amygdala, and stria‑ tum, which is activated during rewarding effects of morphine or cocaine exposure in naïve subjects (Becerra et al. 2006; Breiter et al. 1997), and that activation of the hippocampus is observed during craving situations (Hermann et al. 2006; Kilts et al. 2001; Wang et al. 2007). Furthermore, drug-induced long-term neuroadapta‑ tions and learning-dependent neuroplasticity seem to share common neuronal morphological changes (Nestler 2002) and there is substantial communality between LTP-induced hippocampal learning and neural changes after drugs in the same region (Lu et al. 2000; Ma et al. 2006; Thompson et al. 2002, 2004). In humans, an alteration of genes regulating extracellular matrix remodeling, 4 Stress Disorders 81 which are involved in synaptic plasticity and learning and memory, has been shown to be altered in the hippocampus of cocaine abusers (Mash et al. 2007). In animals, local inhibition of CAMKII within the DG, which impairs learning and memory, attenuates morphine tolerance and dependence (Fan et al. 1999). 4.3.2.4 Drugs of Abuse, Addiction, and Neurogenesis The effects of drugs of abuse and of their misuse on neurogenesis have been docu‑ mented in recent reviews (Eisch and Harburg 2006; Nixon 2006; Powrozek et al. 2004; Eisch and Mandyam 2004). However, to our knowledge, in the context of addiction, or of a transition from drug use and misuse to addiction, very few data are available from experiments using drug self-administration paradigms (see Table 4.2). Most data correspond to pharmacological treatments and were obtained after imposed drug administrations or after a no-choice one-bottle diet, and should thus be considered with caution. Indeed, it has been evidenced that the neurobiological effects of passive drug administration were not the same as those obtained from selfadministration (see, for instance, (Dworkin et al. 1995; Mark et al. 1999; Smith and Dworkin 1990; Stefanski et al. 1999; Wilson et al. 1994; Robinson et al. 2002). As far as research papers and reviews are focused on the process of addiction in relation with hippocampus and more precisely on neurogenesis, it is cogent to first evaluate the data that use appropriate animal models, i.e., self-administration of drug of abuse (Eisch and Harburg 2006). Chronic self-administration of morphine (60 mg/kg per infusion) inhibits cell proliferation in adult rats (Eisch et al. 2000); interestingly, it was shown that this effect was not mediated by changes in the circulating levels of glucocorticoids because similar effects were observed after adrenalectomy and hormone replacement. The impact of chronic nicotine selfadministration has been evaluated with different doses (0.02, 0.04, 0.08 mg/kg per infusion). Neurogenesis was decreased significantly for the highest doses, which also increased cell death (Abrous et al. 2002). Finally, the effects of alcohol have been investigated, which is particularly interesting given the well-known patholo‑ gies implicating the hippocampus after chronic ethanol consumption. In a proto‑ typical experiment alcohol was presented as a two-bottle choice, one bottle containing water, the other 10% ethanol (Crews et al. 2004). Self-administration of ethanol inhibited cell proliferation in the DG by approximately 60%. Interestingly, exercise counteracted this drug effect and increased proliferation. These results were confirmed in other studies presenting ethanol instead of the water bottle, i.e., without choice. Thus, rats fed for 6 weeks with a liquid diet containing ethanol had a 66% decrease of the number of new neurons and a 250% increase in cell death in the DG as compared to water controls. Incidentally, neurogenesis within the olfac‑ tory bulb was not affected. Here, the neurogenesis deficits were prevented when an antioxidant was administered through the diet suggesting an underlying mechanism related to oxidative stress (Herrera et al. 2003). The same paradigm has been used in rats by He et al. with an alcohol liquid diet containing 7% alcohol for 1–4 weeks (He et al. 2005); cell proliferation was inhibited by 50% compared to water-treated Male SD Cocaine Morphine Cocaine Morphine Male SD Male W Male SD Male SD Cocaine 1 × 20 mg/kg for 1 d (ip) 20 mg/kg for 2 w (ip) 3 × 7 mg/kg/w for 22 w (ip) 1 × 10 mg/kg (ip) 75 mg/d for 5 d (pellet) 2 × 230 mg/kg for 1 w (ip) Male W Male SD PND30 Male C57BL/6J Rat Male W Imposed treatments Nicotine 4 × 1 mg/kg over 1 d (ip) Nicotine 1 mg/kg/d for 3 d (ip) Nicotine 6 mg/kg/d for 3 w (pump) Nicotine 0.25 or 4 mg/kg/D for 10 d (pump) Cocaine 20 mg/kg for 8 d (ip) 20 mg/kg for 24 d (ip) Proliferation Proliferation Survival Proliferation Proliferation Proliferation Survival Proliferation Proliferation Proliferation Proliferation Survival Proliferation Day 8 1 w–wd 2 & 4 w–wd Proliferation Day 21 Day 21 4 w–wd 4 w CSA Survival 4 w–wd 4 w CSA Proliferation Proliferation Proliferation Male SD Male SD Male C57BL/6J 0.5 mg/kg/inf for 3 w Neurogenesis Subjects Table 4.2 Influence of drugs of abuse on neurogenesis Drug Duration Self-administration Heroine 60 mg/kg/inf for 26 d Nicotine 0.04–0.08 mg/kg/inf for 42 d Ethanol 12 d of SI 2 h 2 h 1 d 2 h 4 w 3 h 1 d 17–24 d 2 h 1 d 21 d 2 h 4 w 4 w 4 w 2 h 1 d 2 h Delay SVZ: ↑ BrdU ↓ BrdU OB & GCL: ↓ BrdU ↓ BrdU ↓ BrdU, 0 KI67 ↓ KI67 0 BrdU 0 BrdU ↓ BrdU ↓ BrdU ↓ BrdU (SVZ:0) ↓ BrdU ↓ BrdU ↑ BrdU 0 BrdU Eisch et al. (2000) Abrous et al. (2002) Crews et al. (2004) ↓ BrdU ↓ BrdU (SVZ:0) ↓ BrdU SVZ: ↓ BrdU ↓ BrdU SVZ: ↓ Ki67 0 Ki67 0 Ki67 0 BrdU 0 BrdU Kahn et al. (2005) Andersen et al. (2007) Eisch et al. (2000) Yamaguchi et al. (2004) Mudo et al. (2007) Jang et al. (2002) Mechawar et al. (2004) Scerri et al. (2006) Dominguez-Escriba et al. (2006) Noonan et al. (2008) References Results 82 M. Koehl et al. Acute binge (5 g/kg, ip) Chronic binge for 4 d (9.3 g/kg/d, po) Chronic binge for 4 d (8.9 g/kg/d, po) Ethanol Male SD Male SD PND 30 Male SD PND 35–40 Male SD Male SD Male SD Male C57BL/6J Male ICR Male Gerbil Male C57BL/6J Proliferation Survival Proliferation Proliferation Survival Proliferation Survival Proliferation Survival Proliferation Day 4 3 d–wd 1 w–wd 2 & 4 w–wd Survival 1 w–wd Survival Proliferation Survival Proliferation Proliferation/ Survival Proliferation Proliferation 24, 72,76 h ↓ BrdU 0 BrdU 0 BrdU 0 BrdU ↓ BrdU 0 BrdU 0 BrdU 0 BrdU ↓ BrdU 25 d 3 d, 7 d BO: 0 BrdU 0 PCNA 2 w 0 BrdU ↓ BrdU 1 & 3 w ↓ PCNA ↓ BrdU 1 d ↓ BrdU 5 h ↓ BrdU (SVZ: ↓) 28 d ↓ BrdU ↓ BrdU 5 h 0 BrdU 28 d ↓ BrdU Imm ↓ BrdU 28 d ↓ BrdU 4 h 0 BrdU ↑ BrdU & Ki67 (SVZ: 0 BrdU) 0 BrdU ↑ BrdU 28 d 2 h 1 d 5 & 7 d 2 h Nixon and Crews (2004) Nixon and Crews (2002) Jang et al. (2002) Crews et al. (2006) He et al. (2005) Herrera et al. (2003) Maeda et al. (2007) Teuchert-Noodt et al. (2000) Kochman et al. (2006) Fischer et al. (2008) Effects are given by default for the GCL of the dentate gyrus unless otherwise stated. The absence of changes is symbolized by 0 h hours, d days, w weeks, CSA cocaine self-administration, Imm sacrifice immediately after treatment, LD diet Leber–Decarli diet, THC Tetrahydrocannabinol, Wd withdrawal (abstinence) Ethanol Ethanol Ethanol LD diet for 1–3 w (14–15 g/kg/d) 2 g/kg/d for 3 d (ip) 1 × [1 or 2.5 or 5 g/kg] (ip) 1 × 1–30 mg/kg (ip) Escalating dose 20–80 mg/kg for 21 d (po) LD diet for 6 w LD diet for 2 w 2 × 25 mg over 5 d (pellet) 1 × 20 mg/kg (ip) Escalating dose over 2,5,10 d (ip) 1 mg/kg for 2 w (sc) 1 × 25 mg/kg (ip) Ethanol Ethanol THC Amphetamine Amphetamine Morphine 4 Stress Disorders 83 84 M. Koehl et al. rats and the size of the dendritic tree of the DCX-labeled immature neurons was also reduced. Recently, it has been shown that self-administration of cocaine (0.5 mg/kg/infusion) for 3 weeks decreases cell proliferation in the DG without altering the morphology of the immature neurons (Noonan et al. 2008). Four weeks later, independently of the continuation of cocaine self-administration, cell prolif‑ eration and the number of surviving cells were similar to those measured in control animals. Surprisingly, this was accompanied by an increased number of DCXexpressing neurons. In the SVZ, an alteration of cell proliferation was also observed during cocaine self-administration, a change reversed by abstinence. Many more papers have explored the effects of imposed administration and data are summarized Table 4.2. Most of them suggest that repeated or chronic exposures to most addictive drugs impair neurogenesis in the adult hippocampus, mostly by decreasing cell proliferation. In other words, decreased neurogenesis adds itself to the already impressive list of drug-induced neuropathologies that contribute to the transi‑ tion to and maintenance of addiction, and to its propensity to relapse. Although stress modulates neurogenesis and drugs of abuse stimulate the HPA axis, it seems that the drug effects reviewed here are not dependent of the HPA axis activation (Eisch and Harburg 2006; Eisch et al. 2000), but the factors involved remain to be determined. It also seems that chronic drug effects are selective to the SGZ, the other region of adult neurogenesis being relatively spared (Eisch et al. 2000; Herrera et al. 2003; Nixon and Crews 2004) although a decrease (Mechawar et al. 2004; Crews et al. 2006) or an increase (Mudo et al. 2007) in cell genesis have also been reported. These data clearly show that, although they act on different pathways, drugs of abuse, decrease adult neurogenesis. The few studies that have focused on with‑ drawal highlight some compensatory “neurogenesis,” the significance of which is largely unknown. We have proposed that an altered hippocampal neurogenesis, together with other maladaptive plasticity processes occurring within the hip‑ pocampus, could lead to a dysregulation of the DA mesoaccumbens reward system through the hippocampal glutamatergic output that gates information in the nucleus accumbens (Goto and O’Donnell 2001) and/or through the indirect hippocampal glutamatergic output to the mesencephalic DA neurons (Blaha et al. 1997; Legault et al. 2000). Reciprocally, changes in the activity of the DA meso-accumbens system during drug taking or drug seeking could modulate the activity of the hippocampus (Scatton et al. 1980) and neurogenesis (Hoglinger et al. 2004; Kippin et al. 2005). However, this bidirectional relationship between the reward system and adult hippocampus neurogenesis needs specific investigations. 4.3.3 Anxiety Disorders Anxiety is a response expressed whenever one is failing to cope with the threatening aspects of its ongoing life situations. This disorder is the most frequent pathology in clinical psychiatry, representing nearly 30% of all psychiatric disorders. Given that the HF is a brain region associated with the modulation of anxiety state (McNaughton 1997; Bannerman et al. 2004), the impact of a failure of hippocampal 4 Stress Disorders 85 neurogenesis on anxiety-like behavior has been recently examined. In rodents, anxiety-related behaviors are primarily studied by measuring avoidance responses to potentially threatening situations, such as open and brightly lit environments which expose mice to predators, or the presence of an inoffensive predator. A link between neurogenesis and anxiety-related behavior was suggested in models based on the genetic manipulation of Activin or TrkB (Ageta et al. 2008; Bergami et al. 2008). A more direct link was provided by showing that the selective reduction of hippocampal neurogenesis increases anxiety-like behavior (Revest et al. 2009) sug‑ gesting a role of adult neurogenesis in the pathophysiology of anxiety. 4.3.4 Post-Traumatic Stress Disorders PTSD is a pathological state induced by an extreme stressful event representing a direct or indirect threat for the physical integrity of the subject. This disorder is asso‑ ciated with (1) increased anxiety (DSM IV), (2) a general deficit in declarative memory (Golier et al. 2003; Vermetten et al. 2003), and (3) a vulnerability to develop addiction (Schafer and Najavits 2007). Patients display hippocampal alterations, such as a lower hippocampal volume (Shin et al. 2004), although interindividual differ‑ ences in this symptom are observed (Neylan et al. 2004). Furthermore, PTSD would be associated with an increased negative feedback of the HPA axis that certainly implicates an alteration of hippocampal GRs (Yehuda 2000). Altogether, these symp‑ toms suggest that PTSD may be associated with an alteration of neurogenesis. However, the lack of a heuristic animal model hampers advances to test this hypothesis. Indeed, PTSD model is in fact a term in animal studies for almost every stress-induced bio-behavioral alteration. Animal models do not reflect the human disorder closely enough to deserve this term. In particular, they do not provide a model of “pathological emotional memory” with a hyper-mnesia for the source of the trauma together with trauma reliving (flashback) and amnesia for peri-traumatic stimuli. Furthermore, they do not take into account the fact that PTSD is only devel‑ oped by a subpopulation of victims of stressful events, which supposes that an inter-individual variability could also be observed in animals. In conclusion, only the development of new and valid animal models will allow testing of the relation‑ ships between this prevalent disease and neurogenesis. 4.4 Conclusion As reviewed here, there is an abundant literature on the impact of stress on adult neurogenesis. Most studies focused on possible alterations of the new neuronal pool but very few on neurogenesis in “dynamic” conditions, in response to an envi‑ ronmental pressure. For example, both in aged subjects with memory impairments (Drapeau et al. 2007) and in prenatally-stressed rats (Lemaire et al. 2000), the homeostatic regulation of neurogenesis is altered in response to learning. This highlights the importance of studying this metaplasticity in stress-related disorders. 86 M. Koehl et al. From this review, it is obvious that less has been done on stress-related pathologies. The results obtained so far suggest that hippocampal neurogenesis is involved in pathological aging, addiction and anxiety. Much more attention should be given to the fact that stress-related pathologies are not inescapable, and that wide inter-individual differences are observed: some individuals are predisposed to the development of affective disorders, addictive behavior, and age-related cognitive disorders whereas others are resilient. Interestingly, it has been shown that rats that are high behavioral responders to stress (HRs) exhibit prolonged corticosterone secretion in response to stress when they are young, and premature aging of the HPA axis in comparison to rats that are low behavioral responders (LRs) (Dellu et al. 1996). HRs are more prone to develop addictive behavior (Piazza et al. 1989, 2000; Suto et al. 2001) and exhibit an increased propensity to develop age-related memory deficits (Dellu et al. 1994). By comparing these two groups of animals, we found that neurogenesis was higher in LRs in comparison to HRs (Lemaire et al. 1999). Since bio-behavioral reactivity to stress in youth is predictive of drug taking or spatial memory impairments later in life, these individual differences in neurogenesis may account, at least in part, for individual differences in the development of stress-related disorders. In other words, low hippocampal plasticity may render animals more vulnerable to stressrelated disorders (endophenotype HRs). On the other hand, subjects starting off with a high level of neurogenesis (LRs) may be resistant to the development of disorders. These different phenotypic orientations may result from early deleterious life events, which will shape the developmental trajectory of the subjects within a genetic envelope. For example, prenatal stress affects neurogenesis in pathological ways throughout life and renders animals more vulnerable to addiction (Koehl et al. 2000; Deminiere et al. 1992) and to memory impairments (Lemaire et al. 2000). Repeated “traumatic” episodes, together with a genetic predisposition, may further reduce neurogenesis in vulnerable subjects and precipitate the symptoms. Although these propositions are highly speculative, it can be proposed that treatments that increase neurogenesis may help in curing or at least preventing the develop‑ ment of stress-related disorders. Besides pharmacological treatments, stimulating life events such as enriched environment or physical exercise should be considered. The challenge for future research will certainly be to develop therapeutic strategies aimed at stimulating plasticity in the stressed brain and preventing the alteration of affective and cognitive function that may appear in some individuals. References Abou-Samra AB, Harwood JP, Catt KJ, Aguilera G. Mechanisms of action of CRF and other regu‑ lators of ACTH release in pituitary corticotrophs. Ann N Y Acad Sci 1987;512:67–84. Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, et al. Nicotine self-administration impairs hippocampal plasticity. J Neurosci 2002;22(9):3656–3662. Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev 2005;85:523–569. 4 Stress Disorders 87 Ageta H, Murayama A, Migishima R, Kida S, Tsuchida K, Yokoyama M, et al. Activin in the brain modulates anxiety-related behavior and adult neurogenesis. PLoS One 2008;3(4):e1869. Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P. Blockade of CRF1 or V1B receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 2004;9(3):224. Andersen ML, Perry JC, Bignotto M, Perez-Mendes P, Cinini SM, Mello LE, et al. Influence of chronic cocaine treatment and sleep deprivation on sexual behavior and neurogenesis of the male rat. Prog Neuropsychopharmacol Biol Psychiatry 2007;31(6):1224–1229. Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev 2005;29(4–5):525–546. Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett 2004;368(1):7–10. Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissocia‑ tions within the hippocampus – memory and anxiety. Neurosci Biobehav Rev 2004;28(3): 273–283. Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volun‑ teers. Anesth Analg 2006;103(1):208–216, table. Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult pro‑ genitors alters newborn neuron integration into hippocampal circuits and increases anxietylike behavior. Proc Natl Acad Sci USA 2008;105(40):15570–15575. Bernard C. Introduction à la médecine expérimentale. JB Baillierre, New York; 1865. Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci 2003;18(1):215–219. Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell 2004;3(4):227–234. Blaha CD, Yang CR, Floresco SB, Barr AM, Hillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci 1997;9:902–911. Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport 2006;17(6):593–597. Bouchard TJ, Jr. Genes, environment, and personality. Science 1994;264(5166):1700–1701. Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron 1997;19(3):591–611. Brummelte S, Pawluski JL, Galea LA. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of postpartum stress and possible depression. Horm Behav 2006;50(3):370–382. Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature 1994;371(6499):702–704. Canales JJ. Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosci 2007;257(5):261–270. Cannon WB. Organization for physiological homeostasis. Physiol Revi 1929;9:399–431. Chen H, Pandey GN, Dwivedi Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport 2006;17(9):863–867. Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus 2006;16(3):199–207. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992;267(9):1244–1252. Cicchetti D, Cannon TD. Neurodevelopmental processes in the ontogenesis and epigenesis of psychopathology. Dev Psychopathol 1999;11(3):375–393. Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile Rhesus monkeys. Biol Psychiatry 2003;54(10):1025–1034. 88 M. Koehl et al. Crews FT, Nixon K. Alcohol, neural stem cells, and adult neurogenesis. Alcohol Res Health 2003;27(2):197–204. Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell prolifera‑ tion. Alcohol 2004;33(1):63–71. Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, et al. BHT blocks NF-kappaB activa‑ tion and ethanol-induced brain damage. Alcohol Clin Exp Res 2006;30(11):1938–1949. Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA 2001;98(22):12796–12801. Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, et al. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 2002;52(11):1057–1065. Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 2007;32(7):1490–1503. Dagyte G, Van der Zee EA, Postema F, Luiten PG, Den Boer JA, Trentani A, et al. Chronic but not acute foot-shock stress leads to temporary suppression of cell proliferation in rat hip‑ pocampus. Neuroscience 2009;162(4):904–913. Darnaudery M, Perez-Martin M, Belizaire G, Maccari S, Garcia-Segura LM. Insulin-like growth factor 1 reduces age-related disorders induced by prenatal stress in female rats. Neurobiol Aging 2006;27(1):119–127. de Kloet ER. Stress in the brain. Eur J Pharmacol 2000;405(1–3):187–198. de Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology 1987;12(2):83–105. Dellu F, Mayo W, Vallee M, Le Moal M, Simon H. Reactivity to novelty during youth as a predic‑ tive factor of cognitive impairment in the elderly: a longitudinal study in rats. Brain Res 1994;653(1–2):51–56. Dellu F, Mayo W, Vallee M, Maccari S, Piazza PV, Le Moal M, et al. Behavioral reactivity to novelty during youth as a predictive factor of stress-induced corticosterone secretion in the elderly – a life-span study in rats. Psychoneuroendocrinology 1996;21(5):441–453. Deminiere JM, Piazza PV, Guegan G, Abrous N, Maccari S, Le Moal M, et al. Increased locomo‑ tor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res 1992;586(1):135–139. Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, GarciaVerdugo JM, et al. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci 2006;24(2):586–594. Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hip‑ pocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience 2004;127(3):601–609. Drapeau E, Abrous DN. Role of neurogenesis in age-related memory disorders. Aging Cell 2008;doi: 10.1111/j.1474-9726.2008.00369.x. Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci 2007;27(22):6037–6044. Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, et al. The aging hip‑ pocampus: a multi-level analysis in the rat. Neuroscience 2006;139(4):1173–1185. Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing fac‑ tor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry 2003;27(4):625–631. Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogen‑ esis and gene expression. J Pain 2006;7(8):544–555. Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117(3):262–266. 4 Stress Disorders 89 Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: insights for addiction and stem cell biology. Hippocampus 2006;16(3):271–286. Eisch AJ, Mandyam CD. Drug dependence and addiction, II: adult neurogenesis and drug abuse. Am J Psychiatry 2004;161(3):426. Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA 2000;97(13):7579–7584. Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 2002;22(9):3312–3320. Falconer EM, Galea LA. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res 2003;975(1–2):22–36. Fan GH, Wang LZ, Qiu HC, Ma L, Pei G. Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol 1999;56(1):39–45. Fischer SJ, Arguello AA, Charlton JJ, Fuller DC, Zachariou V, Eisch AJ. Morphine blood levels, dependence, and regulation of hippocampal subgranular zone proliferation rely on administra‑ tion paradigm. Neuroscience 2008;151(4):1217–1224. Folkman S, Lazarus R. Stress, appraisal, and coping. New York: Springer Publication Co; 1984. Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorso‑ medial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual rein‑ statement of cocaine seeking in rats. Neuropsychopharmacology 2005;30(2):296–309. Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell 2004;3(6):363–371. Gilbey MP, Michael Spyer K. Essential organization of the sympathetic nervous system. Bailliere’s Clin Endocrinol Metab 1993;7(2):259–278. Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 1982;299(5881):355–357. Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intrave‑ nous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114(1):63–70. Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 2002;159(10):1642–1652. Golier JA, Yehuda R, Lupien SJ, Harvey PD. Memory for trauma-related information in Holocaust survivors with PTSD. Psychiatry Res 2003;121(2):133–143. Goodman A. Neurobiology of addiction. An integrative review. Biochem Pharmacol 2008;75(1):266–322. Goshen I, Kreisel T, Menachem-Zidon O, Licht T, Weidenfeld J, Ben Hur T, et al. Brain interleu‑ kin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 2008;13:717–728. Goto Y, O’Donnell P. Synchronous activity in the hippocampus and nucleus accumbens in vivo. J Neurosci 2001;21(4):RC131. Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 1997;17(7):2492–2498. Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 1998;95(6):3168–3171. Grassi ZG, Cipriani S, Balgkouranidou I, Scattoni R. ‘One night’ sleep deprivation stimulates hippocampal neurogenesis. Brain Res Bull 2006;69(4):375–381. Greisen MH, Altar CA, Bolwig TG, Whitehead R, Wortwein G. Increased adult hippocampal brain-derived neurotrophic factor and normal levels of neurogenesis in maternal separation rats. J Neurosci Res 2005;79(6):772–778. Guzman-Marin R, Suntsova N, Stewart DR, Gong H, Szymusiak R, McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol 2003;549(Pt 2):563–571. 90 M. Koehl et al. Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci 2005;22(8):2111–2116. Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience 2007;148(1):325–333. He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogen‑ esis and dendritic growth of newborn neurons. Eur J Neurosci 2005;21(10):2711–2720. Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci 2004;19(1):131–144. Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci 2005;21(5):1304–1314. Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary– adrenocortical axis. Trends Neurosci 1997;20(2):78–84. Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, et al. Evidence for hip‑ pocampal regulation of neuroendocrine neurons of the hypothalamo–pituitary–adrenocortical axis. J Neurosci 1989;9(9):3072–3082. Herman JP, Wiegand SJ, Watson SJ. Regulation of basal corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid expression in the paraventricular nucleus: effects of selective hypothalamic deafferentations. Endocrinology 1990;127(5):2408–2417. Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative cir‑ cuitry produced by a variable stress paradigm. Neuroendocrinology 1995;61(2):180–190. Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, et al. Blockade of cue-in‑ duced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res 2006;30(8):1349–1354. Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA 2003;100(13):7919–7924. Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci 2006;24(7):1845–1849. Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K. Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J Neurosci Res 2007;85(16):3574–3585. Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 2004;7(7):726–735. Holmes MM, Galea LA. Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behav Neurosci 2002;116(1):160–168. Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic–pituitary–adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci 1990;10(10):3247–3254. Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic– pituitary–adrenocortical axis. Endocr Rev 1991;12(2):118–134. Jang MH, Shin MC, Jung SB, Lee TH, Bahn GH, Kwon YK, et al. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport 2002;13(12):1509–1513. Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 2006;31(11):2395–2404. Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal mono‑ aminergic, glutamatergic and nitrergic neurotransmitter systems. Stress 2007;10(3):227–249. Kahn L, Alonso G, Normand E, Manzoni OJ. Repeated morphine treatment alters polysialylated neural cell adhesion molecule, glutamate decarboxylase-67 expression and cell proliferation in the adult rat hippocampus. Eur J Neurosci 2005;21(2):493–500. Kawamura T, Chen J, Takahashi T, Ichitani Y, Nakahara D. Prenatal stress suppresses cell prolif‑ eration in the early developing brain. Neuroreport 2006;17(14):1515–1518. 4 Stress Disorders 91 Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 2001;58(4):334–341. Kim H, Lee M-H, Chang H-K, Lee T-H, Lee H-H, Shin M-C, et al. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Dev 2006;28:109–114. King RS, DeBassio WA, Kemper TL, Rosene DL, Tonkiss J, Galler JR, et al. Effects of prenatal protein malnutrition and acute postnatal stress on granule cell genesis in the fascia dentata of neonatal and juvenile rats. Brain Res Dev Brain Res 2004;150(1):9–15. Kippin TE, Kapur S, van der KD. Dopamine specifically inhibits forebrain neural stem cell prolif‑ eration, suggesting a novel effect of antipsychotic drugs. J Neurosci 2005;25(24):5815–5823. Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. Eur Arch Psychiatry Clin Neurosci 2007;257(5):271–280. Kochman LJ, dos Santos AA, Fornal CA, Jacobs BL. Despite strong behavioral disruption, Delta9-tetrahydrocannabinol does not affect cell proliferation in the adult mouse dentate gyrus. Brain Res 2006;1113(1):86–93. Koehl M, Bjijou Y, Le Moal M, Cador M. Nicotine-induced locomotor activity is increased by preexposure of rats to prenatal stress. Brain Res 2000;882(1–2):196–200. Koehl M, Lemaire V, Le Moal M, Abrous DN. Age-dependent effect of prenatal stress on hip‑ pocampal cell proliferation in female rats. Eur J Neurosci 2009;29(3):635–640. Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA 2008;105(2):751–756. Koo JW, Park CH, Choi SH, Kim NJ, Kim HS, Choe JC, et al. The postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expres‑ sion. FASEB J 2003;17(11):1556–1558. Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 1999;46(9): 1167–1180. Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science 1997;278(5335): 52–58. Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001;24(2):97–129. Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol 2008;59:29–53. Koob GF, Le Moal M. Neurobiology of addiction. Elsevier; 2006. Lagace DC, Yee JK, Bolanos CA, Eisch AJ. Juvenile administration of methylphenidate attenu‑ ates adult hippocampal neurogenesis. Biol Psychiatry 2006;60:1121–1130. Landfield PW. Hippocampal neurobiological mechanisms of age-related memory dysfunction. Neurobiol Aging 1988;9(5–6):571–579. Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal– pharmacological treatments. Science 1981;214(4520):581–584. Lazarus RS. Progress on a cognitive–motivational–relational theory of emotion. Am Psychol 1991;46(8):819–834. Le Moal M. Historical approach and evolution of the stress concept: a personal account. Psychoneuroendocrinology 2007;32 Suppl 1:S3–S9. Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspec‑ tives. Eur Neuropsychopharmacol 2007;17(6–7):377–393. Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, et al. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry 2001;6(6):610, 725–610, 728. Lee KJ, Kim SJ, Kim SW, Choi SH, Shin YC, Park SH, et al. Chronic mild stress decreases sur‑ vival, but not proliferation, of new-born cells in adult rat hippocampus. Exp Mol Med 2006;38(1):44–54. Legault M, Rompre PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci 2000;20:1635–1642. 92 M. Koehl et al. Lemaire V, Aurousseau C, Le Moal M, Abrous DN. Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur J Neurosci 1999;11(11):4006–4014. Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associ‑ ated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 2000;97(20):11032–11037. Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry 2006;59(9):786–792. Li Y-F, Chen H-X, Liu Y, Zhang Y-Z, Liu Y-Q, Li J. Agmatine increases proliferation of cultured hippocampal progenitor cells and hippocampal neurogenesis in chronically stressed mice. Acta pharmacological Sinica 2006;27:1395–1400. Liu Q, Yu J, Mao-Ying QL, Mi WL, Li B, Wang YQ, et al. Repeated clomipramine treatment reversed the inhibition of cell proliferation in adult hippocampus induced by chronic unpre‑ dictable stress. Pharmacogenomics J 2008;8:375–383. Lopez-Fernandez MA, Montaron MF, Varea E, Rougon G, Venero C, Abrous DN, et al. Upregulation of polysialylated neural cell adhesion molecule in the dorsal hippocampus after contextual fear conditioning is involved in long-term memory formation. J Neurosci 2007;27(17):4552–4561. Lu L, Zeng S, Liu D, Ceng X. Inhibition of the amygdala and hippocampal calcium/calmodulindependent protein kinase II attenuates the dependence and relapse to morphine differently in rats. Neurosci Lett 2000;291(3):191–195. Lucassen PJ, Bosch OJ, Jousma E, Kromer SA, Andrew R, Seckl JR, et al. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: possible key role of placental 11beta-hydroxysteroid dehydrogenase type 2. Eur J Neurosci 2009;29(1):97–103. Luo C, Xu H, Li XM. Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress. Brain Res 2005;1063(1):32–39. Ma YY, Guo CY, Yu P, Lee DY, Han JS, Cui CL. The role of NR2B containing NMDA receptor in place preference conditioned with morphine and natural reinforcers in rats. Exp Neurol 2006;200(2):343–355. Maeda K, Sugino H, Hirose T, Kitagawa H, Nagai T, Mizoguchi H, et al. Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J Pharmacol Sci 2007;103(3):299–308. Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 2005;29(4–5):829–841. Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 2003;28(9): 1562–1571. Mandyam CD, Crawford EF, Eisch AJ, Rivier CL, Richardson HN. Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobiol 2008;68:575–589. Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143(1):47–53. Mash DC, Ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J. Gene Expression in human hip‑ pocampus from cocaine abusers identifies genes which regulate extracellular matrix remodel‑ ing. PLoS One 2007;2(11):e1187. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338(3):171–179. McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allo‑ static load. Metabolism 2006;55 (Suppl 2):S20–S23. McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav 2003;43(1):2–15. McGaugh JL. Memory – a century of consolidation. Science 2000;287(5451):248–251. 4 Stress Disorders 93 McNaughton N. Cognitive dysfunction resulting from hippocampal hyperactivity – a possible cause of anxiety disorder? Pharmacol Biochem Behav 1997;56(4):603–611. Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, et al. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc Natl Acad Sci USA 2004;101(26):9822–9826. Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, et al. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleu‑ kin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neuro‑ genesis. Neuropsychopharmacology 2008;33:2251–2262. Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol 2003;459(2):201–207. Meyers RA, Zavala AR, Speer CM, Neisewander JL. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 2006;120(2):401–412. Mineur YS, Belzung C, Crusio WE. Functional implications of decreases in neurogenesis follow‑ ing chronic mild stress in mice. Neuroscience 2007;150(2):251–259. Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 2005;28:223–50. Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 2004;7:841–846. Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci USA 2006;103(50): 19170–19175. Mitra R, Sundlass K, Parker KJ, Schatzberg AF, Lyons DM. Social stress-related behavior affects hippocampal cell proliferation in mice. Physiol Behav 2006;89(2):123–127. Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging 2006;27(645):654. Mudo G, Belluardo N, Mauro A, Fuxe K. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precur‑ sor cell proliferation. Neuroscience 2007;145(2):470–483. Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 1984;5(1):25–44. Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem 2002;78(3):637–647. Neylan TC, Lenoci M, Rothlind J, Metzler TJ, Schuff N, Du AT, et al. Attention, learning, and memory in posttraumatic stress disorder. J Trauma Stress 2004;17(1):41–46. Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus 2006;16(3):287–295. Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem 2002;83(5):1087–1093. Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neuro‑ genesis in protracted abstinence from alcohol. J Neurosci 2004;24(43):9714–9722. Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normal‑ izes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci 2008;28(10):2516–2526. Nowakowski RS, Hayes NL. CNS development: an overview. Dev Psychopathol 1999;11(3):395–417. Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci 2007;26(12):3395–3401. Oomen CA, Girardi CE, Cahyadi R, Verbeek EC, Krugers H, Joels M, et al. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One 2009;4(1):e3675. 94 M. Koehl et al. Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev 1991;43(4):425–473. Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 2000;425(4):479–494. Park H-J, Lim S, Lee H-S, Lee H-J, Yoo Y-M, Lee HJ, et al. Acupuncture enhances cell prolifera‑ tion in dentate gyrus of maternally-separated rats. Neurosci Lett 2002;319(3):153–156. Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling nega‑ tively modulates stress-induced activation of the hypothalamic–pituitary–adrenal axis. Endocrinology 2004;145(12):5431–5438. Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 2003;17(4):879–886. Pham K, McEwen BS, LeDoux JE, Nader K. Fear learning transiently impairs hippocampal cell proliferation. Neuroscience 2005;130(1):17–24. Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an inter‑ action between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 1996;36:359–378. Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev 1997;25(3):359–372. Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci 1998;19(2):67–74. Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science 1989;245(4925):1511–1513. Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activ‑ ity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predis‑ posed to develop amphetamine self-administration. Brain Res 1991;567(1):169–174. Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensationseeking behaviors. Proc Natl Acad Sci USA 1993;90(24):11738–11742. Piazza PV, Deroche-Gamonet V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose–response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 2000;20(11):4226–4232. Plomin R, Owen MJ, McGuffin P. The genetic basis of complex human behaviors. Science 1994;264(5166):1733–1739. Powrozek TA, Sari Y, Singh RP, Zhou FC. Neurotransmitters and substances of abuse: effects on adult neurogenesis. Curr Neurovasc Res 2004;1(3):251–260. Rapp PR, Amaral DG. Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci 1992;15(9):340–345. Reul JM, de Kloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem 1986;24(1):269–272. Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry 2009;14(10): 959–967. Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of exper‑ imenter-versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse 2002;46(4):271–279. Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem 2007;87(4):688–692. Roman V, Van der BK, Leemburg SA, Van der Zee EA, Meerlo P. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res 2005;1065(1–2):53–59. Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consoli‑ dation. Psychoneuroendocrinology 2000;25(3):213–238. 4 Stress Disorders 95 Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res 2005;1040(1–2):55–63. Roth RH, Tam SY, Ida Y, Yang JX, Deutch AY. Stress and the mesocorticolimbic dopamine sys‑ tems. Ann N Y Acad Sci 1988;537:138–147. Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol 2001;13(3):419–449. Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress 1996;1(1):1–19. Sapolsky RM. Stress, the aging brain, and the mechanisms of neuron death. Cambridge, Massachusetts, London, England: A bradford book, The MIT Press; 1992. Scatton B, Simon H, Le Moal M, Bischoff S. Origin of dopaminergic innervation of the rat hip‑ pocampal formation. Neurosci Lett 1980;18(2):125–131. Scerri C, Stewart CA, Breen KC, Balfour DJ. The effects of chronic nicotine on spatial learning and bromodeoxyuridine incorporation into the dentate gyrus of the rat. Psychopharmacology (Berl) 2006;184(3–4):540–546. Schafer I, Najavits LM. Clinical challenges in the treatment of patients with posttraumatic stress disorder and substance abuse. Curr Opin Psychiatry 2007;20(6):614–618. See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci 2003;985:294–307. Selye H. A syndrome produced by diverse nocious agents. Nature 1936;138:32. Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berl) 1994;114(3):523–527. Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 2000;33(1):13–33. Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus 2004;14(3):292–300. Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry 2007;62(5):487–495. Simon M, Czeh B, Fuchs E. Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Res 2005;1049(2):244–248. Smith JE, Dworkin SI. Behavioral contingencies determine changes in drug-induced neurotransmitter turnover. Drug Dev Res 1990;20(3):337–348. Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminer‑ gic system after active self-administration but not after passive administration of methamphet‑ amine. Eur J Pharmacol 1999;371(2–3):123–135. Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: S. Fisher and J. Reason, editor. Handbook of Life Stress, Cognition and Health.New York: John Wiley & Sons; 1988. Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci 2006;9(4):526–533. Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behav‑ ior in rats. J Neurosci 2003;23(32):10258–10264. Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to selfadminister nicotine. Psychopharmacologia 2001;158(2):175–180. Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell prolif‑ eration in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol 2001;437(4):496–504. Tasker J. Endogenous cannabinoids take the edge off neuroendocrine responses to stress. Endocrinology 2004;145(12):5429–5430. Teuchert-Noodt G, Dawirs RR, Hildebrandt K. Adult treatment with methamphetamine tran‑ siently decreases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm 2000;107(2):133–143. 96 M. Koehl et al. Thomas RM, Urban JH, Peterson DA. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hip‑ pocampus. Exp Neurol 2006;201(2):308–315. Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci 2007;27(11): 2734–2743. Thompson AM, Gosnell BA, Wagner JJ. Enhancement of long-term potentiation in the rat hip‑ pocampus following cocaine exposure. Neuropharmacology 2002;42(8):1039–1042. Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience 2004;127(1):177–185. Tung A, Takase L, Fornal C, Jacobs B. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience 2005;134(3):721–723. Tuvnes FA, Steffenach HA, Murison R, Moser MB, Moser EI. Selective hippocampal lesions do not increase adrenocortical activity. J Neurosci 2003;23(10):4345–4354. Veena J, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci Lett 2009;455(3):178–182. Veenema AH, de Kloet ER, de Wilde MC, Roelofs AJ, Kawata M, Buwalda B, et al. Differential effects of stress on adult hippocampal cell proliferation in low and high aggressive mice. J Neuroendocrinol 2007;19(7):489–498. Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttrau‑ matic stress disorder. Biol Psychiatry 2003;54(7):693–702. Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry 2003;54(10):1035–1040. Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocam‑ pal theta burst stimulation. Science 2001;292(5519):1175–1178. Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, et al. Neural substrates of absti‑ nence-induced cigarette cravings in chronic smokers. J Neurosci 2007;27(51):14035–14040. Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. Hippocampal neurogenesis and behavioural stud‑ ies on adult ischemic rat response to chronic mild stress. Behav Brain Res 2008;189:9–16. Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull 2004;64(4):303–308. Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural–neurobiological concordance in the effects of CMS. Neuropsychobiology 2005;52(2):90–110. Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. Wilson JM, Nobrega JN, Corrigall WA, Coen KM, Shannak K, Kish SJ. Amygdala dopamine levels are markedly elevated after self- but not passive-administration of cocaine. Brain Res 1994;668(1–2):39–45. Xu H, Chen Z, He J, Haimanot S, Li X, Dyck L, et al. Synergetic effects of quetiapine and venla‑ faxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus 2006;16(6):551–559. Xu Y, Ku B, Cui L, Li X, Barish PA, Foster TC, et al. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic fac‑ tor expression in chronically stressed rats. Brain Res 2007;1162:9–18. Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, et al. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci 2004;1025:351–362. Yap JJ, Takase LF, Kochman LJ, Fornal CA, Miczek KA, Jacobs BL. Repeated brief social defeat episodes in mice: effects on cell proliferation in the dentate gyrus. Behav Brain Res 2006;172(2):344–350. Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry 2000;61 Suppl 7:14–21. 4 Stress Disorders 97 Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem 2007;103(5):1843–1854. Zuena AR, Mairesse J, Casolini P, Cinque C, Alema GS, Morley-Fletcher S, et al. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One 2008;3(5):e2170. Chapter 5 Depression Shin Nakagawa and Ronald S. Duman Abstract Depression is one of the most prevalent and costly brain diseases. Available antidepressant drugs are safe and effective, but less than half of all patients attain complete remission with single antidepressant and others exhibit partial, refractory or intolerant responses to treatment. Therefore, these findings emphasize the need to discover new antidepressants. The neurogenic hypothesis postulates that a reduced production of new neurons in the adult hippocampus is involved in pathogenesis of depression and an enhancement of hippocampal neurogenesis is one of mechanisms for the successful antidepressant treatment. This article examines this hypothesis with experimental and clinical data. 5.1 Depression Depression is one of the most prevalent and costly brain diseases, affecting 8–12% of individuals at some point in their lifetime (Andrade et al. 2003; Lopez et al. 2006). In addition, depression is a source of significant mortality, with suicide occurring in up to 15% of individuals suffering from severe major depressive disorder. Epidemiological evidence also suggests that there is a fourfold increase in death rates in depressive individuals who are over age 55 years because they are more likely to develop coronary artery disease and type 2 diabetes (Knol et al. 2006). Therefore, it is an urgent issue to understand both the pathogenesis and treatment for depression. The diagnosis of depression remains purely clinical based on observation, interview, history and collateral information, since there are S. Nakagawa (*) Department of Psychiatry, Hokkaido University Graduate School of Medicine, Kita 15, Nishi 7, Kita-ku, Sapporo 060-8638, Japan e-mail: [email protected] R.S. Duman Division of Molecular Psychiatry, Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, Yale University School of Medicine, New Haven, CT, USA T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_5, © Springer 2011 99 100 S. Nakagawa and R.S. Duman no biological or genetic markers with high specificity and sensitivity at the present time. Consequently, major depressive disorder is defined by final expressions (symptoms) of biological changes in nervous system, falling into three primary categories; mood (e.g., sadness, anhedonia, irritability), basic drives (e.g., sleep, eating) and cognition (e.g., ruminations, guilt, indecisiveness, persistent thoughts of suicide). These diverse symptoms suggest that a number of limbic brain structures such as hippocampus might involve in depression (Drevets 2001). 5.2 Stress Decreases Neurogenesis The relationship between psychosocial stressors and the development of depression in susceptible individuals has long been apparent (Kendler et al. 1999; Gold and Chrousos 2002; Caspi et al. 2003; Pittenger and Duman 2008). Experimental animals with chronic stress paradigms show many of the core behavioral characteristics of depression, and they are responsive to antidepressant treatment (Willner 1990). Although several biological reactions to the stress are seen, neurogenesis in adult hippocampus is also regulated by variety of stress. Simple physical stresses such as restraint, immobilization, foot-shock, and predator odor decrease the cell proliferation (Tanapat et al. 2001; Malberg and Duman 2003; Pham et al. 2003; Rosenbrock et al. 2005). Exposure to paradigms of social subordination results in a decrease in neurogenesis in marmosets (Gould et al. 1998), tree shrews (Czéh et al. 2001; van der Hart et al. 2002) and rats (Czéh et al. 2002). Both acute and chronic stresses decrease neurogenesis in the same fashion (Mirescue and Gould 2006). The survival rate of newly born cells is also reduced by stress (Czéh et al. 2002). Although the stress effects in hippocampal neurogenesis are well known, the underlying mechanisms remain poorly understood. There is substantial evidence that adrenal stress hormones play an important role in this process. Stressful experiences stimulate the hypothalamic–pituitary–adrenal axis (HPA) in animals including human. The paraventricular nucleus of the hypothalamus secretes CRF, which stimulates release of adrenocorticotropic hormone (ACTH) by the anterior pituitary. ACTH then stimulates release of glucocorticoids by the adrenal glands. Glucocorticoid hormones act on two main subtypes of receptors in the brain: the mineralcorticoid receptor (MR) and the glucocorticoid receptor (GR). Upon activation, these receptors translocate to the nucleus of the cell where they trigger changes in gene expression, which have long-lasting effects on the structure and functioning of the cells. Although the elevated level of glucocorticoids is reversed by negative-feedback loop in healthy subjects, the hyperactivity of HPA are often seen in depressive patients (de Kloet et al. 2005). Glucocorticoids are themselves regulators of hippocampal neurogenesis, capable of influencing proliferation (Gould et al. 1992; Ambrogini et al. 2002; Wong and Herbert 2005), differentiation (Wong and Herbert 2006), and survival (Wong and Herbert 2004). Exogenous corticosterone administration reduced neurogenesis (Gould et al. 1992; Murray et al. 2008). The proliferation of precursor cells derived from adult rat 5 Depression 101 hippocampus was interfered with by dexamethasone, an agonist of GRs (Boku et al. 2009). In contrast, inhibition of the HPA by adrenalectomy, which removes circulating adrenal steroids, increased neurogenesis (Cameron and Gould 1994). These data suggest that glucocorticoids are involved in mediating the effect of stress on neurogenesis. Glutamate neurotransmission is another candidate whose release is increased by stress in the hippocampus (Abraham et al. 1998). Activation of NMDA receptors inhibited cell proliferation, while blockade had the opposite effect (Cameron et al. 1995, Gould et al. 1997). On the other hand, MK-801, an NMDA receptor antagonist, prevented the exogenous corticosterone-induced decrease in cell proliferation (Cameron et al. 1998). The pro-inflammatory cytokine interlukin-1b (IL-1b), which is elevated in some stress paradigms (Ben Menachem-Zidon et al. 2008), might also contribute to the effect of stress on neurogenesis. The IL-1b receptor was expressed on hippocampal neural progenitor cells (Koo and Duman 2008). Administration of IL-1b reduced the cell proliferation, while blockade of the IL-1b receptor with antagonist prevented the reductions in neurogenesis caused by chronic unpredictable stress (Koo and Duman 2008). In addition, suppressed neurogenesis induced by chronic stress exposure was reversed using transplantation with neural precursors overexpressing IL-1 receptor antagonist to the hippocampus (Ben Menachem-Zidon et al. 2008). 5.3 Is Decreased Neurogenesis One Pathology of Depression? Although it is well established that stress reduces adult hippocampal neurogenesis, there is an argument whether the decreased neurogenesis could induce the many aspects of depressive symptoms. Magnetic resonance imaging (MRI) studies have demonstrated that the hippocampal volume is decreased in depressed subjects (Sheline et al. 1996, 1999, 2003; Shah et al. 1998; Bremner et al. 2000; Mervaala et al. 2000; Steffens et al. 2000; Frodl et al. 2002; MacQueen et al. 2003; Saarelainen et al. 2003; Vermetten et al. 2003; Campbell et al. 2004; Videbech and Ravnkide 2004; Koolschijn et al. 2009; McKinnon et al. 2009). Because stressful life events are associated with the risk of developing depression in vulnerable persons (Kendler et al. 1999; Gold and Chrousos 2002; Caspi et al. 2003), it has been supposed that cellular changes as observed in animal models with prolonged stress also occur in depressed individuals (McEwen 2000). Since dendritic retraction, cellular shrinkage and decreased numbers of glia cells were seen in experimental animals, they have been thought to be major causative factors underlying the volume reduction of hippocampus for many years (Sapolsky 2000). Recent histological analysis of the dentate gyrus of depressed patients showed a significant increase in packing density of dentate granule cells, a trend toward a reduction in the soma size and only slightly increased neuronal apoptosis without cell loss (Lucassen et al. 2001; Muller et al. 2001; Stockmeier et al. 2004). Hippocampal volume reduction therefore might be attributed to a loss of neuropil, 102 S. Nakagawa and R.S. Duman but not to neurodegeneration or to reduced cell counts. Then, how much does decrease of newborn cells play a role in the hippocampal volume loss? Although the exact number of newborn cells in human hippocampus has not so far been assessed, it is estimated from several studies of rodents and primates (Czéh and Lucassen 2007), that about 5,000 new granule cells appear in the human dentate gyrus per month. This accounts for 0.03% of the entire granule cell population of the dentate gyrus, and for 0.017% of the entire neuron population within the hippocampal formation. The estimated number of newborn cells is very small, so it is unlikely that altered rates of neurogenesis can significantly contribute to volume changes of about 10–15%, as observed in depressed subjects. Reif and colleagues have recently reported no significant difference in proliferation of stem/progenitor cells in the hippocampus of postmortem tissue of depressed patients (Reif et al. 2006). Although this work has had a strong impact, it is unfortunate that the sample size is relatively small and the effect of medication (12 of 15 patients were on medication at time of death) cannot be ignored. More histological studies with greater power and inclusion of postmortem tissue of depressed patients without medications are needed to see precise findings. Studies using the method of ablating neurogenesis are also incompatible with the hypothesis that neurogenesis is associated with depressive-like behavior. Irradiated mice having virtually no newborn cells in the hippocampus show normal behavior in novelty-suppressed feeding and glooming score (Santarelli et al. 2003). Anxiety-related behavior as assessed in conflict-based tests, such as the open field, light–dark choice test, and elevated plus-maze are also not influenced (Saxe et al. 2006). In addition, inescapable stress paradigm shows reduced neurogenesis in all stressed animals, even in those not responding to helplessness (Malberg and Duman 2003). There is therefore no clinical and preclinical evidence so far that an altered rate of adult dentate neurogenesis is critical to the etiology of depression. 5.4 The Involvement of Neurogenesis in the Therapeutic Effects of Antidepressant Treatment Different categories of effective antidepressant medications have been fortuitously discovered. Available antidepressant drugs act in one of three ways: (1) blockade of presynaptic monoamine transporter proteins, which removed released transmitter from the extracellular space, (2) inhibition of monoamine oxidase, which degrades monoamine neurotransmitters, or (3) inhibition or excitation of pre- or postsynaptic receptors that regulate monoamine transmitter release and/or neuronal firing rates. These drugs are safe and effective, but less than half of all patients attain complete remission with a single antidepressant. Others exhibit partial, refractory or intolerant responses to treatment, emphasizing the need to discover new antidepressants. Most available antidepressant drugs increase the concentration of serotonin (5-HT) and/or noradrenaline (NA) in the synaptic cleft immediately based on the mechanism shown above (Morilak and Frazer 2004; Berton and Nestler 2006). However, the vast majority of patients do not respond until at least 5 Depression 103 2–4 weeks after the initiation of treatment. Therefore, this clinical observation has led to the almost universal view that, although monoamine systems are integral to the mechanism of action of antidepressants (Heninger et al. 1996), it is not the final common pathway of the action. It has been shown that chronic treatment with antidepressant drugs such as 5-HT or NA selective reuptake inhibitors (SSRIs or NRIs), tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) increase neurogenesis in adult hippocampus (Malberg et al. 2000; Manev et al. 2001; Nakagawa et al. 2002; Duman 2004). The upregulation of neurogenesis occurs after a prolonged period of antidepressant treatment corresponding to the clinical time course for therapeutic action (Malberg et al. 2000; Nakagawa et al. 2002). With stress paradigms or animal models of depression, chronic antidepressant treatment also prevents the decreased neurogenesis. The effect of chronic psychosocial stress is prevented by the simultaneous administration of tianeptine (Czéh et al. 2001) and imipramine (van der Hart et al. 2002). Chronic treatment with escitalopram reverses the chronic mild stress-induced decrease in neurogenesis (Jayatissa et al. 2006). Chronic fluoxetine treatment blocks the reduction of neurogenesis caused by inescapable footshock in the rat, and there is corresponding reversal of behavioral despair in the learned helplessness model (Rosenbrock et al. 2005). Furthermore, Santarelli and his colleagues showed that preventing antidepressant-induced neurogenesis blocked behavioral responses to antidepressants using genetic and radiological methods. 5-HT1A knockout mice were insensitive to the effects of fluoxetine on behavior or neurogenesis. X-irradiation of a restricted region of mouse brain containing the hippocampus disrupted the behavioral effects of fluoxetine or imipramine on novelty-suppressed feeding (Santarelli et al. 2003). These findings suggest a requirement of hippocampal neurogenesis for the behavioral effects of antidepressant. Another independent study confirmed these initial findings as assessed in the forced-swim test with fluoxetine (Airan et al. 2007). However, several other studies have found no effect of blocking neurogenesis in response to antidepressants in animal models (Bessa et al. 2008; Singer et al. 2009; David et al. 2009). The influence of antidepressants on the different aspects of neurogenesis, including proliferation, survival and differentiation, has been examined. Chronic administration of antidepressants increases the number of proliferating and surviving newborn cells without changing the neuronal lineage (Malberg et al. 2000; Nakagawa et al. 2002). Using a reporter mouse line with the expression controlled by nestin promoter, Encinas and colleagues demonstrated early progenitor cells were targeted by fluoxetine (Encinas et al. 2006). In addition, chronic administration of antidepressants accelerates the maturation of newborn neurons in morphology and gene expressions (Fujioka et al. 2004; Wang et al. 2008). Different 5-HT receptors such as 5-HT1A, 5-HT2A and 5-HT2C and NA receptors could be positively involved in the stimulation of cell proliferation in adult hippocampus (Malberg et al. 2000; Radley and Jacobs 2002; Santarelli et al. 2003; Banasr et al. 2004). Through these receptors, chronic administration of antidepressants regulates intracellular signal transduction cascades and the gene expressions of downstream molecules (Carlenzon et al. 2005). One of the signaling cascades is cAMP–cAMP response element binding protein (CREB) cascade. Enhancement of 104 S. Nakagawa and R.S. Duman this cascade increases neurogenesis, while blockade of CREB function decreases it (Nakagawa et al. 2002). In addition, two growth factors whose expression might be regulated by CREB signaling regulate both the proliferation (vascular endothelial growth factor; VEGF) (Cao et al. 2004; Warner-Schmidt and Duman 2007; Jeon et al. 2007) and survival (brain-derived neurotrophic factor; BDNF) (Sairanen et al. 2005) of newborn neurons. Electroconvulsive therapy (ECT) is another popular treatment for depression, which is usually used to treat resistant depression. Interestingly, electroconvulsive seizures, a model of ECT, have more potent stimulation on cell proliferation than chemical antidepressants paralleling its clinical effects (Madsen et al. 2000; Malberg et al. 2000; Scott et al. 2000; Hellsten et al. 2002). These newborn cells integrate into the granule cell layer and survive for at least 3 months after treatment cessation (Madsen et al. 2000). Recent work showed similar results in adult hippocampus of nonhuman primates (Perera et al. 2007). 5.5 Concluding Remarks Although the relationship between neurogenesis and learning-related functions in the normal brain is an intriguing possibility (Leuner et al. 2006), the link to emotion that is mainly disturbed in depression has not been elucidated. In addition, current evidence indicates that adult hippocampal neurogenesis may not be a major contributor to the development of depression. However, is it really not involved in the pathophysiology of depression? Previous preclinical studies saw the behavioral effect of decreased neurogenesis in wild-type animals, but not in animals with the decreased volume of hippocampus that is observed in depressive subjects. Moreover, they did not consider the genetic contribution. Comparative study of adult hippocampal neurogenesis is needed with depressive subjects. Very recently, a new signal processing method was developed that magnetic resonance spectroscopy could identify neuronal progenitor cells in the hippocampus of live humans (Manganas et al. 2007). On the other hand, neurogenesis may be required for some of the behavioral effects of antidepressants. Although some molecular and cellular mechanisms that underlie the regulation of adult neurogenesis have been identified, much more knowledge is needed to develop new treatments for depression. Acknowledgments This work was supported in part by Grant-in-aid No. 18591269 for Scientific Research from the Ministry of Education, Science and Culture, Japan. References Abraham I, Juhasz G, Kekesi KA et al (1998) Corticosterone peak is responsible for stress-induced elevation of glutamate in the hippocampus. Stress 2:171–181 Airan RD, Meltzer LA, Roy M et al (2007) High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317:819–823 5 Depression 105 Ambrogini P, Orsini L, Mancini C et al (2002) Persistently high corticosterone levels but not normal circadian fluctuations of the hormone affect cell proliferation in the adult rat dentate gyrus. Neuroendocrinology 76:366–372 Andrade L, Caraveo-Anduaga JJ, Berglund P et al (2003) The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res 12:3–21 Banasr M, Hery M, Printemps R et al (2004) Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29:450–460 Ben Menachem-Zidon O, Goshen I, Kreisel T et al (2008) Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology 33:2251–2262 Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev 7:137–151 Bessa JM, Ferreira D, Melo I et al (2008) The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–773 Boku S, Nakagawa S, Masuda T et al (2009) Glucocorticoids and lithium reciprocally regulate the proliferation of adult dentate gyrus-derived neural precursor cells through GSK-3beta and beta-catenin/TCF pathway. Neuropsychopharmacology 34:805–815 Bremner J, Narayan M, Anderson ER et al (2000) Hippocampal volume reduction in major depression. Am J Psychiatry 157:115–118 Cameron HA, Gould E (1994) Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61:203–209 Cameron HA, McEwen BS, Gould E (1995) Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci 15:4687–4692 Cameron HA, Tanapat P, Gould E (1998) Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience 82:349–354 Campbell S, Mariott M, Nahmias C et al (2004) Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 161:598–607 Cao L, Jiao X, Zuzga DS et al (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–835 Carlenzon WA Jr, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28:436–445 Caspi A, Sugden K and Moffitt TE et al (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389 Czéh B, Lucassen PJ (2007) What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci 257:250–260 Czéh B, Michaelis T, Watanabe T et al (2001) Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA 98:12796–12801 Czéh B, Welt T, Fischer AK et al (2002) Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 52:1057–1065 David DJ, Samuels BA, Rainer Q et al (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493 de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475 Drevets W (2001) Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol 11:240–249 Duman RS (2004) Depression: a case of neuronal life and death? Biol Psychiatry 56:140–145 106 S. Nakagawa and R.S. Duman Encinas JM, Vaahtokari A, Enikolopov G et al (2006) Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA 103:8233–8238 Frodl T, Meisenzahl EM, Zetzsche T et al (2002) Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 159:1112–1118 Fujioka T, Fujioka A, Duman RS (2004) Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci 24:319–328 Gold PW, Chrousos GP (2002) Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7:254–275 Gould E, Cameron HA, Daniels DC et al (1992) Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci 12:3642–3650 Gould E, McEwen BS, Tanapat P et al (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498 Gould E, Tanapat P, McEwen BS (1998) Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 95:3168–3171 Hellsten J, Wennstrom M, Mohapel P et al (2002) Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosterone treatment. Eur J Neurosci 16:283–290 Heninger GR, Delgado PL, Charney DS (1996) The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29:2–11 Jayatissa MN, Bisgaard C, Tingstrom A et al (2006) Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 31:2395–2404 Jeon SH, Chae BC, Kim HA et al (2007) The PKA/CREB pathway is closely involved in VEGF expression in mouse macrophages. Mol Cells 23:23–29 Kendler KS, Karkowski LM, Prescott CA (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841 Knol MJ, Twisk JW, Beekman AT et al (2006) Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 49:837–845 Koo JW, Duman RS (2008) IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA 105:751–756 Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ et al (2009) Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 30:3719–3735 Leuner B, Gould E, Shors TJ (2006) Is there a link between adult neurogenesis and learning? Hippocampus 16:216–224 Lopez AD, Mathers CD, Ezzati M et al (2006) Global and regional burden of disease and risk factors (2001) systematic analysis of population health data. Lancet 367:1747–1757 Lucassen PJ, Muller MB, Holsboer F (2001) Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol 158:453–468 MacQueen G, Campbell S, McEwen BS et al (2003) Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 100:1387 Madsen TM, Treschow A, Bengzon J et al (2000) Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry 47:1043–1049 Malberg JE, Duman RS (2003) Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28:1562–1571 Malberg J, Eisch AJ, Nestler EJ et al (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110 Manev H, Uz T, Smalheiser NR et al (2001) Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol 411:67–70 Manganas LN, Zhang X, Li Y et al (2007) Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science 318:980–985 McEwen BS (2000) The neurobiology of stress: from serendipity to clinical relevance. Brain Res 886:172–189 5 Depression 107 McKinnon MC, Yucel K, Nazarov A et al (2009) A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 34:41–54 Mervaala E, Fohr J, Kononen M et al (2000) Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 30:117–125 Mirescue C, Gould E (2006) Stress and adult neurogenesis. Hippocampus 16:233–238 Morilak DA, Frazer A (2004) Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol 7:193–218 Muller MB, Lucassen PJ, Yassouridis A et al (2001) Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci 14:1603–1612 Murray F, Smith DW, Huston PH (2008) Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Neurosci 583:115–127 Nakagawa S, Kim JE, Lee R et al (2002) Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 22:3673–3682 Perera T, Coplan JD, Lisanby SH et al (2007) Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci 27:4894–4901 Pham K, Nacher J, Hof PR et al (2003) Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17:879–886 Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109 Radley JJ, Jacobs BL (2002) 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res 955:264–267 Reif A, Fritzen S, Finger M et al (2006) Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 11:514–522 Rosenbrock H, Koros E, Bloching A et al (2005) Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res 1040:55–63 Saarelainen T, Hendolin P, Lucas G et al (2003) Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 23:349–357 Sairanen M, Lucas G, Ernfors P et al (2005) Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25:1089–1094 Santarelli L, Saxe M, Gross C et al (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809 Sapolsky RM (2000) Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57:925–935 Saxe MD, Battaglia F, Wang JW et al (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 103:17501–17506 Scott BW, Wojtowicz JM, Burnham WM (2000) Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol 165:231–236 Shah P, Ebmeier KP, Glabus MF et al (1998) Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry 172:527–532 Sheline Y, Wany P, Gado MH et al (1996) Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93:3908–3913 Sheline Y, Sanghavi M, Mintun MA et al (1999) Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 19:5034–5043 108 S. Nakagawa and R.S. Duman Sheline Y, Gado MH, Kraemer HC (2003) Untreated depression and hippocampal volume loss. Am J Psychiatry 160:1516–1518 Singer BH, Jutkiewicz EM, Fuller CL et al (2009) Conditional ablation and recovery of forebrain neurogenesis in the mouse. J Comp Neurol 20:567–582 Steffens D, Byrum CE, McQuoid DR et al (2000) Hippocampal volume in geriatric depression. Biol Psychiatry 48:301–309 Stockmeier CA, Mahajan GJ, Konick LC et al (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56:640–650 Tanapat P, Hasting NB, Rydel TA et al (2001) Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol 437:496–504 van der Hart M, Czeh B, de Biurrun G et al (2002) Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry 7:933–941 Vermetten E, Vythilingam M, Southwick SM et al (2003) Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 54:693–702 Videbech P, Ravnkide B (2004) Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966 Wang JW, David DJ, Monckton JE et al (2008) Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28:1374–1384 Warner-Schmidt JL, Duman RS (2007) VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA 104:4647 Willner P (1990) Animal models of depression: an overview. Pharmacol Ther 45:425–455 Wong EY, Herbert J (2004) The corticoid environment: a determining factor for neural progenitors’ survival in the adult hippocampus. Eur J Neurosci 20:2491–2498 Wong EY, Herbert J (2005) Roles of mineralocorticoid and glucocorticoid receptors in the regulation of progenitor proliferation in the adult hippocampus. Eur J Neurosci 22:785–792 Wong EY, Herbert J (2006) Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience 137:83–92 Chapter 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia and Related Mental Diseases Noriko Osumi and Nannan Guo Abstract Neurogenesis is a biological process with multiple steps, and it is critical in brain development and maintenance. The initial step in neurogenesis is the division of neural stem cells to renew themselves, simultaneously producing neuronally committed cells. The integrity of neurogenesis is vulnerable to both genetic and environmental factors. Etiological data imply that impairment in neurogenesis, which is possibly caused by genetic factors as well as by infection, stress, and low nutrition, may underlie the onset of mental diseases. In support of this, an intrigu‑ ing association of abnormal hippocampal neurogenesis and deficits in prepulse inhibition (PPI), one of the compelling endophenotypes (biological markers) of mental disorders including schizophrenia, has been reported in various animal models. If impaired neurogenesis is truly a susceptibility factor for mental diseases, the restoration and improvement of neurogenesis may be beneficial. Here, we introduce an approach using fatty acids to restore neurogenesis. In the future, we hope that noninvasive imaging of neurogenesis will provide biological evidence for diagnosis, treatment, and prognosis of mental diseases. 6.1 Introduction Neurogenesis is a biological process consisting of multiple steps, including prolif‑ eration of neural stem/progenitor cells, differentiation into multiple cell types (i.e., neurons, astrocytes, and oligodendrocytes), maturation of neurons, and integration into neural circuits. It is now widely known that neurogenesis persists throughout life in certain brain regions, such as the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG). However, adult neurogenesis is on a continuum with that of the embryonic stages, when initial proliferation of neural stem/progenitor cells and massive production of N. Osumi (*) and N. Guo Division of Developmental Neuroscience, Tohoku University Graduate School of Medicine, 2-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8575, Japan e-mail: [email protected] T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_6, © Springer 2011 109 110 N. Osumi and N. Guo neurons occur. During the initial postnatal period in rodents when rapid growth takes place, neurogenesis still continues in some brain regions in addition to the SVZ and SGZ, such as the nucleus accumbens (Das and Altman, 1970; Bayer, 1979). Such postnatal neurogenesis occurring until the end of puberty may be critical for the dynamic modification of neural networks in accordance with drastic physical changes in the animal, although there is little research focusing on neurogenesis during this period. If damage occurs in the process of neurogenesis during early embryonic devel‑ opment, the architectures of the central nervous system will be drastically impaired, resulting, for example, in microcephaly. The processes that control the numbers, types, and final locations of neurons and the transition from proliferative to neurogenic cell divisions require a complex network of regulation such that neural specification, cell cycle exit, cell differentiation and neuronal migration should all occur in concert. If some minor disturbance happens during these complex steps, the outcome could also elicit mental disorders. Most recent data have focused on various molecules for synaptic formation with regard to mental disorders (Bennett, 2008, 2009; Ramocki and Zoghbi, 2008; Sudhof, 2008). However, the initial steps of neural circuit formation, i.e., maintenance of neural stem/progenitor cells and their proper cell division and differentiation, could equally be critical. One problem in research for understanding mental disorders at the molecular and cellular levels is the lack of biological markers for these diseases; even though genome-wide association studies have been expected to produce useful knowledge, they have yet to do so. Diagnosis of mental disorders is based on interviews and primarily on the subjective feelings of patients, rather than on biological measures. There is no objective score in psychiatry equivalent to blood pressure, blood compo‑ nents, or specific marker expression as used in diagnosis and prognosis of physical diseases, such as metabolic syndromes and cancers. In addition, there are many arguments about the validity of models for psychiatric illnesses, as rodents cannot tell whether they have auditory hallucinations. However, data are accumulating for potential phenotypes (biological traits) that can be modeled in rodents (see below). In this section, we will discuss the association of neurogenesis and the senso‑ rimotor gating function, which could be a biological trait for mental diseases. Based on this discussion, we will propose the “neurogenesis theory,” our hypothesis that impairments in neurogenesis underlie vulnerability for mental diseases. Finally, we will introduce our approach to increasing neurogenesis using polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA) and arachidonic acid (ARA). We believe that PUFA treatment could be a potential intervention for prevention and therapy of mental disorders. 6.2 Risk Factors for Schizophrenia In the last part of this section, we note that neurogenesis is a key event with regard to the onset of various mental disorders. However, here, we focus mainly on schizophrenia because the disease is a major psychiatric illness; it is relatively 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 111 common – schizophrenic patients occupy most of the beds in psychiatric hospitals – and it presents with a range of symptom severity. It places a great burden on patients, their families and society. Mental illnesses, like other common diseases, are affected by multiple genetic and environmental factors. Schizophrenia is a complex and severe brain disorder with poorly defined etiology and pathophysiology, and it has a high heritability rate of around 60–80% (Weinberger, 1996; Rutter et al., 2006; Porteous, 2008; Tan et al., 2009). Obstetric complications, i.e., various problems at the time of intra‑ uterine period and birth, are among the most established environmental risks for schizophrenia. These risks include complications of pregnancy (bleeding, preeclampsia, diabetes and rhesus incompatibility), abnormal fetal growth and devel‑ opment (low birth weight, congenital minor malformations, and small head circumference), and complications of delivery (asphyxia, uterine atony, and emer‑ gency Cesarean section) (Rapoport et al., 2005a). Some obstetric complications involve specific biological mechanisms that may be more relevant to the pathogen‑ esis of schizophrenia. Bleeding in pregnancy, pre-eclampsia, and delivery compli‑ cations are thought to reflect chronic hypoxia or acute asphyxia. In addition, an association exists between schizophrenia and serologically confirmed in utero influenza, rubella, and respiratory infections. Elevated proinflammatory cytokine levels are a key component of immune responses. Watanabe et al. (2004) reported that the treatment of newborn rats with leukemia inhibitory factor (LIF) led to the manifestation of schizophrenia-like phenotypes in adulthood (Watanabe et al., 2004). Intrauterine malnutrition is associated with an increased risk for schizo‑ phrenia (Verdoux et al., 1997; Dalman et al., 1999) and other psychiatric (Eaton et al., 2001; Hultman et al., 2002) and nonpsychiatric disorders, such as cardiovas‑ cular diseases. Various studies report premorbid risks for schizophrenia, which reflect abnormalities in early brain development in the regions responsible for motor, cognitive, and social/emotional functioning. Here, we introduce unique etiological data showing a fivefold increase in the incidence of schizophrenic patients in the area of Chernobyl that suffered from an accident involving atomic energy (Loganovsky and Loganovskaja, 2000; Loganovsky et al., 2005). Although it is very difficult to correlate the low level of irradiation with the development of schizophrenia, it is notable that gamma irradia‑ tion is used for ablating neural stem/progenitor cells in rodents to model some mental diseases, such as depression and posttraumatic spectrum disorder (Mintz et al., 1998; David et al., 2009; Kitamura et al., 2009). It is therefore possible that weak levels of irradiation could damage neural stem/progenitor cells in the human brain. It is increasingly apparent that environmental factors interact with genetic factors. For example, fetal hypoxia predicts reduced gray matter and increased cerebrospi‑ nal fluid (CSF) volume bilaterally throughout the cortex in schizophrenic patients and their siblings but not in subjects at low genetic risk for schizophrenia (Cannon et al., 2002). A number of reviews include information on the many current genetic factors for schizophrenia (Moffitt et al., 2005; Caspi and Moffitt, 2006; Jaffee and Price, 2007). 112 N. Osumi and N. Guo 6.3 Biomarkers/Endophenotypes for Schizophrenia As mentioned in the Introduction, biological markers are not included in the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders IV), which pro‑ vides the standard diagnostic criteria for mental disorders. This makes it difficult to establish animal models. Recent studies and case reports using brain imaging and postmortem brain analyses, however, have suggested several potentially useful biomarkers that can be replicated (Table 6.1). These biomarkers are measurable and often referred to as “endophenotypes” or “intermediate phenotypes.” They are expected to be better targets for genetic analyses than for psychiatric diseases because they are deemed to be structurally less heterogeneous compared to (syndromic) diseases (Cannon and Keller, 2006; Gould and Gottesman, 2006; Meyer-Lindenberg and Weinberger, 2006). Regarding morphological biomarkers, recent advances in brain imaging tech‑ niques have elucidated several abnormalities in the schizophrenic brain, including decreased gray matter volume (between 2 and 3%), expansion of the lateral ven‑ tricle, and regional volume decreases in the hippocampus, thalamus, and frontal lobes (Shenton et al., 2001; Ho, 2007; Pantelis et al., 2007). Longitudinal studies in adult onset patients also provide evidence for progressive changes in the gray mat‑ ter and lateral ventricle (Ho et al., 2003; Pantelis et al., 2003). Postmortem brain analyses provide further biological findings, such as abnormalities in neuronal size, arborization, and synaptic organization (Zaidel et al., 1997). Glial cell abnormali‑ ties, especially in oligodendrocytes, have also been described (Mintz et al., 1998; Williams et al., 2008). The postmortem brains of schizophrenic patients often show a decreased number of neurites and a smaller number of GABAergic neurons (espe‑ cially parvalbumin positive interneurons) (Benes and Berretta, 2001; Lewis et al., 2001, 2005), although there are disagreements about the latter issue. These mor‑ phological/histological changes in the brain are specific not only for schizophrenic patients but also for mental disorders such as autism and bipolar disorder (Bearden et al., 2001; Glahn et al., 2007; White et al., 2008). Event-related EEG potentials (i.e., P300) and prepulse inhibition (PPI) are fre‑ quently used with regard to physiological markers. P300 is a positive response emitted in the brain within a fraction of a second when an individual recognizes and processes an incoming stimulus that is significant or noteworthy. The response is easily measured in humans, but is difficult to apply for rodents. We are thus paying more attention to PPI as a critical/useful biomarker. PPI is the normal suppression of a startle response when a low intensity stimulus eliciting little or no behavioral response immediately precedes an unexpected stronger startling stimulus (Graham, 1975; Hoffman and Ison, 1980; Geyer and Swerdlow, 2001). PPI has been observed in all mammals tested thus far and has even been shown in invertebrates (Mongeluzi et al., 1998). As a behavioral response tool, the acoustic startle reflex (ASR) is invaluable because it can be measured using accessible electrophysiology tools; importantly, measurements are possible under nearly identical conditions between humans and rodents (Swerdlow et al., 1999). Deficits in PPI are a consistent feature of neuropsychiatric illnesses, including schizophrenia, bipolar disorder, autism, and 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 113 Table 6.1 List of biomarkers/endophenotypes for schizophrenia Biomarkers/ endophenotypes Evidences in favor Anatomy/ Brain structural Dorsolateral prefrontal cortex volume reduction chemistry abnormalities (Prasad et al., 2005) Reduced prefrontal grey matter volume (Gur et al., 2000; Hirayasu et al., 2001) and decreased white matter density (Hulshoff Pol et al., 2004) Increased volume of lateral ventricles (Cahn et al., 2002; Mata et al., 2009) Reduced hippocampal grey matter volume (Lawrie and Abukmeil, 1998) Reduction of interneuronal neuropil (Selemon and Goldman-Rakic, 1999) Dopamine Increased D1 receptor availability (Abi-Dargham dysregulation et al., 2002) Excessive stimulation of striatal dopamine (DA) D2 receptors (Abi-Dargham et al., 1998) Deficient stimulation of prefrontal dopamine (DA) D1 receptors (Karlsson et al., 2002) Neurophysiology Inhibitory failure Deficit in prepulse inhibition of startle (Braff et al., 1992; McDowd et al., 1993) P50 auditory evoked potential suppression (Yee et al.; Wegrzyn, 2004) AS (a saccade) eye movement dysfunction (McDowell and Clementz, 2001; Kang et al., 2011) Impaired deviance Mismatch negativity (Michie, 2001) detection P300 (O’Donnell et al., 1999; van der Stelt et al., 2005) Gamma band synchronization (Light et al., 2006; Uhlhaas and Singer, 2010) Working memory Poor performance at the n-back task, a test of Neurocognition deficits working memory (Abi-Dargham et al., 2002) Impaired visual working memory (Tuulio-Henriksson et al., 2003) Verbal immediate recall impairment (Toulopoulou et al., 2003) Episodic memory Executive dysfunction and impaired memory deficits (Byrne et al., 1999) Significantly poorer delayed verbal memory (O’Driscoll et al., 2001) Impairments in short- and long-term memory functioning (Cannon et al., 2005) ADHD (Perry et al., 2001, 2002, 2007; Hawk et al., 2003; Feifel et al., 2009). Interestingly, the neural circuit relevant to PPI includes specific brain regions, such as the neocortex, amygdala, nucleus accumbens, and the hippocampus (Bast and Feldon, 2003). Lipska et al. induced a decrease in PPI by specifically making lesions of the ventral hippocampus in rat pups (Lipska et al., 1995). Here, the above-mentioned morphological marker (i.e., a smaller hippocampus) could be a result of impaired neurogenesis. We have thus begun to focus on the association between impaired neurogenesis and deficits in PPI. 114 N. Osumi and N. Guo 6.4 Neurogenesis and Prepulse Inhibition Multiple examples demonstrate the association between impaired neurogenesis and deficits in PPI. One report demonstrated PPI deficits in mice deficient in Disheveled (Dvl), a downstream modifier in the canonical Wnt pathway (Lijam et al., 1997). Although this report did not mention neurogenesis, the canonical Wnt pathway is critical for this process (Lie et al., 2005; Solberg et al., 2008). Thus, impaired neu‑ rogenesis in these mice is predictable. Furthermore, altered expression of Wnt signaling molecules is reported in the postmortem brains of schizophrenic patients (see review by Kalkman, 2009). The second mutant is the Small eye rat, a spontaneous mutation of the Pax6 gene (Osumi et al., 1997), whose protein product is a key regulator for proliferation and differentiation of neural stem cells, both in prenatal and in postnatal neurogenesis (Osumi et al., 2008). In the hippocampal DG, Pax6 is expressed in neural stem/early progenitor cells (Fig. 6.1) (Maekawa et al., 2005; Nacher et al., 2005). In Pax6 Fig. 6.1 Expression of the Pax6 and Fabp families in hippocampal neurogenesis. Expression of Pax6 (a) and Fabp7 (b) in neural stem/early progenitor cells in the SGZ of rat hippocampus at 4 weeks. Another member of Fabp, Fabp5 is expressed in the neural progenitor cells (c) 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 115 heterozygous mutant rats, a 30% reduction of cell proliferation is observed in the DG, whereas polysialylated neural cell adhesion molecule (PSA-NCAM)-positive late neural progenitor cells and immature neurons are increased (Maekawa et al., 2005). PPI defects are also reproduced in the Pax6 heterozygous mutant rat (Maekawa et al., 2009a). Here, it is noteworthy that Pax6 regulates the expression of some members of Wnt genes (Maekawa et al., 2005). In addition, involvement of Wnt signaling in hippocampal neurogenesis has been reported (Johnson et al., 2009; Kuwabara et al., 2009). The human PAX6 gene is not reported to be associated with schizophrenia, but Yoshikawa’s group and we have recently reported a missense mutation of PAX6 in an aniridia patient (lacking the iris) with autism (Maekawa et al., 2009b). Clinically, overlapping mechanisms are assumed in schizophrenia and autism (Rutter, 1970; Goldstein et al., 2002). As mentioned above, impaired PPI is regarded as an endophenotype for schizo‑ phrenia, and it is thus hoped that identifying genes that underlie PPI could help decipher the complex polygenic mechanisms that predispose individuals to schizo‑ phrenia and related mental disorders. Along this line, Yoshikawa’s group at the RIKEN Brain Science Institute has long worked to find the responsible loci for PPI by quantitative trait loci (QTL) analyses using extreme mouse strains with regard to PPI. After analyzing 1010 F2 mice with a C57BL/6 (showing high scores in PPI) or a C3H background (low scores in PPI), the investigators have come up with several loci on the mouse chromosomes. Among these loci, intensive mapping has indicated that Fabp7 is a relevant gene for PPI in mice (Watanabe et al., 2007). Fabp7 encodes a fatty acid binding protein and is more widely known to neurobi‑ ologists as brain lipid binding protein (BLBP), a marker of neural stem cells (Feng et al., 1994; Kurtz et al., 1994). Yoshikawa and colleagues have also performed human genetic analyses and have revealed a missense mutation that is significantly associated with schizophrenia (Watanabe et al., 2007). We previously demonstrated that Fabp7 is downstream of Pax6 and crucial for embryonic neurogenesis (Arai et al., 2005). In the DG, Fabp7 is specifically expressed in neural stem/early progenitor cells, and severe impairment in the proliferation of neural stem cells is observed in Fabp7−/− mice (Fig. 6.1) (Watanabe et al., 2007). Fabp7 knockout mouse can there‑ fore be a useful model for schizophrenia. The human FABP7 gene is actually mapped on one of the major schizophrenic loci, 6q13-q26 (SCZD5 in OMIN) (Lewis et al., 2003). Expression of FABP7 mRNA in the postmortem brain is surpris‑ ingly higher in the brains of schizophrenic patients compared with those of control subjects (Watanabe et al., 2007). Interestingly, the amount of Fabp7 transcripts in the prefrontal cortex of C3H/He (showing low PPI) is lower than that of C57BL/6 mice (displaying high PPI) at postnatal day 7; however, in the adult stage (8 weeks old), the expression levels of Fabp7 in C3H/He are higher than those of C57BL/6 mice, consistent with results from humans (Watanabe et al., 2007). A possible explanation for this complex pattern of Fabp7 expressions in mice is that the respon‑ sible neural circuit for PPI could be formed by sufficient Fabp7 expression in the brain during the developmental stages, reminiscent of the “neurodevelopment theory of schizophrenia” (see Sect. 6.5). Another possibility is that there may be excess expression of FABP7/Fabp7 gene products in the adult stage to compensate for the 116 N. Osumi and N. Guo insufficient expression during developmental periods. The latter idea recapitulates the current “fetal reprogramming theory” that is applied to metabolic syndromes (Bertram and Hanson, 2001; Nesterenko and Aly, 2009). During the course of our study, other examples showing defects in both neuro‑ genesis and PPI have accumulated (see Table 6.2). “Disrupted in schizophrenia-1” (DISC1) has been identified as a responsible gene for schizophrenia and bipolar disorder from genetic analyses of a large Scottish family (St Clair et al., 1990). The DISC1 protein interacts with other molecules relevant to the centrosome, cytoskeleton and transcription factors (Mackie et al., 2007; Matsuzaki and Tohyama, 2007; Taya et al., 2007; Kalkman, 2009). A line of ENU-mutagenesis mice that have a mutation in the Disc1 gene exhibited schizophrenic-like behavior, with profound deficits in PPI and latent inhibition that were ameliorated by antip‑ sychotic treatment (Clapcote et al., 2007). Moreover, downregulation of DISC1 leads to accelerated neuronal integration, resulting in aberrant morphological development and mispositioning of newly generated dentate granule cells in a cellautonomous fashion (Duan et al., 2007). Neuregulin-1 (NRG1) is a glycoprotein that interacts with the ErbB receptor tyrosine kinase. NRG1 is produced in numerous isoforms by alternative splicing, which allows it to perform a wide variety of functions. A genetic association study has revealed the gain-of-function mutation in NRG1 type IV as a risk factor for schizo‑ phrenia (Stefansson et al., 2003; Tan et al., 2007). NRG1 signals through the ErbB4 receptor tyrosine kinase that becomes autophosphorylated upon NRG1 binding. There are several reports showing abnormally strong signaling of NRG1-ErbB4 in Table 6.2 Association of impaired neurogenesis and deficits in prepulse inhibition Deficit in prepulse Impaired neurogenesis inhibition Gene Sample studied Brain region Sample studied SGZ Npas3 Npas3−/− mice (Pieper Npas3−/− mice (Brunskill et al., 2005) et al., 2005) SVZ Nrg1 +/− mice (Stefansson Nrg1/ErbB4 ErbB4−/− mice (Ghashghaei et al., et al., 2002) 2006) SGZ Dvl1−/− mice (Lijam Wnt3/Dvl1 Rats with blocked Wnt signaling pathway et al., 1997) (Lie et al., 2005) SGZ Disc1 mutant mice Disc1 Retrovirus mediated (Clapcote et al., Disc1 knockdown 2007) mice (Kim et al., 2009) Pax6 Pax6+/− rats (Maekawa SGZ Pax6+/− rats (Maekawa et al., 2009a) et al., 2009a) SGZ Fabp7 Fabp7−/− mice (Watanabe Fabp7−/− mice (Watanabe et al., 2007) et al., 2007) 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 117 the dorsolateral prefrontal cortex of schizophrenia patients (Hahn et al., 2006; Law et al., 2007; Chong et al., 2008). Hyperactivity of Nrg1-ErbB4 signaling contributes to premature differentiation, accelerated maturation, and eventually aberrant inte‑ gration of neurons into the neuronal network (Ghashghaei et al., 2006). Indeed, adult heterozygous mutant mice with a targeted disruption of type III Nrg1 and ErbB2/ErbB4-deficient mice show deficits in PPI as well as other behavioral and morphologically abnormal phenotypes related to schizophrenia (Chen et al., 2008; Barros et al., 2009). The reason for the discrepancy between results from human postmortem brains and animal models is not currently known. The neuronal PAS domain protein 3 (NPAS3) gene, which encodes a brainenriched transcription factor, has been found to be disrupted in a family suffering from schizophrenia (Kamnasaran et al., 2003; Pickard et al., 2005). Npas1/Npas3deficient mice exhibit abnormal PPI behavior (Erbel-Sieler et al., 2004). Npas3−/− mice show reduced neural precursor cell proliferation in the DG by 84% compared with wild-type littermates (Pieper et al., 2005). Brain-derived neurotrophic factor (BDNF) and related neurotrophins play a role in neurodevelopment and are therefore plausible candidates for the pathophys‑ iology of schizophrenia and other mental diseases. Emx-Cre-driven Bdnf knockout mice, in which BDNF is specifically deleted in the dorsal telencephalon, hip‑ pocampus and parts of the ventral telencephalon and amygdala, do not exhibit altered sensory processing and gating as measured by ASR or PPI, although neu‑ rogenesis has not been examined in these mice (Gorski et al., 2003). In contrast, a site-specific knockdown of BDNF has revealed that a reduction in BDNF expres‑ sion in the DG, but not in the CA3 region of the hippocampus, reduces neurogen‑ esis and affects behaviors associated with depression (Taliaz et al., 2010). It would be interesting to know whether a DG-specific knockdown of BDNF also results in abnormality in PPI. There may be more animal models that exhibit both impaired neurogenesis and deficits in PPI. Through analyses of these genetically modified mice, how‑ ever, we cannot determine whether impaired neurogenesis really causes PPI defects. We have taken a different approach by treating wild-type rats with the antiproliferative drug methylazoxymethanol (MAM) acetate for 1 week from postnatal weeks 4 to 5. At 5 weeks, this treatment transiently reduced neurogen‑ esis in the DG, which recovered to normal levels by 10 weeks. Intriguingly, these MAM-treated rats exhibit deficits in PPI (Maekawa et al., 2009a). A previous study in which MAM treatment was given at fetal stages (embryonic day 17 in rats) also showed PPI defects after puberty (Le Pen et al., 2006). It is therefore reasonable to assume that decreased neurogenesis, especially a reduced pool of neural stem/progenitor cells before adolescence, is highly associated with mani‑ festation of impaired PPI in animal models. A critical question that remains unresolved is whether reduced neurogenesis after puberty or even at adult stages impairs PPI, because we assume that a critical period for the formation of the neural circuit of PPI may occur in the neurodevelopmental stage or before puberty (see above). 118 N. Osumi and N. Guo 6.5 The “Neurogenesis Theory” for Schizophrenia and Other Mental Disorders In the late 1980s, Weinberger and colleagues proposed the “neurodevelopmental theory” for the pathogenesis of schizophrenia (Weinberger, 1987). Their original model showed that rats with the ventral hippocampus ablated at the early postnatal period were hyperactive and displayed PPI deficits (Lipska et al., 1993, 1995). To date, accumulating data on the list of risk genes for schizophrenia and other mental disorders, such as mood disorders and autism, provide further evidence that a large number of neurodevelopmental genes are involved (Rapoport et al., 2005b; Nakatani et al., 2006; Harrison, 2007). Among them, molecules governing neuronal migration, axon extension and synapse formation are currently the most intensively studied (Hattori et al., 2007; Budel et al., 2008; Kobayashi, 2009). We focus especially on the earliest step of neural development, i.e., neurogene‑ sis, among multiple neurodevelopmental processes. As already pointed out in other chapters, states of neurogenesis can correlate with proper learning and memory. As mentioned above, there may be a causal relationship between impaired neuro‑ genesis and decreased PPI. Thus, we propose the hypothesis that impaired neurogenesis may underlie vulnerability for the development of schizophrenia and other mental disorders that exhibit impaired PPI (Fig. 6.2). Because significant neurogenesis occurs during fetal stages and continues in postnatal periods until Neurogenesis Stress, infection, low nutrition, genetic factor Microcephaly Depression, PTSD Schizophrenia PUFA intake →prevention or therapy? embryo postnatal adolescence adulthood aged Time Fig. 6.2 Impaired neurogenesis as a risk factor for the onset of mental diseases. Neurogenesis dramatically occurs in the fetal stage, and continues throughout life. Decrease in neurogenesis in the fetal stage will cause severe brain abnormality such as microcephaly, while impairment of neurogenesis in the postnatal stages will cause mental diseases such as schizophrenia, depression, and post-traumatic stress disorder (PTSD) 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 119 the end of life, various magnitudes in impairment of neurogenesis can affect various mental disorders. Of course, if neurogenesis is greatly reduced during the fetal stage, it results in brain abnormalities such as microcephaly and lissencephaly. Decreased neurogenesis is reported to be related to depression, although there is still debate about its causality (Kempermann and Kronenberg, 2003; Paizanis et al., 2007; Sahay and Hen, 2007). It is therefore reasonable to assume that slight impairment of neurogenesis in the relatively late fetal and/or early postnatal stage may play a role in onset of schizophrenia and possibly of related mental diseases. The advantage of this “neurogenesis theory” is that it can most adequately explain gene/environmental issues involved in the etiology of schizophrenia and other mental disorders because neurogenesis is relatively easily susceptible to both genetic and environmental factors. 6.6 Several Methods for Increasing Neurogenesis Here, we propose a “neurogenesis theory” for the etiology of schizophrenia and possibly other mental disorders. Increasing neurogenesis may be beneficial for treating mental conditions. Although the biological meaning of neurogenesis with regard to mental disorders is not yet fully elucidated, targeting neurogenesis in the endeavor of drug discovery is worthwhile. In fact, many researchers have been searching for the proper target molecules to enhance neurogenesis. Interestingly, lithium chloride (LiCl), a well-known mood stabilizer, increases neurogenesis (Kim et al., 2004; Yan et al., 2007). One of the targets of LiCl is considered GSK3beta, a pivotal player in various signaling pathways, which include the Wnt signaling and PI3 kinase pathways (Silva et al., 2008). As mentioned earlier, BDNF can also improve neurogenesis (Schmidt and Duman, 2007; Choi et al., 2009), and its down‑ stream molecules are considered potential targets. Environmental factors also influence the integrity of neurogenesis. Mice and rats are usually housed in boring cages, but maintaining rodents in physically active conditions can increase neurogenesis (Gould et al., 1999b; Ambrogini et al., 2000; Leuner et al., 2004). For example, voluntary wheel-running dramatically enhances neurogenesis in the hippocampus of rodents (Fabel and Kempermann, 2008; Van Praag, 2008). In addition, neurogenesis can be enhanced in rodents maintained in a cage with various toys, together with running wheels (a so-called enriched environ‑ ment) (Van Praag et al., 2000; Muotri et al., 2009). Furthermore, stimulation with different odors every day results in enhanced neurogenesis in the SVZ of mice (Mouret et al., 2008). Our laboratory has taken different approaches. We have paid attention to nutri‑ tion because first, in the songbird, neurogenesis increases in the brain region related to song-learning (Alvarez-Buylla et al., 1988; Kirn et al., 1994). Male song-learning occurs in the reproductive season and is accompanied by massive food intake. In fact, expression of Fabp7/BLBP, which is important for the transport of fatty acids, also increases in accordance with neurogenesis (Rousselot et al., 1997). 120 N. Osumi and N. Guo Second, neurogenesis is influenced by purines (Cho et al., 2009) which are components of DNA and RNA and basically included in foods such as meat, fish, and beans. It is reasonable to assume that neurogenesis is a very energy-consuming process, especially at the first step of cell proliferation. Third, as already mentioned, etio‑ logical studies have shown that low nutrition is a risk factor for schizophrenia (Susser and Lin, 1992; St Clair et al., 2005). The negative spiral in depression and decreased appetite (Whisman, 1993; Ventegodt et al., 2005) would also suggest the importance of nutrition for the treatment of mental diseases. It is also thought that psychiatric patients may not be willing to do physical exercise or other activities. Finally, our research has revealed that Fabp7 is essential for normal neurogenesis in rodents, and that the Fabp7 gene is considered to be related to the risk of schizophrenia (Watanabe et al., 2007). Recent studies in Yoshikawa’s group have demonstrated that this gene is also associated with bipolar disorder and autism (Iwayama et al., 2010; Maekawa et al., 2010). Further in-depth study of fatty acids and their signal mediators should help reveal the mechanisms involved in neurogenesis and indicate how the impairment of such mechanisms can adversely affect brain functions. Based on the above background, we have focused on PUFAs because they can bind to Fabps that are essential in neurogenesis. In addition, the brain is extremely rich in lipids (60% in dry weight), especially in DHA (17%) and ARA (12%) (Soderberg et al., 1991), and fatty acids are important in brain development (Innis, 2007; Innis and Davidson, 2008). DHA can influence the extension of axons and dendrites, release of neurotransmitters, long-term potentiation of syn‑ apses, and resistance to neuronal cell death (Kim et al., 2001; Cao et al., 2009; Menard et al., 2009). ARA is released from phospholipids in the cell membrane in response to neuronal activity (Farooqui et al., 2004). Furthermore, DHA and ARA are found in various foods such as meat, fish, and eggs that may be unavail‑ able in under conditions of severe famine, which is associated with a risk for schizophrenia. 6.7 Increasing Neurogenesis with PUFAs Based on the above information, we have begun our project to increase neurogen‑ esis by modifying nutritious conditions using rats that are most commonly used for experiments in the field of nutritional research. Rat pups from P2 and older and their mothers were fed on control, ARA(+), DHA(+) and ARA(+)/DHA(+) diets for 4 weeks (Maekawa et al., 2009a). Since these PUFAs are hydrophobic molecules, they can be transferred to a rat pup’s brain across the blood–brain barrier. By administrating ARA, the ratio of ARA/DHA becomes 130% in the hip‑ pocampus of rat pups, while it becomes 75% after treatment with DHA. We found a 32% increase in the total number of BrdU-labeled cells in ARA(+) diet-treated rats compared with control diet-treated rats, whereas there were no significant dif‑ ferences between the control diet- and DHA(+) diet-treated animals or between 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 121 control diet- and ARA(+)DHA(+) diet-treated rats. However, a trend of a slight increase was observed in the DHA(+) diet-treated rats and, to lesser extent, in ARA(+)DHA(+) diet-treated animals. In ARA(+) diet-treated rats, the number of glial fibrillary-associated protein (GFAP)-positive cells was slightly increased, while the number of PSA-NCAM-positive cells was dramatically increased. Both GFAP and PSA-NCAM are markers for hippocampal neural progenitor cells, with the former being the earlier stage marker. Interestingly, we observed an elevated number of mossy fibers that expressed PSA-NCAM (data not shown). Thus, the number of proliferating progenitors and subsequent newly born neurons is considerably increased in the hippocampal SGZ of ARA(+) diet-treated rats. Since dietary ARA improved neurogenesis in normal rats, we embarked on a study to determine whether ARA treatments could restore defective neurogenesis (an approximately 30% decrease) in Pax6(+/−) rats (Maekawa et al., 2009a). Wildtype and Pax6(+/−) rat pups were raised on diets either containing or lacking ARA from P2 to P31. When the ARA(+) diet was given to Pax6(+/−) rats, neurogenesis was increased by 33% at P31 compared to rats fed with the control diet. By continu‑ ously administrating ARA, the reduced PPI observed in Pax6(+/−) rats was par‑ tially recovered with an ARA(+) diet at 15 weeks compared to Pax6(+/−) rats fed with a control diet. There were no significant differences in PPI between wild-type rats fed on an ARA(+) diet and Pax6 mutants fed on a ARA(+) diet, indicating that exogenous ARA does not influence on normal PPI scores. This is the first evidence that PPI deficits can be at least partially ameliorated by manipulating nutritious PUFA contents and thus open a way to future application. Further study will be needed to elucidate the effects of PUFAs on PPI. 6.8 Concluding Remarks Significant debate exists regarding whether neurogenesis occurs in adult brain areas other than the SVZ and SGZ (Gould et al., 1999a; Kornack and Rakic, 2001; Rakic, 2002; Dayer et al., 2005; Ohira et al., 2009). Following an injury such as stroke, neuroblasts generated in the SVZ migrate into areas where neurogenesis does not occur, e.g., the striatum and cerebral cortex (Lindvall and Kokaia, 2008). Moreover, some researchers expect the neurogenic potential upon training with tools in the rodent neocortex (Kumazawa et al., 2009). If the integrity of neurogenesis may be an appropriate biological indicator for a certain mental state, noninvasive monitoring for neurogenesis would be beneficial. Since it takes time that impaired neurogenesis eventually causes decreased volume of the hippocampus as clarified by MRI imaging; conventional MRI may thus be inappropriate for predicting the prognosis of mental disease. The PPI score is a good biomarker for several mental disorders but may be useless for the diagnosis of high-risk people at young ages because PPI abnormalities in many animal models only become evident after puberty (i.e., PPI scores increase with age) (Le Pen et al., 2006; Maekawa et al., 2009a). 122 N. Osumi and N. Guo A unique approach uses biopsied olfactory epithelium (Cascella et al., 2007). The olfactory epithelium contains olfactory receptor neurons that sense odorants. Importantly, these olfactory receptor neurons are continuously generated from the neural stem cells in the olfactory epithelium because they are easily damaged. Sawa’s group at Johns Hopkins University considers that proliferative and differen‑ tiation capacities of these biopsied olfactory epithelial cells may be associated with schizophrenic phenotypes. The majority of the schizophrenic patients show defects in olfaction, and first-degree relatives of schizophrenia patients also show such olfactory defects (Moberg et al., 1999). However, we believe this could reflect the competence of neurogenesis in the brain. In fact, the olfactory epithelium is a highly regenerative tissue that expresses Pax6 and Fabp7, markers for neural stem/ progenitor cells even in adult stages (unpublished results). In other words, neurode‑ velopmental processes continue throughout life in the olfactory epithelium as in SVZ and SGZ. A biopsy of the olfactory epithelium and the following culture assays could therefore be used for diagnosis and even for the prognosis of mental diseases. Pioneering work using a different approach has been performed using magnetic resonance spectroscopy. Using this technology, Manganas and colleagues reported a potential biomarker relevant for neural stem/progenitor cells (Manganas et al., 2007). However, debate continues regarding whether this methodology is truly adequate. In the future, development of more specific and sensitive method‑ ologies for monitoring neurogenesis in the living human brain is desired for the diagnosis and prognosis of mental diseases. Acknowledgments We thank Drs. Tatsunori Seki, Takeshi Sakurai, and Takeo Yoshikawa for critical comments on the manuscript. The work on which this review article is based was sup‑ ported by a Grant-in-Aid for Scientific Research on Priority Areas, “Elucidation of Mechanisms Underlying Brain Development and Learning,” from the Core Research for Evolutional Science and Technology (CREST) of the Japanese Science and Technology Corporation (to N.O.). N.G. is supported by Tohoku Neuroscience Global COE. References Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M (1998) Increased striatal dopamine transmission in schizophrenia: con‑ firmation in a second cohort. Am J Psychiatry 155:761–767. Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22:3708–3719. Alvarez-Buylla A, Theelen M, Nottebohm F (1988) Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc Natl Acad Sci USA 85:8722–8726. Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Del Grande P (2000) Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett 286:21–24. Arai Y, Funatsu N, Numayama-Tsuruta K, Nomura T, Nakamura S, Osumi N (2005) Role of Fabp7, a downstream gene of Pax6, in the maintenance of neuroepithelial cells during early embryonic development of the rat cortex. J Neurosci 25:9752–9761. 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 123 Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Muller U (2009) Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci USA 106:4507–4512. Bast T, Feldon J (2003) Hippocampal modulation of sensorimotor processes. Prog Neurobiol 70:319–345. Bayer SA (1979) The development of the septal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol 183:89–106. Bearden CE, Hoffman KM, Cannon TD (2001) The neuropsychology and neuroanatomy of bipo‑ lar affective disorder: a critical review. Bipolar Disord 3:106–150; discussion 151–103. Benes FM, Berretta S (2001) GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25:1–27. Bennett AOM (2008) Dual constraints on synapse formation and regression in schizophrenia: neu‑ regulin, neuroligin, dysbindin, DISC1, MuSK and agrin. Aust N Z J Psychiatry 42:662–677. Bennett MR (2009) Synapse formation and regression in the cortex during adolescence and in schizophrenia. Med J Aust 190:S14–S16. Bertram CE, Hanson MA (2001) Animal models and programming of the metabolic syndrome. Br Med Bull 60:103–121. Braff DL, Grillon C, Geyer MA (1992) Gating and habituation of the startle reflex in schizo‑ phrenic patients. Arch Gen Psychiatry 49:206–215. Brunskill EW, Ehrman LA, Williams MT, Klanke J, Hammer D, Schaefer TL, Sah R, Dorn GW, 2nd, Potter SS, Vorhees CV (2005) Abnormal neurodevelopment, neurosignaling and behav‑ iour in Npas3-deficient mice. Eur J Neurosci 22:1265–1276. Budel S, Padukkavidana T, Liu BP, Feng Z, Hu F, Johnson S, Lauren J, Park JH, McGee AW, Liao J, Stillman A, Kim JE, Yang BZ, Sodi S, Gelernter J, Zhao H, Hisama F, Arnsten AF, Strittmatter SM (2008) Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci 28:13161–13172. Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC (1999) Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS). Psychol Med 29:1161–1173. Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS (2002) Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry 59:1002–1010. Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, Gasperoni T, Tuulio-Henriksson A, Pirkola T, Toga AW, Kaprio J, Mazziotta J, Peltonen L (2005) Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry 62:1205–1213. Cannon TD, Keller MC (2006) Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol 2:267–290. Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG (2002) Fetal hypoxia and structural brain abnor‑ malities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 59:35–41. Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY (2009) Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem 111:510–521. Cascella NG, Takaki M, Lin S, Sawa A (2007) Neurodevelopmental involvement in schizophrenia: the olfactory epithelium as an alternative model for research. J Neurochem 102:587–594. Caspi A, Moffitt TE (2006) Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci 7:583–590. Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, Rosoklija G, Liu RC, Gingrich JA, Small S, Moore H, Dwork AJ, Talmage DA, Role LW (2008) Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and cor‑ ticostriatal circuit components. J Neurosci 28:6872–6883. 124 N. Osumi and N. Guo Cho MH, Lee JH, Ahn HH, Lee JY, Kim ES, Kang YM, Min BH, Kim JH, Lee HB, Kim MS (2009) Induction of neurogenesis in rat bone marrow mesenchymal stem cells using purine structure-based compounds. Mol Biosyst 5:609–611. Choi SH, Li Y, Parada LF, Sisodia SS (2009) Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol Neurodegener 4:52. Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS (2008) Elevated neureg‑ ulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res 100:270–280. Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC (2007) Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54:387–402. Dalman C, Allebeck P, Cullberg J, Grunewald C, Koster M (1999) Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry 56:234–240. Das GD, Altman J (1970) Postnatal neurogenesis in the caudate nucleus and nucleus accumbens septi in the rat. Brain Res 21:122–127. David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493. Dayer AG, Cleaver KM, Abouantoun T, Cameron HA (2005) New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol 168:415–427. Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H (2007) Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130:1146–1158. Eaton WW, Mortensen PB, Thomsen PH, Frydenberg M (2001) Obstetric complications and risk for severe psychopathology in childhood. J Autism Dev Disord 31:279–285. Erbel-Sieler C, Dudley C, Zhou Y, Wu X, Estill SJ, Han T, Diaz-Arrastia R, Brunskill EW, Potter SS, McKnight SL (2004) Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc Natl Acad Sci USA 101:13648–13653. Fabel K, Kempermann G (2008) Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med 10:59–66. Farooqui AA, Ong WY, Horrocks LA (2004) Biochemical aspects of neurodegeneration in human brain: involvement of neural membrane phospholipids and phospholipases A2. Neurochem Res 29:1961–1977. Feifel D, Minassian A, Perry W (2009) Prepulse inhibition of startle in adults with ADHD. J Psychiatr Res 43:484–489. Feng L, Hatten ME, Heintz N (1994) Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron 12:895–908. Geyer MA, Swerdlow NR (2001) Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci Chapter 8:Unit 8 7. Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, Eisenstat DD, Lai C, Anton ES (2006) The role of neuregulin-ErbB4 interactions on the proliferation and organiza‑ tion of cells in the subventricular zone. Proc Natl Acad Sci USA 103:1930–1935. Glahn DC, Thompson PM, Blangero J (2007) Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Brain Mapp 28:488–501. Goldstein G, Minshew NJ, Allen DN, Seaton BE (2002) High-functioning autism and schizophre‑ nia: a comparison of an early and late onset neurodevelopmental disorder. Arch Clin Neuropsychol 17:461–475. Gorski JA, Balogh SA, Wehner JM, Jones KR (2003) Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience 121:341–354. 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 125 Gould TD, Gottesman, II (2006) Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav 5:113–119. Gould E, Reeves AJ, Graziano MS, Gross CG (1999a) Neurogenesis in the neocortex of adult primates. Science 286:548–552. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999b) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260–265. Graham FK (1975) Presidential Address, 1974. The more or less startling effects of weak pre‑ stimulation. Psychophysiology 12:238–248. Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC (2000) Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 57:761–768. Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE (2006) Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 12:824–828. Harrison PJ (2007) Schizophrenia susceptibility genes and their neurodevelopmental implications: focus on neuregulin 1. Novartis Found Symp 288:246–255; discussion 255–259, 276–281. Hattori T, Baba K, Matsuzaki S, Honda A, Miyoshi K, Inoue K, Taniguchi M, Hashimoto H, Shintani N, Baba A, Shimizu S, Yukioka F, Kumamoto N, Yamaguchi A, Tohyama M, Katayama T (2007) A novel DISC1-interacting partner DISC1-binding zinc-finger protein: implication in the modulation of DISC1-dependent neurite outgrowth. Mol Psychiatry 12:398–407. Hawk LW, Jr., Yartz AR, Pelham WE, Jr., Lock TM (2003) The effects of methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention-deficit hyperactivity disorder. Psychopharmacology (Berl) 165:118–127. Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, YurgelunTodd D, Kikinis R, Jolesz FA, McCarley RW (2001) Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex 11:374–381. Ho BC (2007) MRI brain volume abnormalities in young, nonpsychotic relatives of schizophrenia probands are associated with subsequent prodromal symptoms. Schizophr Res 96:1–13. Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M (2003) Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic reso‑ nance imaging study early in schizophrenia. Arch Gen Psychiatry 60:585–594. Hoffman HS, Ison JR (1980) Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev 87:175–189. Hulshoff Pol HE, Schnack HG, Mandl RC, Cahn W, Collins DL, Evans AC, Kahn RS (2004) Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. Neuroimage 21:27–35. Hultman CM, Sparen P, Cnattingius S (2002) Perinatal risk factors for infantile autism. Epidemiology 13:417–423. Innis SM (2007) Dietary (n-3) fatty acids and brain development. J Nutr 137:855–859. Innis SM, Davidson AG (2008) Cystic fibrosis and nutrition: linking phospholipids and essential fatty acids with thiol metabolism. Annu Rev Nutr 28:55–72. Iwayama Y, Hattori E, Maekawa M, Yamada K, Toyota T, Ohnishi T, Iwata Y, Tsuchiya KJ, Sugihara G, Kikuchi M, Hashimoto K, Iyo M, Inada T, Kunugi H, Ozaki N, Iwata N, Nanko S, Iwamoto K, Okazaki Y, Kato T, Yoshikawa T (2010) Association analyses between brainexpressed fatty-acid binding protein (FABP) genes and schizophrenia and bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 153B:484–493. Jaffee SR, Price TS (2007) Gene–environment correlations: a review of the evidence and implica‑ tions for prevention of mental illness. Mol Psychiatry 12:432–442. Johnson MA, Ables JL, Eisch AJ (2009) Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep 42:245–259. 126 N. Osumi and N. Guo Kalkman HO (2009) Altered growth factor signaling pathways as the basis of aberrant stem cell maturation in schizophrenia. Pharmacol Ther 121:115–122. Kamnasaran D, Muir WJ, Ferguson-Smith MA, Cox DW (2003) Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J Med Genet 40:325–332. Kang SS, Dionisio DP, Sponheim SR (2011) Abnormal mechanisms of antisaccade generation in schizophrenia patients and unaffected biological relatives of schizophrenia patients. Psychophysiology 48:350–361. Karlsson P, Farde L, Halldin C, Sedvall G (2002) PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry 159:761–767. Kempermann G, Kronenberg G (2003) Depressed new neurons – adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 54:499–503. Kim HY, Akbar M, Kim KY (2001) Inhibition of neuronal apoptosis by polyunsaturated fatty acids. J Mol Neurosci 16:223–227; discussion 279–284. Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, Son H (2004) Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem 89:324–336. Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL (2009) DISC1 regulates new neuron development in the adult brain via modulation of AKTmTOR signaling through KIAA1212. Neuron 63:761–773. Kirn J, O’Loughlin B, Kasparian S, Nottebohm F (1994) Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc Natl Acad Sci USA 91:7844–7848. Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K (2009) Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139:814–827. Kobayashi K (2009) Targeting the hippocampal mossy fiber synapse for the treatment of psychi‑ atric disorders. Mol Neurobiol 39:24–36. Kornack DR, Rakic P (2001) Cell proliferation without neurogenesis in adult primate neocortex. Science 294:2127–2130. Kumazawa N, Hama H, Kariya E, Miyawaki A, Iriki A (2009) Effects tool-use learning on hip‑ pocampal synaptic circuits in rodent degu (Octodon degu). Neurosci Res 65:1. Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T (1994) The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 120:2637–2649. Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH (2009) Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci 12:1097–1105. Law AJ, Kleinman JE, Weinberger DR, Weickert CS (2007) Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizo‑ phrenia. Hum Mol Genet 16:129–141. Lawrie SM, Abukmeil SS (1998) Brain abnormality in schizophrenia. A systematic and quantita‑ tive review of volumetric magnetic resonance imaging studies. Br J Psychiatry 172:110–120. Le Pen G, Gourevitch R, Hazane F, Hoareau C, Jay TM, Krebs MO (2006) Peri-pubertal maturation after developmental disturbance: a model for psychosis onset in the rat. Neuroscience 143:395–405. Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ (2004) Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci 24:7477–7481. Lewis DA, Cruz DA, Melchitzky DS, Pierri JN (2001) Lamina-specific deficits in parvalbuminimmunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry 158:1411–1422. Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O’Neill FA, Walsh 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 127 D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O’Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T (2003) Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet 73:34–48. Lewis DA, Hashimoto T, Volk DW (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324. Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH (2005) Wnt signalling regulates adult hippocampal neuro‑ genesis. Nature 437:1370–1375. Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL (2006) Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophre‑ nia patients. Biol Psychiatry 60:1231–1240. Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A (1997) Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell 90:895–905. Lindvall O, Kokaia Z (2008) Neurogenesis following stroke affecting the adult brain. In: Adult neurogenesis (Gage FH et al, eds):22. Lipska BK, Jaskiw GE, Weinberger DR (1993) Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology 9:67–75. Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR (1995) Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 122:35–43. Loganovsky KN, Loganovskaja TK (2000) Schizophrenia spectrum disorders in persons exposed to ionizing radiation as a result of the Chernobyl accident. Schizophr Bull 26:751–773. Loganovsky KN, Volovik SV, Manton KG, Bazyka DA, Flor-Henry P (2005) Whether ionizing radia‑ tion is a risk factor for schizophrenia spectrum disorders? World J Biol Psychiatry 6:212–230. Mackie S, Millar JK, Porteous DJ (2007) Role of DISC1 in neural development and schizophre‑ nia. Curr Opin Neurobiol 17:95–102. Maekawa M, Takashima N, Arai Y, Nomura T, Inokuchi K, Yuasa S, Osumi N (2005) Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neuro‑ genesis. Genes Cells 10:1001–1014. Maekawa M, Takashima N, Matsumata M, Ikegami S, Kontani M, Hara Y, Kawashima H, Owada Y, Kiso Y, Yoshikawa T, Inokuchi K, Osumi N (2009a) Arachidonic acid drives postnatal neuro‑ genesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS One 4:e5085. Maekawa M, Iwayama Y, Arai R, Nakamura K, Ohnishi T, Toyota T, Tsujii M, Okazaki Y, Osumi N, Owada Y, Mori N, Yoshikawa T (2010) Polymorphism screening of brain-expressed FABP7, 5 and 3 genes and association studies in autism and schizophrenia in Japanese subjects. J Hum Genet 55:127–130. Maekawa M, Iwayama Y, Nakamura K, Sato M, Toyota T, Ohnishi T, Yamada K, Miyachi T, Tsujii M, Hattori E, Maekawa N, Osumi N, Mori N, Yoshikawa T (2009b) A novel missense mutation (Leu46Val) of PAX6 found in an autistic patient. Neurosci Lett 462:267–271. Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M (2007) Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science 318:980–985. Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Gonzalez-Mandly A, Vazquez-Barquero JL, Crespo-Facorro B (2009) A neuregulin 1 variant is associated with increased lateral ventricle volume in patients with first-episode schizophrenia. Biol Psychiatry 65:535–540. Matsuzaki S, Tohyama M (2007) Molecular mechanism of schizophrenia with reference to disrupted-in-schizophrenia 1 (DISC1). Neurochem Int 51:165–172. 128 N. Osumi and N. Guo McDowd JM, Filion DL, Harris MJ, Braff DL (1993) Sensory gating and inhibitory function in late-life schizophrenia. Schizophr Bull 19:733–746. McDowell JE, Clementz BA (2001) Behavioral and brain imaging studies of saccadic perfor‑ mance in schizophrenia. Biol Psychol 57:5–22. Menard C, Patenaude C, Gagne AM, Massicotte G (2009) AMPA receptor-mediated cell death is reduced by docosahexaenoic acid but not by eicosapentaenoic acid in area CA1 of hippocam‑ pal slice cultures. J Neurosci Res 87:876–886. Meyer-Lindenberg A, Weinberger DR (2006) Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 7:818–827. Michie PT (2001) What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol 42:177–194. Mintz M, Yovel G, Gigi A, Myslobodsky MS (1998) Dissociation between startle and prepulse inhibition in rats exposed to gamma radiation at day 15 of embryogeny. Brain Res Bull 45:289–296. Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL (1999) Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology 21:325–340. Moffitt TE, Caspi A, Rutter M (2005) Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry 62:473–481. Mongeluzi DL, Hoppe TA, Frost WN (1998) Prepulse inhibition of the Tritonia escape swim. J Neurosci 18:8467–8472. Mouret A, Gheusi G, Gabellec MM, de Chaumont F, Olivo-Marin JC, Lledo PM (2008) Learning and survival of newly generated neurons: when time matters. J Neurosci 28:11511–11516. Muotri AR, Zhao C, Marchetto MC, Gage FH (2009) Environmental influence on L1 retrotrans‑ posons in the adult hippocampus. Hippocampus 19:1002–1007. Nacher J, Varea E, Blasco-Ibanez JM, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS (2005) Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J Neurosci Res 81:753–761. Nakatani N, Hattori E, Ohnishi T, Dean B, Iwayama Y, Matsumoto I, Kato T, Osumi N, Higuchi T, Niwa S, Yoshikawa T (2006) Genome-wide expression analysis detects eight genes with robust alterations specific to bipolar I disorder: relevance to neuronal network perturbation. Hum Mol Genet 15:1949–1962. Nesterenko TH, Aly H (2009) Fetal and neonatal programming: evidence and clinical implica‑ tions. Am J Perinatol 26:191–198. O’Donnell BF, McCarley RW, Potts GF, Salisbury DF, Nestor PG, Hirayasu Y, Niznikiewicz MA, Barnard J, Shen ZJ, Weinstein DM, Bookstein FL, Shenton ME (1999) Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology 36:388–398. O’Driscoll GA, Florencio PS, Gagnon D, Wolff AV, Benkelfat C, Mikula L, Lal S, Evans AC (2001) Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizo‑ phrenic patients. Psychiatry Res 107:75–85. Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S (2009) Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M (2008) Concise review: Pax6 tran‑ scription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells 26:1663–1672. Osumi N, Hirota A, Ohuchi H, Nakafuku M, Iimura T, Kuratani S, Fujiwara M, Noji S, Eto K (1997) Pax-6 is involved in the specification of hindbrain motor neuron subtype. Development 124:2961–2972. Paizanis E, Hamon M, Lanfumey L (2007) Hippocampal neurogenesis, depressive disorders, and antidepressant therapy. Neural Plast 2007:73754. Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK (2003) Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 361:281–288. 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 129 Pantelis C, Velakoulis D, Wood SJ, Yucel M, Yung AR, Phillips LJ, Sun DQ, McGorry PD (2007) Neuroimaging and emerging psychotic disorders: the Melbourne ultra-high risk studies. Int Rev Psychiatry 19:371–381. Perry W, Minassian A, Feifel D, Braff DL (2001) Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry 50:418–424. Perry W, Feifel D, Minassian A, Bhattacharjie I, Braff DL (2002) Information processing deficits in acutely psychotic schizophrenia patients medicated and unmedicated at the time of admis‑ sion. Am J Psychiatry 159:1375–1381. Perry W, Minassian A, Lopez B, Maron L, Lincoln A (2007) Sensorimotor gating deficits in adults with autism. Biol Psychiatry 61:482–486. Pickard BS, Malloy MP, Porteous DJ, Blackwood DH, Muir WJ (2005) Disruption of a brain transcription factor, NPAS3, is associated with schizophrenia and learning disability. Am J Med Genet B Neuropsychiatr Genet 136B:26–32. Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, McKnight SL (2005) The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci USA 102:14052–14057. Porteous D (2008) Genetic causality in schizophrenia and bipolar disorder: out with the old and in with the new. Curr Opin Genet Dev 18:229–234. Prasad KM, Chowdari KV, Nimgaonkar VL, Talkowski ME, Lewis DA, Keshavan MS (2005) Genetic polymorphisms of the RGS4 and dorsolateral prefrontal cortex morphometry among first episode schizophrenia patients. Mol Psychiatry 10:213–219. Rakic P (2002) Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci 3:65–71. Ramocki MB, Zoghbi HY (2008) Failure of neuronal homeostasis results in common neuropsy‑ chiatric phenotypes. Nature 455:912–918. Rapoport JL, Addington A, Frangou S (2005a) The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Curr Psychiatry Rep 7:81–82. Rapoport JL, Addington AM, Frangou S, Psych MR (2005b) The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry 10:434–449. Rousselot P, Heintz N, Nottebohm F (1997) Expression of brain lipid binding protein in the brain of the adult canary and its implications for adult neurogenesis. J Comp Neurol 385:415–426. Rutter M (1970) Autistic children: infancy to adulthood. Semin Psychiatry 2:435–450. Rutter M, Moffitt TE, Caspi A (2006) Gene–environment interplay and psychopathology: multi‑ ple varieties but real effects. J Child Psychol Psychiatry 47:226–261. Sahay A, Hen R (2007) Adult hippocampal neurogenesis in depression. Nat Neurosci 10:1110–1115. Schmidt HD, Duman RS (2007) The role of neurotrophic factors in adult hippocampal neurogen‑ esis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 18:391–418. Selemon LD, Goldman-Rakic PS (1999) The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 45:17–25. Shenton ME, Dickey CC, Frumin M, McCarley RW (2001) A review of MRI findings in schizo‑ phrenia. Schizophr Res 49:1–52. Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, Almeida OF, Sousa N (2008) Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience 152:656–669. Soderberg M, Edlund C, Kristensson K, Dallner G (1991) Fatty acid composition of brain phos‑ pholipids in aging and in Alzheimer’s disease. Lipids 26:421–425. Solberg N, Machon O, Krauss S (2008) Effect of canonical Wnt inhibition in the neurogenic cortex, hippocampus, and premigratory dentate gyrus progenitor pool. Dev Dyn 237: 1799–1811. St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ (1990) Association within a family of a balanced autosomal translocation with major mental illness. Lancet 336:13–16. 130 N. Osumi and N. Guo St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P, He L (2005) Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA 294:557–562. Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, Ingason A, Gulcher JR, Stefansson K, St Clair D (2003) Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet 72:83–87. Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K (2002) Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 71:877–892. Sudhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455:903–911. Susser ES, Lin SP (1992) Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Arch Gen Psychiatry 49:983–988. Swerdlow NR, Braff DL, Geyer MA (1999) Cross-species studies of sensorimotor gating of the startle reflex. Ann N Y Acad Sci 877:202–216. Taliaz D, Stall N, Dar DE, Zangen A (2010) Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 15:80–92. Tan W, Wang Y, Gold B, Chen J, Dean M, Harrison PJ, Weinberger DR, Law AJ (2007) Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and iden‑ tification of a functional promoter variant associated with schizophrenia. J Biol Chem 282:24343–24351. Tan HY, Callicott JH, Weinberger DR (2009) Prefrontal cognitive systems in schizophrenia: towards human genetic brain mechanisms. Cogn Neuropsychiatry 14:277–298. Taya S, Shinoda T, Tsuboi D, Asaki J, Nagai K, Hikita T, Kuroda S, Kuroda K, Shimizu M, Hirotsune S, Iwamatsu A, Kaibuchi K (2007) DISC1 regulates the transport of the NUDEL/ LIS1/14-3-3epsilon complex through kinesin-1. J Neurosci 27:15–26. Toulopoulou T, Morris RG, Rabe-Hesketh S, Murray RM (2003) Selectivity of verbal memory deficit in schizophrenic patients and their relatives. Am J Med Genet B Neuropsychiatr Genet 116B:1–7. Tuulio-Henriksson A, Arajarvi R, Partonen T, Haukka J, Varilo T, Schreck M, Cannon T, Lonnqvist J (2003) Familial loading associates with impairment in visual span among healthy siblings of schizophrenia patients. Biol Psychiatry 54:623–628. Uhlhaas PJ, Singer W (2010) Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11:100–113. van der Stelt O, Lieberman JA, Belger A (2005) Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr Res 77:309–320. Van Praag H (2008) Neurogenesis and exercise: past and future directions. Neuromolecular Med 10:128–140. Van Praag H, Kempermann G, Gage FH (2000) Neural consequences of environmental enrich‑ ment. Nat Rev Neurosci 1:191–198. Ventegodt S, Andersen NJ, Neikrug S, Kandel I, Merrick J (2005) Clinical holistic medicine: holistic treatment of mental disorders. ScientificWorldJournal 5:427–445. Verdoux H, Geddes JR, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, Stober G, Willinger MU, Wright P, Murray RM (1997) Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry 154:1220–1227. Watanabe Y, Hashimoto S, Kakita A, Takahashi H, Ko J, Mizuno M, Someya T, Patterson PH, Nawa H (2004) Neonatal impact of leukemia inhibitory factor on neurobehavioral development in rats. Neurosci Res 48:345–353. 6 Impaired Neurogenesis as a Risk Factor for Schizophrenia 131 Watanabe A, Toyota T, Owada Y, Hayashi T, Iwayama Y, Matsumata M, Ishitsuka Y, Nakaya A, Maekawa M, Ohnishi T, Arai R, Sakurai K, Yamada K, Kondo H, Hashimoto K, Osumi N, Yoshikawa T (2007) Fabp7 maps to a quantitative trait locus for a schizophrenia endopheno‑ type. PLoS Biol 5:e297. Wegrzyn J (2004) [P50 sensory gating disorders of auditory evoked potentials (AEP) in persons with schizophrenia]. Psychiatr Pol 38:833–845. Weinberger DR (1987) Implications of normal brain development for the pathogenesis of schizo‑ phrenia. Arch Gen Psychiatry 44:660–669. Weinberger DR (1996) On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology 14:1S–11S. Whisman MA (1993) Mediators and moderators of change in cognitive therapy of depression. Psychol Bull 114:248–265. White T, Nelson M, Lim KO (2008) Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging 19:97–109. Williams M, Hirsch SR, Ansorge O, Thom M, Maier M (2008) Oligodendrocyte abnormalities in mood and affective disorders in the anterior cingulate cortex and corpus callosum in the postmortem brain. Schizophr Res 98:1. Yan XB, Hou HL, Wu LM, Liu J, Zhou JN (2007) Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology 53:487–495. Yee CM, Williams TJ, White PM, Nuechterlein KH, Ames D, Subotnik KL (2010) Attentional modulation of the P50 suppression deficit in recent-onset and chronic schizophrenia. J Abnorm Psychol 119:31–39. Zaidel DW, Esiri MM, Harrison PJ (1997) Size, shape, and orientation of neurons in the left and right hippocampus: investigation of normal asymmetries and alterations in schizophrenia. Am J Psychiatry 154:812–818. Chapter 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke: A Future Therapeutic Target to Promote Functional Restoration? Olle Lindvall and Zaal Kokaia Abstract Recent experimental evidence obtained mainly in rodents has indicated that the stroke-damaged adult brain makes an attempt to repair itself by producing new neurons from its own neural stem cells. Here, we summarize the current status of this research with an emphasis on how, in the future, optimization of this potential self-repair mechanism could become of therapeutical value to promote functional restoration after stroke. Currently, our knowledge about the mechanisms regulating the different steps of neurogenesis after stroke is incomplete. Despite a lot of circumstantial evidence, we also do not know if stroke-induced neurogenesis contributes to functional improvement and to what extent the new neurons are integrated into existing neural circuitries. It is highly likely that, in order to have a substantial impact on the recovery after stroke, neurogenesis has to be markedly enhanced. Based on available data, this should primarily be achieved by increasing the survival and differentiation of the generated neuroblasts. Moreover, for maximum functional recovery, optimization of neurogenesis most likely needs to be combined with stimulation of other endogenous neuroregenerative responses, e.g., protection and sprouting of remaining mature neurons, and transplantation of stem cell-derived neurons and glia cells. O. Lindvall (*) Laboratory of Neurogenesis and Cell Therapy, Section of Restorative Neurology, Wallenberg Neuroscience Center, University Hospital, 221 84, Lund, Sweden and Lund Strategic Research Center for Stem Cell Biology and Cell Therapy, Lund, Sweden e-mail: [email protected] Z. Kokaia Laboratory of Neural Stem Cell Biology, Section of Restorative Neurology, Stem Cell Institute, University Hospital, 221 84, Lund, Sweden and Lund Strategic Research Center for Stem Cell Biology and Cell Therapy, Lund, Sweden T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_7, © Springer 2011 133 134 O. Lindvall and Z. Kokaia 7.1 Introduction In stroke, occlusion of a cerebral artery gives rise to focal ischemia with irreversible injury in a core region and partially reversible damage in the surrounding penumbra zone. Stroke is a leading cause of chronic disability in humans. No effective treatment to promote recovery in patients exists. Many different types of neurons and glial cells die in stroke, and to repair the stroke-damaged brain may, therefore, seem unrealistic. However, even re-establishment of only a fraction of damaged neuronal circuitries could have important clinical implications. During recent years, stroke has been shown to induce the formation of new neurons in the adult rodent brain from neural stem cells located in two regions: the subventricular zone (SVZ), lining the lateral ventricle, and the subgranular zone (SGZ) in the dentate gyrus (for review, see Jin and Galvan, 2007; Zhao et al., 2008). Stroke-induced neurogenesis is triggered both in areas where new neurons are normally formed, such as the dentate gyrus, and in areas which are non-neurogenic in the intact brain, e.g., the striatum. These findings have raised the possibility that the adult brain makes an attempt to repair itself, which may be of clinical importance, but two major issues need to be addressed. First, what are the consequences of ischemia-induced neurogenesis; does it contribute to disease symptomatology, or counteract symptoms, improving functional recovery? Second, because the neurogenic response is minor and recovery after stroke incomplete, how can this presumed self-repair mechanism be boosted? In this chapter, we will summarize research on neurogenesis after stroke with an emphasis on our own recent findings, and discuss the scientific problems that need to be addressed before this potential self-repair mechanism could be considered in a clinical–therapeutical perspective. We will focus on stroke-induced formation of new neurons in damaged areas where neurogenic mechanisms do not normally operate, i.e., striatum and cerebral cortex. The influence of stroke on hippocampal neurogenesis will not be covered here. For more comprehensive reviews about neurogenesis after cerebral ischemia, the reader is referred to Lindvall et al. (2004), Lichtenwalner and Parent (2006), Taupin (2006) and Greenberg (2007). 7.2 Stroke-Induced Neurogenesis in Rodents 7.2.1 Proliferation of Neural Stem/Progenitor Cells in Subventricular Zone In the most common model for neurogenesis research, stroke is induced by transient middle cerebral artery occlusion (MCAO) in rats or mice, which is accomplished by insertion of a filament through the internal carotid artery to the origin of the middle cerebral artery (MCA). Recirculation is restored by withdrawal of the filament. Depending on the duration of the occlusion, only the dorsolateral part of 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke 135 the striatum will be damaged (30 min MCAO in rats), or the lesion will extend into the overlying parietal cortex (2 h MCAO). Stroke stimulates cell proliferation in the ipsilateral SVZ (Fig. 7.1), which is elevated 1–2 weeks after MCAO (Jin et al., 2001; Arvidsson et al., 2002; Parent et al., 2002; Zhang et al., 2004a,b), but has returned to baseline at 6 weeks (Thored et al., 2006). However, the ipsilateral SVZ is expanded up to 16 weeks after MCAO (Thored et al., 2006, 2007), and the number and size of neurospheres isolated from the ipsilateral SVZ are increased. These findings indicate that stroke leads to longterm alterations in the stem cell niche in the SVZ. Many compounds and treatments have been reported to increase SVZ cell proliferation after stroke (for references, see, e.g., Lindvall and Kokaia, 2008). Much less is known about endogenous mechanisms regulating progenitor proliferation in the SVZ. It has been shown that Notch1 and its naturally occurring activator, Jagged1, are expressed in the adult SVZ (Stump et al., 2002), and that administration of Notch ligands increases the number of proliferating cells in the SVZ after stroke (Androutsellis-Theotokis et al., 2006). Insulin-like growth factor-1 (IGF-1) has also been proposed to be a mediator of the increased SVZ progenitor proliferation after stroke (Yan et al., 2007). Our recent data indicate that tumor necrosis factor-a (TNF-a), through one of its receptor subtypes, TNF-R1, can influence SVZ progenitor proliferation after stroke (Iosif et al., 2008). TNF-a is a major player in many neurodegenerative diseases including stroke (Hallenbeck, 2002), its main source being activated microglia (Gebicke-Haerter, 2001). Concomitantly with the increased proliferation 1 week after stroke, we found elevated microglia numbers and TNF-a and TNF-R1 gene expression in mouse SVZ. TNF-R1 was expressed on SVZ progenitor cells. In animals lacking TNF-R1, stroke-induced SVZ cell proliferation and neuroblast formation were enhanced. In support of the in vivo data, addition of TNF-a reduced size and numbers of SVZ neurospheres through a TNF-R1-dependent mechanism. Our results identify TNF-R1 as the first negative regulator of stroke-induced SVZ progenitor proliferation. Blockade of TNF-R1 signaling might be a novel strategy to promote the proliferative response in SVZ after stroke. 7.2.2 Duration of Neurogenic Response In the initial studies, stroke-induced neurogenesis was believed to be a short-lasting response with a duration of only a few weeks. There is now experimental evidence indicating that the formation of new striatal neurons can continue at least up to 1 year after 2 h MCAO in rats (Kokaia et al., 2006; Thored et al., 2006). Neuroblasts expressing the marker doublecortin (Dcx) in the damaged striatum were already more numerous at 1 week, and the number was stable thereafter up to 4 months following the insult. Dcx is a reliable marker of neurogenesis, which is transiently expressed (during about 2–3 weeks) in newly formed neuroblasts in both the intact and injured brain (Brown et al., 2003; Couillard-Despres et al., 2005). The number 136 O. Lindvall and Z. Kokaia Fig. 7.1 Schematic representation of stroke-induced striatal and cortical neurogenesis. Neural stem/ progenitor cells reside in the SVZ adjacent to the lateral ventricle (a). The focal ischemic insult leads to loss of either striatal and cortical (b, c), or only cortical (d, e) neurons, which gives rise to increased proliferation of the progenitors (b, d). Neuroblasts formed after, and to some extent also before, the stroke migrate to the damaged part of the striatum (b) or cortex (d) where they differentiate and express markers of mature neurons (c, e). In some studies, evidence for cortical neurogenesis has been obtained in animals with ischemic damage in both striatum and cortex. LV Lateral ventricle 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke 137 of Dcx+ cells then declined up to the 1-year time point, when it corresponded to about one-quarter of that observed during the first 4 months. At all time points, the Dcx+ cells migrated from the SVZ laterally and ventrally into the damaged area (Fig. 7.1). We confirmed that the Dcx+ neuroblasts also formed long-term after the insult differentiated into mature neurons using BrdU injections. Interestingly, the ratio between NeuN+/BrdU+ cells and Dcx+ cells at 1 year was similar to that at 6 weeks. Taken together, our findings indicate that striatal neurogenesis after stroke continues at least for 1 year, and that despite a decline in the formation of new neuroblasts at this late time point, the yield of differentiated neurons seems to be unchanged. 7.2.3 Phenotype of New Neurons The phenotype of the stroke-generated neurons is only partly known. In most studies, co-expression of the mature neuronal marker NeuN in BrdU-labeled cells has been the only evidence for the formation of new differentiated neurons. Following 2 h MCAO, 42% of the mature, new neurons expressed DARPP-32 (Arvidsson et al., 2002), which is a specific marker for striatal medium-sized spiny neurons. This is the phenotype of most of the neurons destroyed by the ischemic lesion. Similarly, Parent et al. (2002) reported that a majority of new neurons in the peri-infarct area after stroke were DARPP-32-positive. In another model of striatal injury (excitotoxic lesion by quinolinic acid), Collin et al. (2005) also identified newly generated cells expressing markers characteristic of striatal interneurons (parvalbumin and neuropeptide Y). Such cells were not detected following MCAO in rats (Parent et al., 2002). In contrast, Teramoto et al. (2003) did not observe any BrdU+ cells expressing DARPP-32 but a substantial number of BrdU+ cells co-labeled with parvalbumin after stroke in mice, which had been infused with EGF. It will be of major interest in future studies to determine to what extent the whole repertoire of striatal neurons with their specific phenotypic characteristics can be generated from SVZ stem/progenitor cells after stroke. 7.2.4 Influence of Aging It is well established that stroke mainly affects older people. Thus, if cell replacement from endogenous neural stem cells should have any impact on functional restoration in the majority of stroke patients, it is of major importance that the new neurons can also be recruited to the ischemically damaged area in the aged brain. We found no differences between young (3 months) and old (15 months) rats in the number and distribution of neuroblasts and mature neurons in the damaged striatum after stroke (Darsalia et al., 2005). Our findings also indicate that the stroke-damaged aged brain is permissive for neuroblast migration and that this potential self-repair mechanism seems to operate similarly in young and old brains. 138 O. Lindvall and Z. Kokaia 7.2.5 Influence of Inflammation A major problem with endogenous neurogenesis as a potential mechanism for cell replacement after stroke is that only a fraction of the new neurons will survive longterm. We have estimated that about 80% of the stroke-generated striatal neurons die during the first 2 weeks after their formation (Arvidsson et al., 2002). The mechanisms underlying the poor survival of the new neurons are incompletely understood. Caspases probably play a role because administration of caspase inhibitors has been shown to lead to markedly improved survival of neuroblasts in the damaged striatum (Thored et al., 2006). It is also conceivable that the inflammatory changes accompanying the ischemic damage contribute to the poor survival of the new striatal neurons similar to what has been described for new hippocampal neurons early after they have been born in an inflammatory environment (Ekdahl et al., 2003; Monje et al., 2003). Consistent with a cytotoxic action of microglia after cerebral ischemia, administration of the anti-inflammatory drug indomethacin improved the survival of stroke-generated neuroblasts in the striatum (Hoehn et al., 2005). Similarly, chronic delivery of minocycline, which reduced microglia activation, increased the number of new neuroblasts and mature neurons in the dentate gyrus after MCAO (Liu et al., 2007). The role of inflammation for stroke-induced neurogenesis has, however, turned out to be much more complex, and evidence is now accumulating which indicates that microglia can also be beneficial. In support, chronically activated microglia are permissive to neuronal differentiation and survival in adult mouse SVZ cultures (Cacci et al., 2008). Microglia and microglia-conditioned medium rescue the in vitro formation of neuroblasts from SVZ NSCs, which otherwise is lost with continued culture (Aarum et al., 2003; Walton et al., 2006). Furthermore, we have recently demonstrated increased numbers of activated microglia in the ipsilateral SVZ concomitant with neuroblast migration into the striatum up to 16 weeks following 2 h MCAO in rats (Thored et al., 2009). In the SVZ, microglia exhibited ramified or intermediate morphology, signifying a downregulated inflammatory profile, whereas amoeboid or round phagocytic microglia were frequent in the periinfarct striatum. Strong long-term upregulation of the IGF-1 gene and elevated numbers of IGF-1-expressing microglia were found in the ipsilateral SVZ up to 16 weeks after stroke. IGF-1 protein has been reported to mitigate apoptosis and promote proliferation and differentiation of neural progenitors (Camarero et al., 2003; Otaegi et al., 2006; Kalluri et al., 2007), probably directly via IGF-1 receptors on the NSCs themselves (Yan et al., 2006). The long-term accumulation of microglia with proneurogenic phenotype in the SVZ implies a supportive role of these cells for the continuous neurogenesis after stroke. Consistent with this idea, overexpression of IGF-1 via adeno-associated virus-mediated gene transfer enhanced neurogenesis in the SVZ after stroke in mice (Zhu et al., 2008). It has been proposed that autoimmune, CNS-specific T cells, by interacting with resident microglia, can promote progenitor proliferation in the SGZ and SVZ and possibly also neuronal survival and differentiation (Ziv et al., 2006). 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke 139 In the dentate gyrus, enriched environment stimulated neurogenesis and led to increased numbers of microglia expressing MHC-II and IGF-1 and recruitment of T lymphocytes (Ziv et al., 2006). Lack of T cells reduced progenitor proliferation, which was restored by T cell replenishment. Moreover, modulation of the immune system by downregulation of the activity of regulatory T cells using subcutaneous injection of the copolymer poly-YE directly after induction of permanent MCAO in rats increased hippocampal and induced cortical neurogenesis (Ziv et al., 2007). This procedure probably enhanced the rapid recruitment of the relevant T cells recognizing CNS antigens. Consistent with the findings of Ziv et al. (2006), we have found that, following stroke, SVZ contained increased numbers of microglia expressing MHC-II and IGF-1, i.e., molecules that are believed to be associated with a neuroprotective microglial phenotype. However, very few lymphocytes were detected in the SVZ at all time points although lymphocytes were readily found in the peri-infarct striatal area, especially during the first days after MCAO. Thus, our data do not provide supportive evidence for the idea that T lymphocyte– microglia interaction in the neurogenic niche in SVZ is of significant importance for long-term neurogenesis after stroke. 7.2.6 Recruitment to Damaged Area If the new neurons generated in the SVZ after stroke should contribute to the functional restoration, they must efficiently reach those areas in which the old neurons have died as a result of the insult. Radial glia are distributed both in the SVZ and possibly the ischemic striatum after stroke and are probably involved in guiding the migrating neuroblasts (Zhang et al., 2007). There is also a close association between the newly formed neurons and the vasculature when they migrate towards the damaged area. At 18 days following 30 min MCAO in mice, Yamashita et al. (2006) found chains of neuroblasts being wound around endothelial cells, the neuroblasts being elongated parallel to the blood vessels and the chains oriented in the mediolateral direction. In another model, at 7 days after focal cortical stroke in mice (Ohab et al., 2006), neuroblasts were located in large numbers in physical proximity to endothelial cells in the peri-infarct cortex, where active vascular remodeling occurred. We have found (Thored et al., 2007) that the newly formed neuroblasts are located in the vicinity of blood vessels throughout long-term neurogenesis (at least up to 16 weeks after the insult). The majority of the neuroblasts migrated towards the ischemic damage through the striatal area which selectively, as compared to other striatal areas adjacent to SVZ, exhibited long-lasting increase of vessel density and, during the first 2 weeks after the insult, endothelial cell proliferation, consistent with angiogenesis. In further support of neurogenesis-angiogenesis interaction, the study of Ohab et al. (2006) indicated that neurogenesis and angiogenesis in the peri-infarct cortex were causally linked through vascular production of stromal-derived factor 1 (SDF-1) and angiopoietin 1. However, the blood vessels could also play a role for the survival and differentiation of the 140 O. Lindvall and Z. Kokaia closely located neuroblasts during their migration by endothelial release of other factors such as brain-derived neurotrophic factor (Louissaint et al., 2002; Wang et al., 2007). Also, other molecular mechanisms are involved in recruiting the new neurons to the damaged area. The most well-established one is SDF-1a and its cognate receptor CXCR4. Following stroke, SDF-1a is expressed in reactive atrocytes and activated microglia in the damaged area (Thored et al., 2006) and SDF-1a levels are increased in the stroke hemisphere (Robin et al., 2006). CXCR4 is expressed on the neural progenitors and stroke-generated neuroblasts (Robin et al., 2006). When blocking CXCR4, the migration of neuroblasts was markedly attenuated (Robin et al., 2006; Thored et al., 2006). Taken together, these data indicate that SDF-1a/CXCR4 signaling regulates the directed migration of new striatal neurons towards the ischemic damage. Similarly, monocyte chemoattractant protein-1 (MCP-1) (Yan et al., 2007) is upregulated in activated microglia and reactive astrocytes in the cortex and striatum, and the new neuroblasts express the MCP-1 receptor CCR2 after stroke. Consistent with a role of this factor in the migration, transgenic mice lacking MCP-1 or CCR2 showed less recruitment of neuroblasts into the striatum after stroke (Yan et al., 2007). Finally, Lee et al. (2006) showed that the extracellular protease matrix metalloproteinase-9 (MMP-9) is colocalized with the neuroblast marker Dcx in the SVZ and striatum following stroke in mice. Administration of an MMP inhibitor markedly reduced neuroblast migration. Thus, extracellular proteolysis through the action of MMP seems to be involved in neuroblast migration through the ischemic and partially damaged striatum after stroke (Lee et al., 2006). We have found that the extension of the ischemic damage plays an important role for the recruitment of neuroblasts to the striatum after stroke. Following 30 min MCAO, which gave rise to a lesion restricted to the dorsolateral striatum, the new striatal neuroblasts were markedly fewer as compared to following a 2 h insult, which caused more extensive striatal damage and, in addition, injury to the overlying parietal cortex (Thored et al., 2006). The number of neuroblasts correlated significantly with the volume of striatal injury. We conclude that the extent of the ischemic lesion influences the degree of activation of the molecular and cellular mechanisms that promote recruitment of new neurons to the striatum after stroke (Fig. 7.1). Therefore, small lesions in the striatum as well as isolated cortical lesions, which trigger cell proliferation in the SVZ, will not give rise to significant neuroblast migration into the striatum. It remains to be established how the underlying mechanisms should be optimized in order to promote migration of neuroblasts, 7.2.7 Cortical Neurogenesis In the first study reporting neuronal replacement from endogenous precursors following injury to the adult mammalian brain, selective apoptotic degeneration of neurons in cerebral cortex was found to give rise to formation of new corticothalamic neurons 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke 141 (Magavi et al., 2000). However, the number of new neurons which could be detected in the ischemic cerebral cortex in the initial studies describing stroke-induced striatal neurogenesis was scarce (Zhang et al., 2001; Arvidsson et al., 2002; Parent et al., 2002). It is now well established that limited recruitment of new neurons may occur to the cerebral cortex after stroke (Fig. 7.1). Jin and co-workers (Jin et al., 2003) reported migration of neuroblasts from SVZ to the boundaries of an ischemic cortical lesion. Other studies have described the occurrence of new cells expressing markers of neuroblasts or mature neurons in the ischemia-damaged cerebral cortex (Jiang et al., 2001; Zhang et al., 2006a; Ziv et al., 2007). Leker and coworkers (Leker et al., 2007) using the distal MCAO model, which causes selective cortical damage, reported that the majority of new cells in the peri-infarct cortex at 1 week were positive for neural stem cell markers such as Sox2+ and nestin+ but 3 months later about 12% of them expressed the mature neuronal marker NeuN+. This finding most likely reflects neuronal differentiation of the precursors detected in the cortex shortly after the insult. Finally, stroke-induced cortical neurogenesis can be triggered (Kolb et al., 2006) or enhanced (Taguchi et al., 2004; Zhang et al., 2006b; Wang et al., 2007; Ziv et al., 2007) by additional manipulation, e.g., growth factor infusion. It is conceivable that the high variability in the magnitude of cortical neurogenesis is dependent on the stroke model and the location, extent and dynamics of the ischemic lesion, and the resulting differences in cues regulating migration and neuronal differentiation of the generated cells. 7.3 Stroke-Induced Neurogenesis in Humans Although it cannot be excluded that local precursors also contribute to the neurogenic response after stroke, available experimental evidence indicates that the SVZ is the main source of neuroblasts which migrate towards the damage in rodents. Interestingly, the SVZ in the adult human brain contains neural stem cells (Eriksson et al., 1998; Sanai et al., 2004; Quinones-Hinojosa et al., 2006) and, similar to rodents, progenitor cells and neuroblasts reach the olfactory bulb via the rostral migratory stream to become mature neurons (Curtis et al., 2007). Thus, also in humans, the reservoir of neural stem cells in the SVZ could potentially produce new neurons for repair of the stroke-damaged brain. The evidence for strokeinduced neurogenesis in the human brain after stroke is so far based on a limited number of studies. Consistent with a neurogenic response to human stroke, Macas et al. (2006) found increased numbers of cells expressing the cell cycle marker Ki67 in the ipsilateral SVZ and of neural progenitor cells in the parenchyma close to the ventricular wall. Jin et al. (2006) have reported that cells expressing markers of cell proliferation and immature neurons, which were preferentially located close to blood vessels, were found in the area surrounding cortical infarcts. Finally, in an 84-year-old patient who suffered a stroke 1 week prior to death, a large number of cells expressing neural stem/progenitor cell markers, such as nestin, Sox2, stagespecific embryonic antigen 4, Musashi, and PSA-NCAM were observed around the 142 O. Lindvall and Z. Kokaia region of infarction, but not in adjacent “healthy” white matter or corresponding brain areas of the healthy control (Minger et al., 2007). 7.4 Functional Consequences of Stroke-Induced Neurogenesis For optimum functional restoration, the new neurons which replace those neurons which have died after stroke should become morphologically and functionally integrated into existing neural circuitries. To what extent the stroke-generated neurons form afferent and efferent connections is currently unclear. Yamashita et al. (2006) reported that the axons of the new striatal neurons contain abundant presynaptic vesicles and form synapses with neighboring cells, indicating some level of integration. A crucial question is whether the new cells develop into neurons with the functional properties and synaptic connectivity characteristic of those neurons which they should replace. Due to technical difficulties, it has not yet been possible to perform electrophysiological recordings on stroke-generated, SVZ-derived cells expressing neuronal markers. Therefore, it is not known if they really become functional neurons and are synaptically integrated into remaining neural circuitries. Another important issue is whether the new neurons develop their functional synaptic connectivity to improve or worsen pathological brain function. For stroke-generated striatal neurons, it has not been possible to address this question. We have reported that the environmental conditions encountering new neurons, formed from endogenous stem cells in the adult hippocampus, influence the development of their synaptic connectivity (Jakubs et al., 2006). New dentate granule cells were generated after a physiological stimulus, i.e., running, or a brain insult, i.e., status epilepticus, which gave rise to neuronal death and inflammation. The new cells formed in the epileptic brain exhibited functional connectivity consistent with decreased excitability, i.e., reduced excitatory and increased inhibitory drive. Thus, new neurons born in adult brain exhibit a high degree of plasticity in their afferent synaptic inputs, which can act to mitigate pathological brain function. Circumstantial evidence indicates that endogenous neurogenesis can contribute to functional recovery after stroke. Spontaneous motor recovery after stroke in rats occurred over several months concomitantly with striatal neurogenesis (Thored et al., 2006). Administration of molecules that promote neural proliferation in the SVZ and striatal neurogenesis after stroke (for references, see Lindvall and Kokaia, 2008) has been reported to be associated with improved functional outcome. However, with available methods, it is not possible to prove a causal relationship between neurogenesis and behavioral improvement after stroke. Suppression or enhancement of stroke-induced neurogenesis through delivery of mitosis inhibitors or trophic factors, respectively, will also affect other processes. Generation of conditional transgenic mice, in which the newly formed neuroblasts carry a gene for selective ablation, could solve this problem. 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke 143 7.5 Neurogenesis as a Potential Therapeutic Target After Stroke When trying to develop novel approaches to stimulate cell replacement from endogenous precursors, it should be remembered that neurogenesis after stroke is not just one process but comprises several steps: (1) proliferation of stem/progenitor cells; (2) survival of immature or mature neurons; (3) migration of new neuroblasts to appropriate location; (4) differentiation of new neuroblasts to phenotype of neurons which need to be replaced; and (5) development of functional synaptic connectivity counteracting disease symptoms. Our findings of long-term neurogenesis after stroke provide evidence that the main problem for effectiveness is not insufficient production of new neuroblasts. Therefore, the proliferation step may not be the most suitable target, but the major emphasis should be put on approaches improving the survival of the new neurons. The neurogenesis-modifying drugs should preferably act in either of three different ways: first, by improving the survival of the new neuroblasts or mature neurons; available data indicate that this could be achieved by the administration, e.g., of anti-inflammatory agents, caspase inhibitors, or neurotrophic factors; second, by increasing the migration of the new neurons to the damaged area; delivery of molecules like SDF-1a could be useful to attract the stroke-generated neuroblasts; and finally, by stimulating the differentiation of the new neuroblasts to mature neurons with cortical or striatal phenotype. How this should be achieved is currently unknown. It is conceivable that, in order to optimize the replacement of neurons which have died after stroke with new mature neurons, the neurogenesismodifying drugs would have to be administered during several months. Some drugs may be administered systemically, whereas others have to be delivered locally, in the vicinity of the ischemic damage. This could be carried out by intracerebral infusion of the protein, injection of a viral vector carrying the therapeutic gene, or transplantation of encapsulated cells which have been genetically modified to secrete the protein. It may also be possible to use some of the molecules which promote neurogenesis in animal models of stroke (Lindvall and Kokaia, 2008) in a clinical setting. It should be pointed out, though, that currently no drugs which specifically act on neurogenesis have been developed. Thus, delivery of these molecules could lead to functional restoration by several mechanisms, e.g., by promoting the survival and function of the remaining mature neurons. For example, granulocyte colony-stimulating factor (G-CSF), which is currently being applied clinically to promote recovery in stroke patients, has been shown to increase the recruitment of new neuroblasts to the ischemic area of the neocortex in a rodent stroke model (Schneider et al., 2005). The beneficial effects of G-CSF administration may be due to multiple actions such as protection of both remaining and new neurons, stimulation of blood vessel formation, and influences on inflammation (Schabitz and Schneider, 2007). Another factor, 144 O. Lindvall and Z. Kokaia glial cell line-derived neurotrophic factor (GDNF), has been infused intrastriatally in patients with Parkinson’s disease. The GDNF protein was shown to have biological effects in the human brain, i.e., to induce sprouting of the remaining dopaminergic neurons in a Parkinson’s patient (Love et al., 2005). We have found that similar intrastriatal GDNF infusion in rats increased cell proliferation in ipsilateral SVZ and recruitment of new neuroblasts into the striatum after MCAO, and improved survival of new mature neurons (Kobayashi et al., 2006). Thus, administration of this factor may become of therapeutic value to promote neuroregenerative responses in stroke patients. 7.6 Clinical Perspectives To target the adult brain’s own neural stem cells and their progeny in order to promote neurogenesis and functional restoration after stroke would be an exciting, novel therapeutic strategy. It is a major challenge to determine if and how the reservoir of neural stem cells located in the SVZ could be efficiently recruited for repair of the stroke-damaged human brain. However, at this stage, our knowledge about mechanisms regulating neurogenesis after stroke is limited and we do not understand its functional consequences. Most likely, in order to have a substantial impact on the recovery after stroke, this potential mechanism for self-repair needs to be markedly enhanced, primarily by increasing the survival and differentiation of the generated neuroblasts. It is unlikely, though, that stimulation of neurogenesis from endogenous stem cells per se could be developed into a competitive clinical approach and provide optimum repair of the stroke-damaged brain. Such a strategy probably needs to be combined with transplantation of neural stem cells or their progeny in the vicinity of the damaged area. For efficient repair, it may also be necessary to provide the new cells, generated from endogenous and grafted stem cells, with a platform in the form of synthetic extracellular matrix so that they can reform appropriate brain structure. From the present chapter, it should be obvious that much experimental work remains to be carried out before neurogenesis-based therapeutic approaches could become a reality in stroke patients. Four different steps can be defined in this roadmap towards the clinic: (1) clarify the mechanisms of neurogenesis from endogenous neural stem cells after stroke; (2) demonstrate that the new neurons are functionally integrated in the diseased brain and contribute to recovery; (3) optimize degree of behavioral recovery induced by neurogenesis in animal models of stroke; and (4) select patients for cell therapy and start clinical trials. Close links between basic and clinical research will be necessary in order to promote effective progress in this development. Acknowledgments Our own research was supported by the Swedish Research Council, Juvenile Diabetes Research Foundation, EU project LSHB-2006-037526 (StemStroke), and the Söderberg, Crafoord, Segerfalk, and Kock Foundations. The Lund Stem Cell Center is supported by a Center of Excellence grant in Life Sciences from the Swedish Foundation for Strategic Research. 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke 145 References Aarum J, Sandberg K, Haeberlein SL, Persson MA (2003) Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci USA 100:15983–15988. Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD (2006) Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442:823–826. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970. Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1–10. Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L (2008) In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia 56:412–425. Camarero G, Leon Y, Gorospe I, De Pablo F, Alsina B, Giraldez F, Varela-Nieto I (2003) Insulinlike growth factor 1 is required for survival of transit-amplifying neuroblasts and differentiation of otic neurons. Dev Biol 262:242–253. Collin T, Arvidsson A, Kokaia Z, Lindvall O (2005) Quantitative analysis of the generation of different striatal neuronal subtypes in the adult brain following excitotoxic injury. Exp Neurol 195:71–80. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21:1–14. Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van RoonMom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS (2007) Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315:1243–1249. Darsalia V, Heldmann U, Lindvall O, Kokaia Z (2005) Stroke-induced neurogenesis in aged brain. Stroke 36:1790–1795. Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003) Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA 100:13632–13637. Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317. Gebicke-Haerter P (2001) Microglia in neurodegeneration: molecular aspects. Microsc Res Tech 54:47–58. Greenberg DA (2007) Neurogenesis and stroke. CNS Neurol Disord Drug Targets 6:321–325. Hallenbeck JM (2002) The many faces of tumor necrosis factor in stroke. Nat Med 8:1363–1368. Hoehn BD, Palmer TD, Steinberg GK (2005) Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke 36:2718–2724. Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, Kokaia Z, Lindvall O (2008) Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab 28:1574–1587. Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O (2006) Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron 52:1047–1059. Jiang W, Gu W, Brannstrom T, Rosqvist R, Wester P (2001) Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke 32:1201–1207. Jin K, Galvan V (2007) Endogenous neural stem cells in the adult brain. J Neuroimmune Pharmacol 2:236–242. Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA (2001) Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA 98:4710–4715. 146 O. Lindvall and Z. Kokaia Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA (2003) Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 24:171–189. Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA (2006) Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA 103:13198–13202. Kalluri HS, Vemuganti R, Dempsey RJ (2007) Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. Eur J Neurosci 25:1041–1048. Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O (2006) Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke 37:2361–2367. Kokaia Z, Thored P, Arvidsson A, Lindvall O (2006) Regulation of stroke-induced neurogenesis in adult brain--recent scientific progress. Cereb Cortex 16 Suppl 1:i162–i167. Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S (2006) Growth factorstimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab 27:983–997. Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH (2006) Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci 26:3491–3495. Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD (2007) Longlasting regeneration after ischemia in the cerebral cortex. Stroke 38:153–161. Lichtenwalner RJ, Parent JM (2006) Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab 26:1–20. Lindvall O, Kokaia Z, Martinez-Serrano A (2004) Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med 10 Suppl:S42–S50. Lindvall O, Kokaia Z (2008) Neurogenesis following stroke affecting the adult brain. In Adult neurogenesis. F. Gage, G. Kempermann, and H. Song, editors: Cold Spring Harbor Laboratory Press. 549–570. Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J (2007) Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke 38:146–152. Louissaint A, Jr., Rao S, Leventhal C, Goldman SA (2002) Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34:945–960. Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS (2005) Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med 11:703–704. Macas J, Nern C, Plate KH, Momma S (2006) Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci 26:13114–13119. Magavi SS, Leavitt BR, Macklis JD (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405:951–955. Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG (2007) Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med 2:69–74. Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760–1765. Ohab JJ, Fleming S, Blesch A, Carmichael ST (2006) A neurovascular niche for neurogenesis after stroke. J Neurosci 26:13007–13016. Otaegi G, Yusta-Boyo MJ, Vergano-Vera E, Mendez-Gomez HR, Carrera AC, Abad JL, Gonzalez M, de la Rosa EJ, Vicario-Abejon C, de Pablo F (2006) Modulation of the PI 3-kinase-Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci 119:2739–2748. Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM (2002) Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 52:802–813. Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A (2006) Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol 494:415–434. 7 Neurogenesis from Endogenous Neural Stem Cells After Stroke 147 Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M (2006) Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab 26:125–134. Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A (2004) Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427:740–744. Schabitz WR, Schneider A (2007) New targets for established proteins: exploring G-CSF for the treatment of stroke. Trends Pharmacol Sci 28:157–161. Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR (2005) The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 115:2083–2098. Stump G, Durrer A, Klein AL, Lutolf S, Suter U, Taylor V (2002) Notch1 and its ligands Deltalike and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev 114:153–159. Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T (2004) Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 114:330–338. Taupin P (2006) Stroke-induced neurogenesis: physiopathology and mechanisms. Curr Neurovasc Res 3:67–72. Teramoto T, Qiu J, Plumier JC, Moskowitz MA (2003) EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest 111:1125–1132. Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O (2006) Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24:739–747. Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O (2007) Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38:3032–3039. Thored P, Heldmann U, Gomes-Leal W, Gisler R, Ekdahl CT, Kokaia Z, Lindvall O (2009) Longterm accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia 57:835–849. Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, II, Scheffler B, Steindler DA (2006) Microglia instruct subventricular zone neurogenesis. Glia 54: 815–825. Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA (2007) VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res 85:740–747. Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K (2006) Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci 26:6627–6636. Yan YP, Sailor KA, Vemuganti R, Dempsey RJ (2006) Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci 24:45–54. Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ (2007) Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab 27:1213–1224. Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M (2001) A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol 50:602–611. Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M (2004a) Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci 24:5810–5815. 148 O. Lindvall and Z. Kokaia Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M (2004b) Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab 24:441–448. Zhang L, Zhang Z, Zhang RL, Cui Y, LaPointe MC, Silver B, Chopp M (2006a) Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res 1118:192–198. Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM, Huang YL, Signore AP, Chen J, Sun FY (2006b) Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion. Neurobiol Dis 24:345–356. Zhang RL, Zhang ZG, Wang Y, Letourneau Y, Liu XS, Zhang X, Gregg SR, Wang L, Chopp M (2007) Stroke induces ependymal cell transformation into radial glia in the subventricular zone of the adult rodent brain. J Cereb Blood Flow Metab 27:1201–1212. Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660. Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, Yang GY, Chen Y (2008) Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke 39:1254–1261. Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M (2006) Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9:268–275. Ziv Y, Finkelstein A, Geffen Y, Kipnis J, Smirnov I, Shpilman S, Vertkin I, Kimron M, Lange A, Hecht T, Reyman KG, Marder JB, Schwartz M, Yoles E (2007) A Novel Immune-Based Therapy for Stroke Induces Neuroprotection and Supports Neurogenesis. Stroke 38:774–782. Chapter 8 Perspectives of “PUFA-GPR40 Signaling” Crucial for Adult Hippocampal Neurogenesis Tetsumori Yamashima Abstract Both cognitive functions and mental health are known to be influenced by diet, although the underlying mechanisms are not well understood. However, there is a consensus that the brain responds to changes in the fatty acid composi‑ tion of diet, and that simultaneous changes in neural membrane components occur to affect membrane fluidity, gene expression and neuronal functions. For polyun‑ saturated fatty acids (PUFA), various functions in the neurons have been proposed such as lipid storage, membrane synthesis, b-oxidation, enzyme activity and transcription programs. PUFA have recently emerged as a new class of modulator for the synaptic transmission and plasticity in the brain. As chaperones, fatty acid binding proteins (FABP) facilitate the transport of PUFA to specific compartments in the neurons. Both PUFA and FABP are nowadays known to be major regulators of adult neurogenesis, and circumstantial evidence implicates adult hippocampal neurogenesis in learning, memory and emotions. The survival of newborn neurons is increased by enriched environment and hippocampus-dependent stimuli. The key molecule that can explain synergistic effects of PUFA and stimuli should be brainderived neurotrophic factor (BDNF), because this molecule is synthesized predomi‑ nantly in hippocampal neurons for the structural remodeling and synaptic plasticity. In response to exercise, dietary energy restriction, or cognitive stimulation, levels of BDNF are increased in the hippocampus to promote adult neurogenesis. The recent discovery that newborn neurons and glia in the primate hippocampus express the free fatty acid-receptor, G protein-coupled receptor 40 (GPR40), was a minor breakthrough in the research on adult neurogenesis, because PUFA-GPR40 binding can lead to BDNF production via activations of cAMP-response element binding protein (CREB). CREB-dependent gene expression is crucial for a variety of neu‑ ronal functions such as learning, memory and emotions through BDNF synthesis. GPR40 may be one of the candidates explaining the effects of PUFA upon neuronal T. Yamashima (*) Department of Restorative Neurosurgery, Kanazawa University Graduate School of Medical Science, Takara-machi 13-1, Kanazawa 920-8641, Japan e-mail: [email protected] T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_8, © Springer 2011 149 150 T. Yamashima differentiation and synaptogenesis. By paying special attention to available evidence of the “PUFA-GPR40 signaling,” this review aims to understand one of the main cascades of adult neurogenesis. 8.1 Background Using tritiated thymidine, Joseph Altman (1963) provided autoradiographic evi‑ dence for the generation of new neurons in the dentate gyrus (DG) of the adult rat hippocampus. Thirty years after this discovery, Seki and Arai (1991, 1993a,b) first introduced bromodeoxyuridine (BrdU) and polysialylated-neural cell adhesion molecule (PSA-NCAM) as less time-consuming and more precise markers to label newborn neurons in the adult brain. BrdU labels only the nucleus of newborn neu‑ rons, while anti-PSA-NCAM antibody can stain the cytoplasm, dendrites and axons. Owing to these novel markers, research on adult neurogenesis faced a turn‑ ing point by stimulating many investigations using various experimental animals and paradigms. The mammalian adult neurogenesis has been firmly established across distinct species, and new neurons were demonstrated to be produced even in adult humans (Eriksson et al. 1998). Thus, interest in adult neurogenesis has increased remarkably worldwide in the past decade. The hippocampal neurogenesis has long been considered to be associated with learning and memory, but nowadays there is increasing evidence that it is also involved in modulating emotional responses (Santarelli et al. 2003). One of the most striking features of adult neuro‑ genesis is that this can be up- or downregulated by a variety of environmental, endocrine or pharmacological factors, and most importantly by diet. The brain must maintain behavior and thus preserve the underlying circuitry, whereas it must allow circuits to adapt to environmental challenges. This dilemma between stability and plasticity is a subject of debate (Abrous et al. 2005). Plasticity of the brain exists at the levels of dendritic spines, dendrites, and axons, which are supported by two divergent aspects, i.e., long-term dendrite stability and experi‑ ence-dependent synaptic plasticity (Abrous et al. 2005). To achieve stability and plasticity, the brain utilizes polyunsaturated fatty acids (PUFA) for the membrane phospholipid synthesis. However, the signaling mechanisms that influence prolif‑ eration, survival and differentiation of neural progenitors in the adult hippocampus have not been fully elucidated. PUFA primarily contribute to the membrane synthe‑ sis, and also function as signals for transcriptional networks to modulate gene expression, growth and survival pathways. Concerning the PUFA control of gene expression in the brain, the search for PUFA-specific signaling pathways has now become an important focus in the research on adult neurogenesis. Not only nuclear transcription factors but also cell surface receptors should act as “sensors” of free fatty acids (FFA). In addition to the gene transcription, direct effects of PUFA upon membranes must be considered, particularly in the context of the possible role of PUFA as a signal messenger (Yamashima 2008). 8 Perspectives of “PUFA-GPR40 Signaling” 151 Utilizing the human genome database, the G protein-coupled receptors (GPCR) deorphanizing strategy successfully identified multiple receptors of FFA. G proteincoupled receptor 40 (GPR40), which is preferentially expressed in pancreatic b-cells, mediates the majority of the effects of FFA on the insulin secretion from rodents to humans. However, its role in another abundant organ “brain” still remains completely unknown because of lack of appropriate rodent experiments, as GPR40 is not expressed in the rodent brain (Briscoe et al. 2003; Itoh et al. 2003). Identification of GPR40 in the neurogenic niche of the adult monkey hippocampus by the author’s group (Ma et al. 2007, 2008; Yamashima 2008; Boneva and Yamashima 2011), has led to considerable interest in its function. One answer leads to another question: why is GPR40 expressed in the newborn neurons and what is its function? Here, the author would like to review available data supporting the idea that the PUFA-GPR40 signaling might be crucial for both the activation/phosphory‑ lation of cAMP response element-binding protein (CREB) and changes in brainderived neurotrophic factor (BDNF) expression (Boneva and Yamashima 2011). 8.2 PUFA At least six categories of PUFA effects on brain functions have been noted to date, including modifications of (1) membrane fluidity, (2) activity of membrane-bound enzymes, (3) number and affinity of receptors, (4) function of ion channels, (5) production and activity of neurotransmitters, and (6) signal transduction which controls neurotransmitters and neuronal growth factors (Yehuda et al. 2002). The n-3 fatty acids, such as eicosapentaenoic acid (EPA, [20:5(n-3)]) and docosa‑ hexaenoic acid (DHA, [22:6(n-3)]) and the n-6 fatty acids such as arachidonic acid [20:4(n-6)], are of particular importance in the nervous system (Beltz et al. 2007). EPA, DHA, and arachidonic acid can be produced through the elongation and desaturation of a-linolenic acid [18:3(n-3)a]) and linoleic acid [18:2(n-6)], respec‑ tively (Fig. 8.1). However, the conversion of a-linolenic acid to EPA or DHA is limited, since about 75% of available a-linolenic acid is shunted to b-oxidation in mitochondria for ATP synthesis (Brenna 2002). Furthermore, the commercial oils such as safflower, sunflower, and corn oils, contain very high proportions of lino‑ leic acid. Accordingly, the Western diet using abundant plant oils contributes to generating larger amounts of arachidonic acid than necessary by consuming the delta-6 desaturase enzyme. Consequently, its consumption decreases the conver‑ sion of a-linolenic acid to EPA and DHA, because this conversion competes for the same enzyme converting linoleic acid to arachidonic acid (Wurtman et al. 2009). The modernization of food manufacturing has dramatically altered the balance of essential fatty acids in the Western diet (Simopoulos 1991). There has been an dramatic increase in the intake of n-6 PUFA and a concomitant decrease of n-3 PUFA (Sanders 2000). The difference between n-3 and n-6 fatty acid-derived eico‑ sanoids is that most of the mediators synthesized from EPA and DHA are 152 T. Yamashima Fig. 8.1 Biosynthetic pathway of unsaturated fatty acids, and their structure. In saturated fatty acids such as palmitic and stearic acids, all available carbon atom positions are filled. In contrast, unsaturated fatty acids (UFA) are so-called, because 1–6 carbon atoms are not saturated with hydrogen atoms. Mono-UFA (MUFA) contain a single carbon double bond, while poly-UFA (PUFA) contain two or more double bonds. MUFA are n-9 (w-9 or omega-9) oleic acid such as olive oil, while PUFA are comprised of n-6 linoleic acids such as plant oil and n-3 a-linolenic acid such as fish oil. UFA are classified by the position of the first double bond from the methyl end (n) of the fatty acid carbon chain. Linoleic acid can be converted sequentially via a biosynthetic pathway into other n-6 fatty acids such as g-linolenic acid, dihomogammalinolenic acids (DGLA) and arachidonic acid. Similarly, a-linolenic acid can be converted into longer chain n-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). But, a-linolenic acid is not efficiently converted to EPA and DHA in humans. Accordingly, linoleic acid, arachidonic acid, a-linolenic acid, EPA and DHA must be obtained largely from dietary sources as “essential fatty acids.” As mammalian cells lack D-15 or D-12 desaturase, they are almost unable to synthesize these fatty acids de novo. Since conversion of n-3 and n-6 PUFA shares the same series of enzymes, a competition exists between the n-3 and n-6 series for metabolism with an excess of one causing a significant decrease in the conversion of the other. Major sources of n-6 fatty acids are vegetable oils such as corn, safflower and soybean oil, while n-3 fatty acid sources are fish oils such as sardine, mackerel, salmon and tuna. The dietary supply of fatty acids in the former days contained a 1:1 ratio of n-6 to n-3 PUFA. However, the present ratio in the Western countries is greater than 10:1, causing an absolute deficiency of n-3 PUFA. One should note that this is one of the major causes of cognitive and mental disorders in recent years a nti-inflammatory, whereas those from arachidonic acid are pro-inflammatory or show other disease-propagating effects (Schmitz and Ecker 2008). Plasma levels of the total n-3 PUFA and the n-3/n-6 ratio are significantly lower in the dementia group, compared with normal elderly controls. In contrast, total n-6 fatty acids are higher in patients with Alzheimer dementia or cognitive impairment associated with aging (Conquer et al. 2000). A deficiency of the long-chain n-3 PUFA has been linked to a variety of diseases including physical diseases such as cancer, 8 Perspectives of “PUFA-GPR40 Signaling” 153 cardiovascular disease, rheumatoid arthritis, and asthma (Dyall and Michael-Titus 2008). A link was suggested between a high n-3 PUFA intake and a decreased risk of cognitive decline and dementia in middle and old age groups (Kalmijn et al. 1997a,b, 2004; Johnson and Schaefer 2006), particularly of Alzheimer’s disease in Western countries (Kalmijn et al. 1997a,b; Grant 1997). Recently, another important link between n-3 PUFA deficiency and mood disor‑ ders has been suggested. Rodents fed an n-3 PUFA-deficient diet show not only poor responses in the brightness discrimination, shock avoidance and water maze tasks but also an increased anxiety in their elevated plus-maze performance (Yamamoto et al. 1987; Umezawa et al. 1995; Frances et al. 1996; Jensen et al. 1996; Moriguchi et al. 2000; Takeuchi et al. 2003; Owada et al. 2006). Epidemiological data suggest that consumption of n-3 PUFA is inversely correlated to the prevalence and severity of depression, whereas recent clinical studies provide evidence for the benefits of n-3 PUFA in the treatment of depressive disorders (Freeman et al. 2006; Montgomery and Richardson 2008; Owen et al. 2008). Over the past 100 years, the intake of linoleic acid, trans fatty acids and saturated fatty acids has dramatically increased in Western civilizations, whereas the consumption of n‑3 fatty acids has simultaneously decreased. This might have explained both the elevated incidence of major depression in the Western countries and the minimum incidence in Japan (Hibbeln 1998; GómezPinilla 2008). However, in the past 50 years, the consumption of n‑3 fatty acids has decreased remarkably even in Japan, which, the author speculates, might be one of the causative factors of major depression (approximately 700,000/year) and suicide (approximately 30,000/year) recently increasing in this country. For instance, patients with depression that plays an important role in the epidemiology of suicide, were reported to have increased from 100,000 (83.1 per 100,000 population) to 430,000 (340.0 per 100,000) between 1984 and 1998 in Japan (Nakao et al. 2008). Mammalian brain tissues are predominantly composed of lipids including satu‑ rated, monounsaturated, and PUFA. The principal n-3 fatty acid found in the brain is DHA, and it comprises 10–20% of total fatty acid composition. As PUFA such as DHA show a kink at the double-bond, this kink facilitates a molecular space for receptors and/or channels to be interwoven within the membrane phospholipids. Within the brain tissue, DHA preferentially accumulates in growth cones, synapto‑ somes, astrocytes, myelin, and microsomal and mitochondrial membranes. In syn‑ aptic membranes, DHA accumulates in phosphatidylethanolamine and phosphatidylserine phospholipids. Because phosphatidylserine plays an important role in mediating binding of signal transduction proteins with the plasma mem‑ brane, reductions of phosphatidylserine concentrations in the synaptic membrane would lead to deficits in the growth cone motility and synaptogenesis. Binding of protein kinase C (PKC) with phosphatidylserine is necessary for the membrane binding and maximal kinase activity. Then, reductions of phosphatidylserine con‑ centrations in the synaptic membrane would also lead to impairments of the PKC signaling (McNamara and Carlson 2006). The combination of fish oil and exercise elevates levels of hippocampal BDNF and its downstream effectors synapsin I and CREB (Wu et al. 2008). Bousquet et al. (2009) reported that n-3 PUFA, especially DHA, can enhance the Bdnf mRNA 154 T. Yamashima expression in the striatum of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonian mice model. Furthermore, the DHA-induced improvement of spatial learning ability was associated with increased levels of pro-BDNF and mature-BDNF in the hippocampus (Wu et al. 2008). In contrast, BDNF mRNA and protein levels in the hippocampus were decreased in a rat model with a deficient intake in DHA (Levant et al. 2008). Notably, the DHA effect is associated with increases not only in BDNF mRNA and protein expression but also in newborn cells and neurons in the DG, synaptophysin expression in CA3, and the volume of hippocampus (Venna et al. 2009). In humans, optimized voxel-based morphometry on high-resolution MRI showed a significant correlation between n-3 PUFA consumption and regional gray-matter volume in amygdala, hippocampus and anterior cingulate cortex (Conklin et al. 2007). Upregulation of BDNF appears to be the initial event of the n-3 PUFA effects, then a question emerges as to why n-3 PUFA is capable of upregulating BDNF. Concerning the DHA-induced BDNF increment, a few possibilities have been reported: (1) “the action of DHA on plasma membranes” may activate signaling mechanisms that can result in more BDNF synthesis; (2) DHA is converted to Neuroprotectin D1, and elevates levels of BDNF; (3) its antioxidant capacity may reduce oxidative stress that decreases BDNF; and (4) DHA may help glucose transport across the blood–brain barrier to provide an energy source for the neurons (Wu et al. 2008). The author speculates that the possibility of (1) is most important, but at present no one knows in detail about the action of DHA on plasma membranes. As DHA is one of the major PUFA involved in the brain and retina (Rouse and Fleischer 1969), it is considered to be essential for the proper neuronal develop‑ ment and function (McKenna and Campagnoni 1979; Morgan et al. 1981; Minami et al. 1997). Most of the communications that take place between pairs of neurons occur at synapses that form between a presynaptic terminal originating on an axon and the postsynaptic membrane on a dendrite or cell body. Such synapses can survive for only days to months, and thus must be renewed periodically throughout the individual’s life span. This continuing synaptogenesis is crucial for both brain plasticity and the individual’s ability to learn (Wurtman et al. 2009). The forma‑ tion of dendritic spines in the hippocampus is induced by synaptic inputs that induce long-term potentiation (LTP) in CA1 pyramidal neurons. Synaptogenesis can be enhanced by the activation of CREB, which induces synapse formation. DHA, but not arachidonic acid, administered orally to rodents promotes synaptic membrane synthesis or dendritic spine formation (Sakamoto et al. 2007; Wurtman et al. 2009). By using transgenic fat-1 mice rich in endogenous n-3 PUFA, He et al. (2009) showed that increased brain DHA significantly enhances hippocam‑ pal neurogenesis and neuritogenesis, accompanied by a better spatial learning performance. The effects of DHA on neurogenesis and neuritogenesis have been explained mainly through two mechanisms: one is modulation of membrane prop‑ erties while the other is regulation of gene expression that is involved in neurogen‑ esis and neuritogenesis (He et al. 2009). First, DHA is a key building material for membrane synthesis and is esterified into phospholipids of the plasma mem‑ brane bilayer. This significantly alters membrane fluidity, flexibility, permeability, 8 Perspectives of “PUFA-GPR40 Signaling” 155 electrostatic behavior, and/or direct interaction with membrane proteins, and consequently regulates neurotransmission and signal transduction. Second, DHA exerts complex changes in the gene expression that is involved in neurogenesis and neuritogenesis by its interactions with transcriptional factors such as retinoid X receptor (RXR) (de Urquiza et al. 2000) and peroxisome proliferator-activated receptor (PPAR) (Göttlicher et al. 1993). For the transport of DHA in the newborn neurons, Boneva et al. (2011) recently reported that fatty acid binding proteins (FABP) were preferentially expressed in the newborn astrocytes, but not in the newborn neurons, within the neurogenesis niche of the adult monkey hippocampus. Then, DHA alone cannot gain access into the nuclei of newborn neurons of the primate hippocampus. Arachidonic acid is mainly derived from meats, egg yolk, and some fish, while its precursor linoleic acid is widespread in plants and seed oils. Arachidonic acid is abundant in the brain gray matter and has various physiological functions as another major component of cell membranes. Release of arachidonic acid from membrane phospholipids can serve as an intercellular messenger, which may activate PKC, modulate ion channels, transporters and receptors (Kawasaki et al. 2002; Wang et al. 2006). Arachidonic acid plays a role in generating LTP, presum‑ ably by stimulating tyrosine kinase and then activating phospholipase C (PLC) (Lynch 1998). In aged animals, there is a decrease in membrane arachidonic acid concentration especially in the hippocampus, then the decrease in membrane fluid‑ ity occurs simultaneously, which might be expected to adversely affect membrane function (Lynch 1998). Fluorescent molecules in a small region of the cell are irre‑ versibly photobleached using a high-power laser beam, and subsequent movement of the surrounding non-bleached fluorescent molecules into the photobleached area can be recorded with lower laser power. Using such a live cell imaging technique of fluorescent recovery after photobleaching, Fukaya et al. (2007) demonstrated that long-term administration of arachidonic acid to senescent rats preserves membrane fluidity and maintains hippocampal plasticity. Accordingly, water-maze performance and synaptic plasticity are improved with dietary supplementation of arachidonic acid in aged rats (Kotani et al. 2003). Administration of arachidonic acid successfully and dramatically increases adult hippocampal neurogenesis not only in wild-type but also in Pax6(+/−) rats. Maekawa et al. (2009) explained this by the following two scenarios. One scenario, although confined to rodents express‑ ing neuronal FABP, is that arachidonic acid itself, being transmitted from FABP3 to nuclear receptor proteins (Veerkamp and Zimmerman 2001), augments prolifera‑ tion of neural progenitor cells by controlling the transcription of cell proliferation genes. As small intracellular proteins, FABP can gain access into the nucleus and potentially target fatty acids to transcription factors such as members of the PPAR family (Furuhashi and Hotamisligil 2008). PPAR coordinates the expression of proteins that are involved in the uptake, synthesis, transport, storage, degradation and elimination of lipids (Chawla et al. 2001). The second scenario is that prosta‑ glandin E2 plays an important role in neurogenesis (Uchida et al. 2002), and stimu‑ lates endocannabinoid receptor CB2, as both prostaglandin E2 and endocannabinoids are metabolites of arachidonic acid. 156 T. Yamashima 8.3 FABP The precise mechanisms for the role of PUFA in promoting adult neurogenesis remain unknown. Two possibilities, however, have been considered likely as a mechanism of PUFA effects on adult neurogenesis. One is that PUFA themselves augment proliferation of neural progenitor cells, while another is that PUFA, being transmitted by various FABP to nuclear receptor proteins, control the transcription of genes related to the cell proliferation. FABP are ~15-kDa intracellular proteins that bind long-chain fatty acids with high affinity, being expressed in almost all mammalian tissues (Storch and Corsico 2008). Amino acid sequence homology of each FABP varies from 20 to 70%; however, they show almost identical threedimensional structures, consisting of a ten-stranded antiparallel b-barrel structure formed by two orthogonal five-stranded b-sheets (Chmurzynska 2006). The bind‑ ing pocket is located inside the b-barrel, and PUFA is bound to the interior cavity (Furuhashi and Hotamisligil 2008). Since the discovery of FABP (Ockner et al. 1972), at least nine members of FABP have been identified, and their names have been assigned according to the tissue where each was first recognized: e.g., liver (L-), intestinal (I-), heart (H-), adipocyte (A-), epidermal (E-), ileal (Il-), brain (B-), myelin (M-) and testis (T-) (Furuhashi and Hotamisligil 2008). However, naming and/or classification after the tissues in which they have been discovered or are prominently expressed are often misleading (Haunerland and Spener 2004). Accordingly, a numerical nomenclature for the different FABP genes was recently introduced; e.g., brain-type FABP (FABP7). Most FABP bind only a single fatty acid, and many FABP are prominently expressed in a single tissue or cell type, but heart-type (FABP3) and epidermal-type (FABP5) FABP display a broad tissue distribution. For example, the brain most abundantly expresses at least three differ‑ ent FABP, i.e., FABP3, -5 and -7. FABP3 (also known as H-FABP) is abundant in the myocardium, and has been isolated from a wide range of tissues including brain, skeletal muscle, renal cortex, lung, testis, aorta, adrenal gland, mammary gland, placenta, ovary, and brown adipose tissue (Zanotti 1999; Chmurzynska 2006). FABP5 (also known as E-FABP) is abun‑ dantly expressed in epidermal cells of the skin, and also in other tissues such as brain, tongue, adipose tissue, mammary gland, kidney, liver, lung, and testis (Makowski and Hotamisligil 2005; Chmurzynska 2006; Rolph et al. 2006). FABP5 is expressed in astrocytes and glia of the prenatal and perinatal brain, and, unlike FABP7, also in neurons (Veerkamp and Zimmerman 2001). Neurons of the gray matter express FABP3 and FABP5 but not FABP7. FABP7 (also known as B-FABP) is strongly expressed in radial glia cells of the developing brain, especially in the pre-perinatal stage, but the expression decreases as differentiation progresses (Feng et al. 1994). FABP7 is distinguished from other FABP by its strong affinity for n-3 PUFA, in par‑ ticular DHA (Xu et al. 1996). FABP3, 5 and 7 bind fatty acids and, additionally, retinoids and eicosanoids (Schaap et al. 2002). In the primate hippocampus, intrigu‑ ingly, all of the FABP3, 5 and 7 were expressed not in neurons but in the newborn glia surrounding the newborn neurons (Fig. 8.2) (Boneva et al. 2011). 8 Perspectives of “PUFA-GPR40 Signaling” 157 FABP have various functions such as the promotion of cellular uptake and transport of fatty acids toward mitochondrial b-oxidation systems, cell membrane synthesis, and participation in the regulation of gene expression and cell growth (Fig. 8.2) (Haunerland and Spener 2004). Many of the effects of n-3 and n-6 fatty acids are exerted through altered gene expression, although n-3 fatty acids are more potent than n-6 fatty acids. Nuclear receptors are a family of fatty acid-activated transcription factors that control several genes of fatty acid synthesis or degradation and inflammatory signaling. As these nuclear receptors are part of the family of steroid hormone receptors, called nuclear hormone receptors (NHR). NHR involve farnesoid X receptor (FXR), liver X receptor (LXR), nuclear factor kB (NFkB), PPAR, RXR, and sterol regulatory element binding protein 1c (SREBP-1c). Upon ligand binding, NHR undergo a conformational change that dissociates corepres‑ sors and facilitates recruitment of coactivator proteins to enable transcriptional Fig. 8.2 Role of FABP in the neurogenesis niche of adult primates. Left Schematic drawing of the role of FABP in the neurogenic niche of the subgranular zone (SGZ) of adult primates. FABP in the newborn astrocytes (As) conceivably contribute to the transport of PUFA from the blood vessel (BV) to the neuronal progenitor cells (NPC). With the aid of PUFA being supplied by FABP of the surrounding astrocytes, NPC can proliferate in the subgranular zone (SGZ), migrate into the granule cell layer (GCL), and make synaptic connections with pre-existing mature granular cells (cited from Boneva et al. 2011). Right PUFA, transported by FABP, contribute to the cell mem‑ brane synthesis, ATP production and nuclear signaling especially in the astrocytes. In the primate neurons, however, because of the lack of FABP and presence of GPR40, PUFA bind with the surface GPR40 receptor to trigger the intrinsic pathways leading to the activation of cAMP response element-binding protein (CREB) 158 T. Yamashima activation (Schmitz and Ecker 2008). Astrocytes are known to regulate the number of structurally mature, functional synapses and are necessary to maintain synaptic stability (Ullian et al. 2001). In view of the expression and localization of FABP confined to cells of astrocyte lineage (Owada et al. 1996; Boneva et al. 2011), it remains to be elucidated how FABP in astrocytes eventually affect neuronal synap‑ tic activity. PPAR in astrocytes is a ligand-activated transcription factor that het‑ erodimerizes with RXR. Both PPAR and RXR in astrocytes can modulate expression of genes involved in lipid metabolism (Cristiano et al. 2005). FABPmediated intracellular transport of PUFA into PPAR may be closely related to the activation process of PPAR–RXR, thereby regulating expression of the lipid metab‑ olism gene. Then, the resultant alteration of lipid environment in the astrocytes may eventually influence neuronal synapses. Despite many studies, the precise functions of each FABP have thus far not been fully explored. FABP7 exhibits high binding affinity for n-3 PUFA, such as DHA, EPA and a-linolenic acid, and for monounsaturated n-9 oleic acid over n-6 PUFA (Balendiran et al. 2000). In contrast to other FABP, FABP7 has a 40-fold greater affinity for DHA than for arachidonic acid (Xu et al. 1996), which suggests a spe‑ cific role for FABP7 in DHA metabolism. Although histological changes were not detected in the brain, the FABP7-knockout mice showed enhanced anxiety and impaired fear memory as well as decreased (~4%) content of DHA in the neonatal brain compared to the wild-type mice. The impaired fear memory and increased anxiety should be related eventually to the changes in the neuronal synaptic activity through N-methyl-d-aspartate (NMDA) receptors. There was a significant decrease of DHA-induced enhancement of NMDA-induced cation current in hippocampal neurons disassociated from these FABP7-knockout mice compared to the wild-type mice (Owada et al. 2006). Such a modulatory effect of DHA is thought to be exerted by altering the lipid environment of NMDA receptor (Nishikawa et al. 1994). A startle response to an unexpected, strong stimulus can be suppressed by an immediately-preceding low-intensity stimulus, thereby eliciting little on no behav‑ ioral response. This phenomenon, called “prepulse inhibition,” has been observed in all mammals which have been tested to date, and is known to be diminished in human schizophrenic patients (Watanabe et al. 2007). FABP7-deficient mice showed a decreased prepulse inhibition and a shortened startle response latency, which are typical of proposed effects of the chromosome 10-quantitative trait loci analysis. Furthermore, FABP7-deficient mice showed a decreased adult neurogen‑ esis in the hippocampus, and schizophrenic patients showed upregulation of Fabp7 mRNA in brains. Accordingly, it is possible that disturbance of FABP7-mediated essential fatty acid metabolism and signaling in the developing brain may be one risk factor in the occurrence of schizophrenia, with a greater effect in males (Watanabe et al. 2007). Gerstner et al. (2008) showed abundant Fabp7 mRNA accumulation in the nestin-positive precursor cells in the SGZ of adult mice. Interestingly, higher levels of Fabp7 were evident during lights-on. In adult monkeys after ischemia showing an increased hippocampal neurogenesis, FABP7 was most frequently expressed in the S-100b-positive astrocytes and nestin-positive neural progenitors, but not in bIII-tubulin- or PSA-NCAM-positive newborn 8 Perspectives of “PUFA-GPR40 Signaling” 159 n eurons. These results support a role of astrocyte- and/or neural progenitor-derived FABP7 as a component of the molecular machine regulating the neurogenesis niche in the adult brain (Fig. 8.2) (Boneva et al. 2011). However, the mechanism of FABP7 action upon newborn neurons should be distinct between rodents and primates. 8.4 GPR40 Much vertebrate physiology is based on the signal transduction of GPCR; a family of seven-transmembrane-helix, heterotrimeric guanine nucleotide-binding proteins. These receptors are among the essential nodes of communication between the inter‑ nal and external environments of cells across the plasma membrane. GPCR are responsible for the transmission of extracellular signals to intracellular responses by diverse stimuli such as hormones, neurotransmitters and environmental stimu‑ lants (Bockaert and Pin 1999; Maller 2003; Rosenbaum et al. 2009). Signaling by GPCR is ubiquitous in the brain and retina, and includes many neurotransmitter and channel signaling pathways that are related to cognitive function, vision, taste, and odor (Salem et al. 2001; Niu et al. 2004). Despite intensive research over the past three decades, little is known about GPCR function. GPR40 had been one of the orphan (that is, its ligands being unidentified) GPCR that were isolated originally from a human genomic DNA fragment (Sawzdargo et al. 1997). Sawzdargo et al. (1997) first identified GPR40–43 in a search for novel human galanin receptor subtypes, using human genomic DNA as a template and sets of degenerate primers based on conserved sequences in human and rat galanin receptors. Subsequently, three independent groups identified almost simultaneously long-chain saturated and unsaturated fatty acids as ligands for GPR40 (Briscoe et al. 2003; Itoh et al. 2003; Kotarsky et al. 2003). A variety of fatty acids were found to act agonistically at GPR40 in the micromolar concentration range. Molecular analysis of Gpr40 mRNA in the human tissues revealed ubiquitous distribution with the highest expression in the brain, and next in the pancreas (Briscoe et al. 2003). Recently, understanding of the functions of the GPR40 and their potential mechanisms of action has begun to emerge especially in the pancreas. As GPR40 was found to be enriched 2- to 100-fold in the pancreatic islets as compared with the whole pancreas tissue (Briscoe et al. 2003), it should be related to the insulin secretion. Itoh et al. (2003) demonstrated that long-chain fatty acids amplify glucose-stimulated insulin secretion from pancreatic b-cells by activating GPR40. In contrast, the role of GPR40 in the brain still remains almost unknown, although Gpr40 mRNA (Briscoe et al. 2003) and GPR40 proteins (Ma et al. 2007) were ubiquitously expressed in the CNS. One hypothesis is that brain GPR40 acts as the target receptor for FFA, and regulates neural functions such as the lipidsensing mechanism in the hypothalamus and the control of energy balance (Obici et al. 2002; Lam et al. 2005; Pocai et al. 2006). As Lam et al. (2005) stated, it is probable that selective regions of the brain including the hypothalamus can function 160 T. Yamashima as a sensor of nutrient (for instance, glucose and lipids) availability that can integrate multiple nutritional and hormonal signals, and complementary fatty acid-sensing mechanisms may also operate within the CNS. It is possible that GPR40, being expressed in neurons of the hypothalamus, hippocampus, etc. (Ma et al. 2007, 2008), might function as a sensor of circulating FFA (Fig. 8.2). Although direct evidence for the in vivo PUFA-protein interaction is limited, there is a representa‑ tive interaction between PUFA and GPCR. Briscoe et al. (2003) studied pEC50 values of fatty acids in HEK293 cells stably expressing human GPR40. Notably, DHA shows a pEC50 of 5.37 for GPR40 while for arachidonic acid it is 4.92. Rhodopsin is one of GPCR responsible for sensing light in the retina. DHA forms tight associations with rhodopsin, weakening its interhelical packaging and thus effecting its stability and enhancing its kinetics (Schmitz and Ecker 2008). GPR40 is coupled to Gaq, which results in the activation of PLC (Hardy et al. 2005) and subsequent increases in the intracellular Ca2+ concentration ([Ca2+]i) (Itoh et al. 2003). In pancreatic b cells, long-chain FFA serve as secondary stimuli to glucose because they are ineffective alone, but increase insulin release in the presence of elevated glucose. GPR40 mediates the increase in [Ca2+]i and insulin secretion through the Gaq-PLC pathway in b cells, which results in internal mobili‑ zation of Ca2+ from the endoplasmic reticulum (ER) and leads to increment of Ca2+ influx via voltage-dependent L-type Ca2+ channels (LTCC) (Shapiro et al. 2005). As newborn cells in the SGZ of the primate hippocampus also express abundant GPR40 (Fig. 8.3) (Ma et al. 2008; Yamashima 2008), PUFA-GPR40 signaling might be implicated in adult neurogenesis. By reconstructing pEGFP-N1 vectorexpressing Gpr40 gene in cultured rat neural stem cells, Ma et al. (2010) recently demonstrated that DHA can induce neuronal differentiation, neurite growth and branching of adult rat stem cells partly through GPR40. They also showed that DHA/GPR40 can induce the PLC/IP3 signaling pathway to modulate intracellular Ca2+ mobilization independent of extracellular Ca2+ concentration. Furthermore, the author’s group has recently confirmed that bone marrow-derived mesenchymal stem cells positive for GPR40 can proliferate with the aid of b-FGF, and subse‑ quently differentiate into immature neurons with the aid of DHA (Kaplamadzhiev et al. 2010). Interestingly, in the primate hippocampus, GPR40 expression in the newborn neurons was initially seen in the cytoplasm, dendrites and synaptic but‑ tons, but after maturation it was transferred to the nucleus and perinuclear cyto‑ plasm (Fig. 8.3). A considerable downregulation of GPR40 receptor expression was found along with neuronal differentiation of the bone marrow-derived mesenchy‑ mal stem cells when DHA was applied. The transfer of the membrane surface receptor after activation has been called “receptor internalization” (Freedman and Lefkowitz 1996; Ferguson et al. 1996; Goodman et al. 1996; Pierce and Lefkowitz 2001; Marchese et al. 2008), and this was thought to be characteristic of GPCR. Rapid desensitization and receptor trafficking are well known to occur for tightly controlling the temporal and spatial regulation of GPCR signaling. As so-called “receptor internalization” was confirmed in GPR40-positive immature and mature neurons of both in vivo and in vitro experimental paradigms (Ma et al. 2008; Yamashima 2008; Kaplamadzhiev et al. 2010; Boneva and Yamashima 2011), the implication of GPR40 for adult neurogenesis is highly probable. 8 Perspectives of “PUFA-GPR40 Signaling” 161 Fig. 8.3 GPR40 expression in the normal and post-ischemic monkey hippocampus. Upper (a–c) Double-staining for GPR40 (green) and PSA-NCAM (red), bIII-tubulin (red), or doublecortin (DCX) (red), shows GPR40 immunoreactivity in the PSA-NCAM (a), bIII-tubulin (b) and DCX (c) positive newborn neurons in the SGZ (arrow) of the control monkeys. Lower (d1–d3) Double-staining for PSA-NCAM (red) and GPR40 (green) of the control (d1) and post-ischemic day 9 (d2) or day 15 (d3) shows that PSA-NCAM/GPR40 double-positive newborn neurons increased significantly within the SGZ after transient global ischemia. Please, note that in both non-ischemic (a, b, c, d1) and post-ischemic (d2, d3) states, newborn neurons show GPR40 immunoreactivity in the cytoplasm, dendrites and synaptic buttons, whereas the mature granular neurons show it especially in the nuclei and perinuclear cytoplasm. This, so-called “receptor internalization” is generally characteristic for GPCR. Scale bars 20 mm (a, b, c); 50 mm (d1–d3). Right lower Increment of GPR40 expression in the SGZ after ischemia. Western blot‑ ting with densitometric quantitative analysis shows that upregulation of GPR40 occurs in the first 2 weeks after ischemia, being maximal on day 15 (d15). C, Non-ischemic control; Pan, pancreas as a positive control for GPR40 (cited from Ma et al. 2008) 8.5 CREB The CREB, being localized within the nucleus, is a leucine zipper transcription factor critical for stimulus-transcription coupling, which transmits signals at the cell membrane to alter gene expression. Altering gene expression affects the func‑ tion of neurons and neuronal circuits by regulating multiple proteins including BDNF, etc. Expression of these CREB targets might be region-specific; each protein is not necessarily expressed in all areas of the brain. Both CREB and cAMP-response element (CRE)-mediated gene expression are important in devel‑ opment, survival and differentiation of neurons, and are involved in learning and memory, and synaptic transmission (Lonze and Ginty 2002; Carlezon et al. 2005; Kitagawa 2007). CREB affects learning and memory processes, because they are crucial mediators of experience-based neuroadaptations. The mechanisms that underlie the ability of CREB to facilitate memory involve processes such as the induction of LTP or depression of synaptic strength, the growth and formation of 162 T. Yamashima new synaptic connections, or protein synthesis-dependent processes being involved in the retrieval and reconsolidation of memory (Carlezon et al. 2005). Numerous signaling pathways, such as adenylate cyclase (AC) and cAMP, Ca2+ and calmodu‑ lin, and MAPK, are involved in transmitting information initiated by membrane receptor-mediated actions to the CREB within the nucleus (Carlezon et al. 2005). Binding of neurotransmitters and neurotrophins to the membrane receptors such as TrkB, AMPA receptors, NMDA receptors and GPCR, triggers intracellular signal‑ ing cascades that culminate in CREB phosphorylation. Zhu et al. (2004) found that activation of CREB is responsible for recruiting new neurons into the dentate circuits of adult rats that have been subjected to cerebral ischemic stroke. Expression of a constitutively-active CREB, VP16-CREB, using pSFV-based gene expression vector facilitated survival of newly-generated granule neurons in the DG of adult rats. As the increased number of new neurons induced by VP16-CREB was similar to that induced by ischemic injury, Zhu et al. (2004) suggested that CREB activation fulfills a necessary and sufficient condition for ischemia-induced neurogenesis in the DG of adult rats. Specific gene targets must underlie the induction of neurogenesis by CREB. It is likely that the common final event of both CREB activation- and ischemia-induced neurogenesis is upregulation of BDNF. Both CREB activation-induced neurogenesis of adult rats (Zhu et al. 2004) and ischemia-induced neurogenesis of adult monkeys (Yamashima et al. 2004) correlate with upregulation of BDNF and increased neurogenesis in the DG. Intriguingly, CREB-dependent expression of BDNF is also elevated by exercise and spatial learning (Tokuyama et al. 2000) as well as by antidepressants (WarnerSchmidt and Duman 2006). Adult neurogenesis in the DG is well known to be upregulated not only by stress, exercise and learning but also by antidepressants (Sairanen et al. 2005) or dietary restriction (Lee et al. 2000, 2002a,b). Antidepressant treatments in humans increase the expression of BDNF in the hippocampus (Nibuya et al. 1995). Nakagawa et al. (2002) found that chronic administration of fluoxetine, a serotonin-selective reuptake inhibiting antidepressant that is known to increase pCREB in the adult hippocampus (Thome et al. 2000), also increases the survival of BrdU-labeled newborn cells. Increases in BDNF activity within the hippocampus can stimulate processes such as dendritic sprouting and neurogenesis. Then, these five conditions (stress, exercise, learning, antidepressant, and dietary restriction) may share a common mechanism of action in increasing the number of newlygenerated dentate cells, and the common factor might be BDNF signaling (Tokuyama et al. 2000; Lee et al. 2000, 2002a,b; Zhu et al. 2004; Yamashima et al. 2004; Sairanen et al. 2005; Warner-Schmidt and Duman 2006). CREB is activated by various physiological stimuli and plays important roles in many biological processes (Lonze and Ginty 2002; Johannessen et al. 2004). Bioamine (dopamine, serotonin, and norepinephrine)-induced CREB activation is dependent on Gas-coupled bioamine receptors through an AC-cAMP-dependent protein kinase A (PKA) signaling pathway. One of the signaling pathways also regulated by antidepressants that increase synaptic levels of serotonin and norepinephrine is the cAMP–CREB cascade (Warner-Schmidt and Duman 2006). PLC hydrolyses phosphatidylinositol 4,5-bisphosphate (PIP2) (Rebecchi and 8 Perspectives of “PUFA-GPR40 Signaling” 163 Pentyala, 2000; Rhee 2001) to generate two second messengers; one is inositol 1,4,5-trisphosphate (IP3) which releases Ca2+ from ER and/or mitochondria, while the other is diacylglycerol (DAG) which acts together with Ca2+ to activate PKC (Berridge and Irvine 1984, 1989). Although very little is known about the GaqCREB pathway, Thonberg et al. (2002) suggested that Gaq can activate CREB in cultured adipocytes through the PKC signaling pathway. Also in cultured neurons, Mao et al. (2007) confirmed PKC-regulated CREB phosphorylation. Furthermore, in vivo experimental paradigms, Suo et al. (2006) demonstrated that an amine neu‑ rotransmitter can activate CREB through Gaq, using a CRE reporter and reverse genetic approaches in Caenorhabditis elegans. Using rat neural stem cells primarily devoid of GPR40, Ma et al. (2010) have very recently confirmed that the DHAinduced neuronal differentiation of GPR40 gene-transfected cells occur by the activation of Gaq. Fig. 8.4 A possible “PUFA-GPR40 signaling” cascade. The mechanism involves a signal trans‑ duction cascade that includes binding of certain PUFA with GPR40, activation of the GPR40 receptor, Gaq-mediated activation of phospholipase C (PLC), breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), Ca2+ release from the endoplasmic reticulum (ER), and activations of cAMP-dependent protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinases (CaMK) IV. Lastly, activations of PKA and CaMKIV increase phosphorylation of CREB and expression of BDNF by stimulation of gene expression. Inset (cited from He et al. 2009): n-3 PUFA increase the spine density in CA1 pyra‑ midal neurons, and this is mediated by BDNF 164 T. Yamashima In brain neurons, CREB phosphorylation occurs under a wide variety of external stimuli that include (1) growth factors during development, (2) depolarization and synaptic activity during normal neuronal function, and (3) hypoxia and stress during ischemic or traumatic injuries (Lonze and Ginty 2002). However, this characteristic implicates CREB as a key participant in biological phenomena such as BDNF production. Presumably, through the common final CREB-BDNF activation, diverse stimuli such as stress, learning, exercise, enriched environment, etc. can independently result in upregulation of adult neurogenesis. In hippocampal neurogenesis, the ability of newborn neurons to communicate with their environ‑ ment, by converging onto nuclear transcription factors and expressing new appro‑ priate genes, is critical for ensuring their survival, proliferation and differentiation of developing neurons. In the rat frontal cortex, Rao et al. (2007) demonstrated that dietary n-3 PUFA can influence DHA level, phosphorylated p38 MAPK activity and CREB-DNA binding activity to regulate BDNF expression. The author would like to suggest that CREB phosphorylation with the resultant BDNF synthesis can be induced also by “PUFA-GPR40 signaling” (Fig. 8.4). It is possible that PUFA, GPR40, pCREB and BDNF may be engaged in the same signaling pathway to promote neuronal maturation and plasticity in the adult neurogenic niche of primates (Boneva and Yamashima 2011). 8.6 BDNF Neurotrophic factors (neurotrophins) are extracellular signaling molecules that are crucial not only in the developing brain but also in the adult brain. The multiple neurotrophic factors play pivotal roles for finely regulating all the stages of adult hippocampal neurogenesis. Among several classes of neurotrophic factors includ‑ ing BDNF, neurotrophin-3 (NT-3), NT-4/5, ciliary neurotrophic factor (CNTF), and NGF, BDNF is most peculiar for enhancing survival of newborn neurons (Balu and Lucki 2009). Chronic administration of BDNF directly into the hippocampus increased neurogenesis of granule cells (Scharfman et al. 2005). Furthermore, BDNF contributes to adult hippocampal neurogenesis by promoting effects of an enriched environment (Kempermann et al. 1997, 1998; Rossi et al. 2006), exercise (van Praag et al. 1999, 2002), dietary restriction (Lee et al. 2002b), and antidepres‑ sants (Sairanen et al. 2005). For example, a reduction in calorie intake of 30–40% increases adult hippocampal neurogenesis in rodents, and this effect is partly medi‑ ated by BDNF (Lee et al. 2002a,b). Flavonoids, which are enriched in foods such as cocoa and blueberries, have been shown to increase hippocampal neurogenesis in chronically-stressed rats (An et al., 2008). Williams et al. (2008) have identified BDNF as a potential mediator of the effect of flavonoids on cognition. Stress decreases expression of BDNF to cause depression or mood disorders, whereas antidepressants increase BDNF expression (Duman and Monteggia 2006). Coincidentally, adult hippocampal neurogenesis is decreased in stressed animals, whereas it is increased in antidepressant-treated animals (Warner-Schmidt and 8 Perspectives of “PUFA-GPR40 Signaling” 165 Duman 2006). This correlation suggests a pivotal role of BDNF in cognition, mood, and neuroplasticity. The neurotrophin tyrosine kinase receptor type 2 (TrkB) is critical for diverse functions of the mammalian nervous system in health and disease as a neurotrophin receptor. TrkB serves an important role not only in the neuronal survival and dif‑ ferentiation but also in the synaptic structure, function, and plasticity (McAllister et al. 1999; Poo 2001). Neurotrophins that activate TrkB include NT-4 and the prototypic ligand, BDNF. These ligands bind with the ectodomain of TrkB to induce its activation by dimerization, increased intrinsic kinase activity, autophos‑ phorylation of the receptor, and initiation of downstream signaling pathways (Huang and Reichardt 2003). BDNF-TrkB signaling should induce local synthesis of proteins important for spine growth and plasticity within and/or underneath the stimulated spines. Then, these locally-synthesized proteins presumably promote actin dynamics and AMPA receptor trafficking in the stimulated spines, which leads to growth of spine heads and formation of stable LTP (An et al. 2008, Waterhouse and Xu 2009). The brain produces two BDNF transcripts, with either short or long 3¢untrans‑ lated regions (3¢UTR). The short and long 3¢UTR Bdnf mRNAs are involved in different cellular functions; the former being restricted to somata, the latter to den‑ drites. Bdnf mRNA containing the same coding sequence but distinct 3¢UTR can have distinct physiological functions because of their selective subcellular localiza‑ tion and translation. Translation of the short Bdnf mRNA may produce BDNF protein required for the neuronal survival and maintenance in the soma, whereas that of the long Bdnf mRNA may regulate synaptic structure and function in den‑ drites (An et al. 2008). Most dendritically-translated BDNF are secreted as proBDNF, and converted to mature-BDNF by tissue plasminogen activator (Pang et al. 2004) or matrix metalloproteinases (Lee et al. 2001). Since these proteases are secreted from stimulated spines (Lochner et al. 2006; Lu et al. 2008), the conver‑ sion of pro-BDNF to mature BDNF is limited to the stimulated spines to activate TrkB on these spines in an autocrine manner (An et al. 2008). Long-lasting synaptic changes in transmission and morphology, require delivery of newly-synthesized BDNF to affected synapses (Fig. 8.4). A single BDNF protein being produced from several mRNA variants, has a different subcellular localization; soma, proximal or distal dendrites. This may represent a spatial code for the local control of BDNF availability to achieve synaptic plasticity in relation to neuronal development, and to learning and memory (Tongiorgi 2008). 8.7 Conclusion Our understanding of the role of “PUFA-GPR40 signaling” in adult neurogenesis is still at an early stage. What are the main signal transduction pathways that control adult neurogenesis? Keeping an open mind to the role of GPR40 signaling in the neurogenic niche, we should be cautious about the possible role of PUFA in adult 166 T. Yamashima neurogenesis. Ultimately, intimate knowledge of the PUFA-GPR40-CREB-BDNF signaling cascade would provide precise pharmacological and cellular interventions to manipulate the process, resulting in specific treatments to repair the damaged brain. Additional studies are necessary to further define the role of the PUFA/ GPR40 signaling pathways in adult hippocampal neurogenesis. Acknowledgments This work was supported by a grant (Creative Scientific Research: 17GS0317, Kiban-Kennkyu (B): 18390392) from the Japanese Ministry of Education, Culture, Sports, Science and Technology. References Abrous DN, Koehl M, Le Moal M (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569 Altman J (1963) Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec 145:573–591 An JJ, Gharami K, Liao GY et al (2008) Distinct role of long 3¢ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134:175–187 Balendiran GK, Schnutgen F, Scapin G et al (2000) Crystal structure and thermodynamic analysis of human brain fatty acid-binding protein. J Biol Chem 275:27045–27054 Balu DT, Lucki I (2009) Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev 33:232–252 Beltz BS, Tlusty MF, Benton JL et al (2007) Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett 415:154–158 Berridge MJ, Irvine RF (1984) Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312:315–321 Berridge MJ, Irvine RF (1989) Inositol phosphates and cell signalling. Nature 341:197–205 Bockaert J, Pin JP (1999) Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J 18:1723–1729 Boneva NB, Kaplamadzhiev DB, Sahara S et al (2011) Expression of fatty acid-binding pro‑ teins in adult hippocampal neurogenic niche of postischemic monkeys. Hippocampus 21:162–171 Boneva NB, Yamashima T (2011) New insights into ‘GPR40-CREB interaction in adult neuro‑ genesis’ specific for primates. Hippocampus (in press) Bousquet M, Gibrat C, Saint-Pierre M et al (2009) Modulation of brain-derived neurotrophic factor as a potential neuroprotective mechanism of action of omega-3 fatty acids in a parkin‑ sonian animal model. Prog Neuropsychopharmacol Biol Psychiatry 33:1401–1408 Brenna JT (2002) Efficiency of conversion of a-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care 5:127–132 Briscoe CP, Tadayyon M, Andrews JL et al (2003) The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278:11303–11311 Carlezon WA Jr, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28:436–445 Chawla A, Repa JJ, Evans RM et al (2001) Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866–1870 Chmurzynska A (2006) The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 47:39–48 Conklin SM, Gianaros PF, Brown SM et al (2007) Long-chain omega-3 fatty acid intake is associ‑ ated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett 421:209–212 8 Perspectives of “PUFA-GPR40 Signaling” 167 Conquer JA, Tierney MC, Zecevic J et al (2000) Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 35:1305–1312 Cristiano L, Cimini A, Moreno S et al (2005) Peroxisome proliferator-activated receptors (PPARs) and related transcription factors in differentiating astrocyte cultures. Neuroscience 131:577–587 de Urquiza AM, Liu S, Sjöberg M et al (2000) Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 290:2140–2144 Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127 Dyall SC, Michael-Titus AT (2008) Neurological benefits of omega-3 fatty acids. Neuromol Med 10:219–235 Eriksson PS, Perfilieva E, Eriksson TB et al (1998) Neurogenesis in the adult human hippocam‑ pus. Nature Med 4:1313–1317 Feng L, Hatten ME, Heintz N (1994) Brain lipid-binding protein (BLBP): a novel signaling sys‑ tem in the developing mammalian CNS. Neuron 12:895–908 Ferguson SS, Downey WE, Colapietro AM et al (1996) Role of b-arrestin in mediating agonist promoted G protein-coupled receptor internalization. Science 271:363–366 Frances H, Monier C, Clement M et al (1996) Effect of dietary a-linolenic acid deficiency on habituation. Life Sci 58:1805–1816 Freedman NJ, Lefkowitz RJ (1996) Desensitization of G protein-coupled receptors. Recent Prog Horm Res 51:319–351 Freeman MP, Hibbeln JR, Wisner KL et al (2006) Omega-3 fatty acids: evidence basis for treat‑ ment and future research in psychiatry. J Clin Psychiatry 67:1954–1967 Fukaya T, Gondaira T, Kashiyae Y et al (2007) Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats. Neurobiol Aging 28:1179–1186 Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7:489–503 Gerstner JR, Bremer QZ, Vander Heyden WM et al (2008) Brain fatty acid binding protein (Fabp7) is diurnally regulated in astrocytes and hippocampal granule cell precursors in adult rodent brain. PLoS ONE 3:e1631 Gómez-Pinilla F (2008) Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9:568–578 Goodman OB Jr, Krupnick JG, Santini F et al (1996) b-arrestin acts as a clathrin adaptor in endo‑ cytosis of the b2-adrenergic receptor. Nature 383:447–450 Göttlicher M, Demoz A, Svensson D et al (1993) Structural and metabolic requirements for activa‑ tors of the peroxisome proliferator-activated receptor. Biochem Pharmacol 46:2177–2184 Grant WB (1997) Dietary links to Alzheimer’s disease. Alzheimer Dis Rev 2:42–55 Hardy S, St-Onge GG, Joly E et al (2005) Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. J Biol Chem 280:13285–13291 Haunerland NH, Spener F (2004) Fatty acid-binding proteins – insights from genetic manipula‑ tions. Prog Lipid Res 43:328–349 He C, Qu X, Cui L et al (2009) Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci USA 106:11370–11375 Hibbeln JR (1998) Fish consumption and major depression. Lancet 351:1213 Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642 Itoh Y, Kawamata Y, Harada M et al (2003) Free fatty acids regulate insulin secretion from pan‑ creatic b cells through GPR40. Nature 422:173–176 Jensen CL, Chen H, Fraley JK et al (1996) Biochemical effects of dietary linoleic/alpha-linolenic acid ratio in term infants. Lipids 31:107–113 Johannessen M, Delghandi MP, Moens U (2004) What turns CREB on? Cell Signal 16:1211–1227 Johnson EJ, Schaefer EJ (2006) Potential role of dietary n-3 fatty acids in the prevention of dementia and macular degeneration. Am J Clin Nut 83(6 Suppl):1494S–1498S 168 T. Yamashima Kalmijn S, Launer LJ, Ott A et al (1997a) Dietary fat intake and the risk of incident dementia in the Rotterdam study. Ann Neurol 42:776–782 Kalmijn S, Feskens EJM, Launer LJ et al (1997b) Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol 145:33–41 Kalmijn S, van Boxtel MP, Ocke M et al (2004) Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 62:275–280 Kaplamadzhiev DB, Hisha H, Adachi Y et al (2010) Bone marrow-derived stromal cells can express neuronal markers by DHA/GPR40 signaling. BioSci Trends 4:119–129 Kawasaki A, Han MH, Wei JY et al (2002) Protective effects of arachidonic acid on glutamate neurotoxicity in rat retinal ganglion cells. Invest Opthalmol Vis Sci 43:1835–1842 Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495 Kempermann G, Kuhn HG, Gage FH (1998) Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18:3206–3212 Kitagawa K (2007) CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J 274:3210–3217 Kotani S, Nakazawa H, Tokimasa T et al (2003) Synaptic plasticity preserved with arachidonic acid diet in aged rats. Neurosci Res 46:453–461 Kotarsky K, Nilsson NE, Flodgren E et al (2003) A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun 301:406–410 Lam TK, Schwartz GJ, Rossetti L (2005) Hypothalamic sensing of fatty acids. Nat Neurosci 8:579–584 Lee J, Duan W, Long JM et al (2000) Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci 15:99–108 Lee R, Kermani P, Teng KK et al (2001) Regulation of cell survival by secreted proneurotrophins. Science 294:1945–1948 Lee J, Seroogy KB, Mattson MP (2002a) Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 80:539–547 Lee J, Duan W, Mattson MP (2002b) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82:1367–1375 Levant B, Ozias MK, Davis PF et al (2008) Decreased brain docosahexaenoic acid content pro‑ duces neurobiological effects associated with depression: interactions with reproductive status in female rats. Psychoneuroendocrinology 33:1279–1292 Lochner JE, Honigman LS, Grant WF et al (2006) Activity-dependent release of tissue plasmino‑ gen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol 66:564–577 Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–623 Lu Y, Christian K, Lu B (2008) BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89:312–323 Lynch MA (1998) Age-related impairment in long-term potentiation in hippocampus: a role for the cytokine, interleukin-1b? Prog Neurobiol 56:571–589 Ma D, Tao B, Warashina S et al (2007) Expression of free fatty acid receptor GPR40 in the CNS of adult monkeys. Neurosci Res 58:394–401 Ma D, Lu L, Boneva NB et al (2008) Expression of free fatty acid receptor GPR40 in the neuro‑ genic niche of adult monkey hippocampus. Hippocampus 18:326–333 Ma D, Zhang M, Larsen CP et al (2010) DHA promotes the neuronal differentiation of rat neural stem cells transfected with GPR40 gene. Brain Res 1330:1–8 Maekawa M, Takashima N, Matsumata M et al (2009) Arachidonic acid drives postnatal neuro‑ genesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS ONE 4:e5085 8 Perspectives of “PUFA-GPR40 Signaling” 169 Makowski L, Hotamisligil GS (2005) The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr Opin Lipidol 16:543–548 Maller JL (2003) Signal transduction. Fishing at the cell surface. Science 300:594–595 Mao L, Tang Q, Wang JQ (2007) Protein kinase C-regulated cAMP response element-binding protein phosphorylation in cultured rat striatal neurons. Brain Res Bull 72:302–308 Marchese A, Paing MM, Temple BRS et al (2008) G protein-coupled receptor sorting to endo‑ somes and lysosomes. Annu Rev Pharmacol Toxicol 48:601–629 McAllister AK, Katz LC, Lo DC (1999) Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22:295–318 McKenna MC, Campagnoni AT (1979) Effect of pre- and postnatal essential fatty acid deficiency on brain development and myelination. J Nutr 109:1195–1204 McNamara RK, Carlson SE (2006) Role of omega-3 fatty acids in brain development and func‑ tion: Potential implications for the pathogenesis and prevention of psychopathology. Prostoglandins Leukot Essent Fatty Acids 75:329–349 Minami M, Kimura S, Endo T et al (1997) Dietary docosahexaenoic acid increases cerebral ace‑ tylcholine levels and improves passive avoidance performance in stroke-prone spontaneously hypertensive rats. Pharmacol Biochem Behav 58:1123–1129 Montgomery P, Richardson A (2008) Omega-3 fatty acids for bipolar disorder. Cochrane Database Syst Rev 2:CD005169 Morgan BLG, Oppenheimer J, Winick E (1981) Effects of essential fatty acid deficiency during late gestation on brain N-acetylneuraminic acid metabolism and behave in pregnancy. Br J Nutr 46:223–230 Moriguchi T, Greiner RS, Salem Jr N (2000) Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem 75:2563–2573 Nakagawa S, Kim JE, Lee R et al (2002) Localization of phosphorylated cAMP response elementbinding protein in immature neurons of adult hippocampus. J Neurosci 22:9868–9876 Nakao M, Takeuchi T, Yoshimasu K (2008) A proposed approach to suicide prevention in Japan: the use of self-perceived symptoms as indicators of depression and suicidal ideation. Environ Health Prev Med 13:313–321 Nibuya M, Morinobu S, Duman RS (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15:7539–7547 Nishikawa M, Kimura S, Akaike N (1994) Facilitatory effect of docosahexaenoic acid on N-methyld-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol 475:83–93 Niu SL, Mitchell DC, Lim SY et al (2004) Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J Biol Chem 279:31098–31104 Obici S, Feng Z, Morgan K et al (2002) Central administration of oleic acid inhibits glucose pro‑ duction and food intake. Diabetes 51:271–275 Ockner RK, Manning JA, Poppenhausen RB et al (1972) A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science 177:56–58 Owada Y, Yoshimoto T, Kondo H (1996) Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J Chem Neuroanat 12:113–122 Owada Y, Abdelwahab SA, Kitanaka N et al (2006) Altered emotional behavioral responses in mice lacking brain-type fatty acid binding protein gene. Eur J Neurosci 24:175–187 Owen C, Rees AM, Parker G (2008) The role of fatty acids in the development and treatment of mood disorders. Curr Opin Psychiatry 21:19–24 Pang PT, Teng HK, Zaitsev E et al (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306:487–491 Pierce KL, Lefkowitz RJ (2001) Clssical and new roles of b-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci 2:727–733 Pocai A, Lam TK, Obici S et al (2006) Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 116:1081–1091 Poo MM (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2:24–32 170 T. Yamashima Rao JS, Ertley RN, Lee HJ et al (2007) n-3 Polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry 12:36–46 Rebecchi MJ, Pentyala SN (2000) Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 80:1291–1335 Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70:281–312 Rolph MS, Young TR, Shum BO et al (2006) Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein AP2. J Immunol 177:7794–7801 Rosenbaum DM, Rasmussen SG, Kobilka BK (2009) The structure and function of G-proteincoupled receptors. Nature 459:356–363 Rossi C, Angelucci A, Costantin L et al (2006) Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrich‑ ment. Eur J Neurosci 24:1850–1856 Rouse G, Fleischer S (1969) Phospholipid composition of human, bovine and frog myelin isolated on a large scale from brain and spinal cord. Lipids 4:239–242 Sairanen M, Lucas G, Ernfors P et al (2005) Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25:1089–1094 Sakamoto T, Cansev M, Wurtman RJ (2007) Oral supplementation with docosahexaenoic acid and uridine-5-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res 1182:50–59 Salem N Jr, Litman B, Kim HY (2001) Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 36:945–959 Sanders TA (2000) Polyunsaturated fatty acids in the food chain in Europe. Am J Clin Nutr 71:176S–178S Santarelli L, Saxe M, Gross C et al (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809 Sawzdargo M, George SR, Nguyen T et al (1997) A cluster of four novel human G protein-cou‑ pled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun 239:543–547 Schaap FG, van der Vusse GJ, Glatz JF (2002) Evolution of the family of intracellular lipid bind‑ ing proteins in vertebrates. Mol Cell Biochem 239:69–77 Scharfman H, Goodman J, Macleod A et al (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192:348–356 Schmitz G, Ecker J (2008) The opposing effects of n−3 and n−6 fatty acids. Prog Lipid Res 47:147–155 Seki T, Arai Y (1991) The persistent expression of a highly polysialylated NCAM in the dentate gyrus of the adult rat. Neurosci Res 12:503–513 Seki T, Arai Y (1993a) Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci 13:2351–2358 Seki T, Arai Y (1993b) Highly polysialylated NCAM expression in the developing and adult rat spinal cord. Brain Res Dev Brain Res 73:141–145 Shapiro H, Shachar S, Sekler I et al (2005) Role of GPR40 in fatty acid action on the b cell line INS-1E. Biochem Biophys Res Commu 335:97–104 Simopoulos AP (1991) Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr 54:438–463 Storch J, Corsico B (2008) The emerging functions and mechanisms of mammalian fatty acidbinding proteins. Annu Rev Nutr 28:73–95 Suo S, Kimura Y, Van Tol HHM (2006) Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine–Gq signaling in Caenorhabditis elegans. J Neurosci 26:10082–10090 Takeuchi T, Iwanaga M, Harada E (2003) Possible regulatory mechanism of DHA-induced antistress reaction in rats. Brain Res 964:136–143 8 Perspectives of “PUFA-GPR40 Signaling” 171 Thome J, Sakai N, Shin KH et al (2000) cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci 20:4030–4036 Thonberg H, Fredriksson JM, Nedergaard J et al (2002) A novel pathway for adrenergic stimula‑ tion of cAMP-response-element-binding protein (CREB) phosphorylation: mediation via a1adrenoceptors and protein kinase C activation. Biochem J 364:73–79 Tokuyama W, Okuno H, Hashimoto T et al (2000) BDNF upregulation during declarative memory formation in monkey inferior temporal cortex. Nat Neurosci 3:1134–1142 Tongiorgi E (2008) Activity-dependent expression of brain-derived neurotrophic factor in den‑ drites: facts and open questions. Neurosci Res 61:335–346 Uchida K, Kumihashi K, Kurosawa S et al (2002) Stimulatory effects of prostaglandin E2 on neurogenesis in the dentate gyrus of the adult rat. Zoolog Sci 19:1211–1216 Ullian EM, Sapperstein SK, Christopherson KS et al (2001) Control of synapse number by glia. Science 291:657–661 Umezawa M, Ohta A, Tojo H et al (1995) Dietary a-linolenate/linoleate balance influences learn‑ ing and memory in the senescence-accelerated mouse (SAM). Brain Res 669:225–233 Van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neuro‑ genesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270 van Praag H, Schinder AF, Christie BR et al (2002) Functional neurogenesis in the adult hip‑ pocampus. Nature 415:1030–1034 Veerkamp JH, Zimmerman AW (2001) Fatty acid-binding proteins of nervous tissue. J Mol Neurosci 16:133–142; discussion 151–157 Venna VR, Deplanque D, Allet C et al (2009) PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology 34:199–211 Wang ZJ, Liang C., Li GM et al (2006) Neuroprotective effects of arachidonic acid against oxida‑ tive stress on rat hippocampal slices. Chem Biol Inter 163:207–217 Warner-Schmidt JL, Duman RS (2006) Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus 16:239–249 Watanabe A, Toyota T, Owada Y et al (2007) Fabp7 maps to a quantitative trait locus for a schizo‑ phrenia endophenotype. PLoS Biol 5:2469–2483 Waterhouse EG, Xu B (2009) New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci 42:81–89 Williams CM, El Mohsen MA, Vauzour D et al (2008) Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain‑ derived neurotrophic factor (BDNF) levels. Free Radic Biol Med 45:295–305 Wu A, Ying Z, Gomez-Pinilla F (2008) Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 155:751–759 Wurtman RJ, Cansev M, Sakamoto T et al (2009) Use of phosphatide precursors to promote syn‑ aptogenesis. Annu Rev Nutr 29:59–87 Xu LZ, Sanchez R, Sali A et al (1996) Ligand specificity of brain lipid-binding protein. J Biol Chem 271:24711–24719 Yamamoto N, Saitoh M, Moriuchi A et al (1987) Effect of dietary a-linolenate/linoleate balance on brain lipid compositions and learning ability of rats. J Lipid Res 28:144–151 Yamashima T (2008) A putative link of PUFA, GPR40 and adult-born hippocampal neurons for memory. Prog Neurobiol 84:105–115 Yamashima T, Tonchev AB, Vachkov IH et al (2004) Vascular adventitia generates neuronal pro‑ genitors in the monkey hippocampus after ischemia. Hippocampus 14:861–875 Yehuda S, Rabinovitz S, Carasso RL et al (2002) The role of polyunsaturated fatty acids in restor‑ ing the aging neuronal membrane. Neurobiol Aging 23:843–853 Zanotti G (1999) Muscle fatty acid-binding protein. Biochim Biophys Acta 1441:94–105 Zhu DY, Lau L, Liu SH et al (2004) Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 101:9453–9457 Chapter 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex* Noriyuki Kishi, U. Shivraj Sohur, Jason G. Emsley, and Jeffrey D. Macklis Abstract It was long believed that the adult central nervous system (CNS) was incapable of generating new neurons or having neurons added to its post-mitotic circuitry. However, recent development of new experimental techniques and approaches has shown that the adult brain contains neural precursors (sometimes termed “neural stem cells”; comprising partially heterogeneous, fate-restricted progenitors plus theoretical primitive totipotent CNS “stem cells”) that are capable of generating new neurons, astroglia, and oligodendroglia. Understanding the specific controls over the generation of the wide diversity of neuronal subtypes (both projection neurons and local circuit interneurons), each with specific projection/ efferent targets, afferent connectivity, functions, and neurotransmitters will be critical, as the field advances, toward directed and controlled differentiation of neural precursors, and toward potential cellular circuit repair for specific nervous system degenerative and acquired diseases of specific neuronal circuitry. In this review, we provide an overview of prior work on induced neurogenesis in the adult neocortex from endogenous multipotent precursors, and also discuss emerging knowledge regarding a “molecular logic” of combinational moleculargenetic controls over projection neuron subtype specification and differentiation in the developing neocortex. Together, this developmental and regenerative biology – the earlier “proof-of-concept” experiments on induction of neurogenesis, plus the * This review was updated, expanded in scope, integrated, and modified from previously published review articles for different readerships, with only minor or no modification of some subsections (Emsley et al., 2005; Sohur et al., 2006; Kishi et al., 2009) and with some information integrated from a much more extensive review of cortical neuron subtype specification (Molyneaux et al., 2007). N. Kishi, U.S. Sohur, J.G. Emsley, and J.D. Macklis (*) MGH-HMS Center for Nervous System Repair, Departments of Neurosurgery and Neurology, and Program in Neuroscience, Harvard Medical School and Nayef Al-Rodhan Laboratories, Massachusetts General Hospital and Department of Stem Cell and Regenerative Biology, and Harvard Stem Cell Institute, Harvard University, Boston, MA 02114, USA e-mail: [email protected] T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_9, © Springer 2011 173 174 N. Kishi et al. newly and rapidly developing knowledge of pre- and post-mitotic cell-intrinsic transcriptional controls and cell-extrinsic peptide/growth factor controls over subtype specification, differentiation, and connectivity of disease-vulnerable circuit neurons – provides a roadmap toward potential future functional circuit repair of specific projection neuron circuitry of the adult neocortex. 9.1 Introduction Over most of the past century of modern neuroscience, it was thought that the adult brain was incapable of generating new neurons or having neurons added to its complex circuitry. However, the recent development of new experimental tech‑ niques and approaches has resulted in an explosion of research demonstrating that neurogenesis, the birth of new neurons, constitutively occurs (as described earlier in this volume) at significant levels in two specific regions of the adult mammalian brain: olfactory bulb and hippocampal dentate gyrus. This olfactory bulb and dentate gyrus adult neurogenesis has demonstrated theoretical roles in learning and response to novelty (Magavi et al., 2005; Aimone et al., 2006; Kee et al., 2007). Intriguingly, however, there are significant numbers of multipotent neural precursors, or “stem cells” (diverse populations of neural precursors are often termed “neural stem cells”, though many types of data indicate that these are quite heterogeneous by region and developmental stage), in many parts of the adult mammalian brain (Altman and Das, 1965; Altman, 1969; Reynolds and Weiss, 1992; Lois and Alvarez-Buylla, 1993; Palmer et al., 1995; McKay, 1997; Gage, 2000; van der Kooy and Weiss, 2000; Alvarez-Buylla et al., 2001). The rise of neural precursor cell biology, dramatically increasing knowledge of developmental and adult neurogenesis, and building evidence that neurogenesis can be induced (albeit so far relatively crudely) in normally non-neurogenic regions (Magavi et al., 2000; Scharff et al., 2000; Arvidsson et al., 2002; Nakatomi et al., 2002; Parent et al., 2002; Emsley et al., 2005; Sohur et al., 2006; Brill et al., 2009) increasingly support the idea of cellular replacement strategies to treat diseases of the brain. The idea of ‘making new neurons’ is appealing for neurodegenerative diseases, or selective neuronal loss associated with some acquired disorders. One goal of neural precursor biology is to learn from this regionally limited, constitutive neurogenesis how to manipulate neural precursors toward therapeutic neuronal or glial repopulation. Elucidation of the relevant molecular controls might allow both the control over transplanted precursor cells and the development of neuronal replacement therapies based on the recruitment of endogenous cells. Unlike other tissues, there are thousands of intermixed neuronal subtypes in the mammalian CNS, hundreds in the cerebral cortex alone. During the development of the CNS, neural precursors undergo precise stepwise differentiation to ultimately produce the complex variety of neuronal subtypes that populate the mature brain. Morphologically, neurons are divided into two broad categories: (1) projection neurons, which extend axons to distant target areas, and (2) interneurons, which make local connections. Projection neurons and interneurons are further subclassified by location, 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex 175 neurotransmitter production or sensitivity, electrophysiological characteristics, and axonal connectivity and neuronal circuits within which they send projections. Therefore, at least in the CNS, repair of diseased brain circuitry would require rebuilding and re-establishing new and appropriate circuitry, instead of simply replacing diseased neurons with new “genetic” neurons without correct connectivity and function. Thus, subtype specificity and connectivity are critically important. In this chapter, we will provide a brief overview of prior work on induced neurogenesis in the adult neocortex from endogenous multipotent precursors, and also discuss emerging knowledge regarding a “molecular logic” of combinational molecular-genetic controls over projection neuron subtype specification and differentiation in the developing neocortex. 9.2 Adult Neurogenesis: Historical Perspective Ramon y Cajal has been widely quoted as writing that “in the adult centres the nerve paths are something fixed, ended and immutable. Everything may die, nothing may be regenerated.” The relative lack of recovery from CNS injury and neurode‑ generative disease, and the relatively subtle and extremely limited distribution of neurogenesis in the adult mammalian brain resulted in the entire field reaching the conclusion that neurogenesis does not occur in the adult mammalian brain. Joseph Altman and colleagues were the first to use techniques sensitive enough to detect the ongoing cell division that occurs in select areas of the adult mammalian brain. Using tritiated thymidine as a mitotic label, they published evidence that neurogenesis constitutively occurs in the hippocampus (Altman and Das, 1965) and olfactory bulb (Altman, 1969) of the adult mammalian brain. However, the absence of neuron-specific immunocytochemical markers at the time resulted in identification of putatively newborn neurons being made on purely morphological criteria. These limitations led to a widespread lack of acceptance of these results, and made research in the field difficult. The field of adult neurogenesis was rekindled in 1992, when it was shown that precursor cells isolated from the forebrain can differentiate into neurons in vitro (Reynolds and Weiss, 1992; Richards et al., 1992). These results and technical advances such as the development of immunocytochemical reagents that could more easily and accurately identify the phenotype of various neural cells, led to an explosion of research in the field. Normally occurring neurogenesis in the subven‑ tricular zone (SVZ)/olfactory bulb and the dentate gyrus subregion of the hippocampus have now been well characterized in the adult mammalian brain. 9.3 Controversy on Adult Neurogenesis in the Neocortex The vast majority of studies investigating potential neurogenesis in the neocortex of the well-studied rodent brain do not report normally occurring adult cortical neuro‑ genesis. Rigorous studies employing serial-section analysis and three-dimensional 176 N. Kishi et al. confocal reconstruction demonstrate a complete absence of constitutively occurring neurogenesis in the murine neocortex (Magavi et al., 2000; Ehninger and Kempermann, 2003; Chen et al., 2004). These experiments provide evidence that satellite glial cells, closely apposed to neurons in normal neocortex, could be mistakenly interpreted as adult-born neurons when, in fact, a newborn glial cell overlies a pre-existent neuronal nucleus. However, a few studies report low level constitutively occurring neurogenesis in specific regions of the neocortex of adult primates (Gould et al., 1999a, 2001) and in the visual cortex of adult rats (Kaplan, 1981). In Gould et al. (1999a,b,c), neuro‑ genesis of 2–3 new neurons per mm3 was reported in prefrontal, inferior temporal and posterior parietal cortex of the adult macaque, but not in striate cortex, interpreted by the authors as being because this is a simpler primary sensory area. These authors later reported that these cells are transient and do not survive (Gould et al., 2001). In contrast, other recent reports, analyzed with more rigorous methods as noted earlier, are unable to reproduce these findings, and report a complete absence of constitutive cortical neurogenesis in both rodents and primates (Magavi et al., 2000; Kornack and Rakic, 2001; Ehninger and Kempermann, 2003; Koketsu et al., 2003; Chen et al., 2004). There exists a single report of neurogenesis in the visual cortex of the adult rat (Kaplan, 1981), but this study used tritiated thymidine and purely morphological cell-type identification, and has not been confirmed by any other group. It is unclear whether even very low-level neurogenesis occurs normally in the neocortex of any mammals (though most data indicate none in rodents or primates). However, examination of the potential for constitutive neurogenesis in what are considered normally to be non-neurogenic regions will be required perhaps to assess definitively the potential existence of extremely low-level neurogenesis. The situation in the literature is not clear, but it is important to remain critical and cautious in interpretation, maintaining ‘reserved optimism’. After all, the brain does regenerate poorly. Although these newer results might fundamentally change our view on pathology-induced neuroplasticity, it should not be forgotten that there is little evidence to date that neurogenesis outside the normally neurogenic regions could by itself significantly contribute to brain repair. Attempts toward endogenous repair will likely require exogenous de-repression, instructional signals, and neuronal survival support. Restorative neurobiology focusing on this approach aims at re-activating the developmental potential of precursor cells in the neurogenic and nonneurogenic regions of the adult brain. 9.4 Induction of Neurogenesis in the Adult Neocortex Induced neurogenesis in non-neurogenic regions can be envisioned in two basic forms: (1) local parenchymal precursor cells might be activated to generate new neurons upon complex activation associated with either a set of directed molecular and/or genetic interventions or a pathological event; or (2) precursor cells from normally neurogenic regions, e.g. the SVZ, might respond to either type of stimulus and migrate into the parenchyma. Already, there are reports of examples for both the possibilities. 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex 177 The first evidence that regenerative neurogenesis is generally possible was reported by our lab (Magavi et al., 2000) in the neocortex; this work served as a ‘proof of concept’ for the induction of adult mammalian neurogenesis. Work by our lab and other groups has extended this general concept importantly in new direc‑ tions, in the striatum, hippocampus, corticospinal system, and cortical callosal system (Arvidsson et al., 2002; Nakatomi et al., 2002; Parent et al., 2002; Chen et al., 2004; Brill et al., 2009). Whereas multipotent precursors are concentrated in the anterior SVZ and the dentate gyrus, they can be found in lower densities in several other regions of the adult brain, including widely in small numbers in the parenchyma. As discussed earlier, these precursors have broad potential; they can differentiate into astroglia, oligodendroglia and neurons; receive afferents; and extend axons to their targets, given an appropriate in vitro or in vivo environment. Prior results demonstrated that cortex undergoing targeted apoptotic degeneration could direct multipotent precursors to integrate into adult cortical microcircuitry (Snyder et al., 1997; Sheen et al., 1999). The population specificity of neuronal death was found to be essential, allowing maintenance of local circuitry and survival of surrounding cells that alter gene expression to direct precursors (Wang et al., 1998; Shin et al., 2000; Fricker-Gates et al., 2002). These properties of endogenous multi‑ potent precursors led us (Magavi et al., 2000) to investigate the fate of these precursors in an adult cortical environment manipulated in a manner previously demonstrated to support neurogenesis (Wang et al., 1998; Scharff et al., 2000). In agreement with the prior data, it was found that endogenous multipotent precursors could be induced to differentiate into neurons in the adult mouse neocortex, without transplantation (Magavi et al., 2000). These experiments induced synchronous apoptotic degeneration of corticothalamic projection neurons and examined the fates of newborn cells within cortex using markers of progressive neuronal differentiation. The results demonstrated that endogenous neural precursors (mostly from the SVZ, and possibly some from the parenchyma of the cortex itself) could be induced in situ to differentiate into cortical neurons, survive for many months and form appropriate long-distance connections in the adult mammalian brain. Interestingly, Guo et al. recently reported that proteolipid promoter-expressing NG2+ cells in the parenchyma have the ability to produce cells of different lineages, including glutamatergic neurons in the early postnatal mouse (Guo et al., 2009), and Rivers et al. reported related results that NG2+ cells generate small numbers of piriform cortex projection neurons in adult mice (Rivers et al., 2008). These results indicated that the normal absence of constitutive cortical neurogenesis does not reflect an intrinsic limitation of the endogenous neural precursors’ potential, but more likely results from a lack of appropriate microenvironmental signals necessary for neuronal differentiation or survival. Elucidation of these signals could enable CNS repair. Other groups have reported similar and complementary results in hippocampus (Nakatomi et al., 2002), striatum (Arvidsson et al., 2002; Parent et al., 2002), and again in cerebral cortex (Brill et al., 2009), confirming and further supporting this direction of research. Nakatomi and colleagues used an in vivo ischemia model by which it was found that repopulation of neurons in the CA1 region of the hippocampus is possible following the elimination of these neurons in the CA1 178 N. Kishi et al. region (Nakatomi et al., 2002). It was found that the overwhelming majority of adult-born neurons repopulating the damaged CA1 region originated from a prolif‑ erative response in the posterior periventricle, and that this proliferative response could be augmented by infusion of high levels of EGF and FGF-2, dramatically increasing the number of neurons able to migrate into and repopulate (or survive within) the damaged CA1 region. While the levels of EGF and FGF-2 substantially exceeded those reasonable for human application, these experiments serve as a proof of principle for enhancement of an endogenous neurogenic response. Lately, our lab (Chen et al., 2004) demonstrated the induction of low-level neurogenesis of corticospinal motor neurons, with progressive extension of axons into the cervical spinal cord over 3–4 months. Most recently, Gotz and colleagues demonstrated recruitment of adult mouse subependymal zone progenitors into the cerebral cortex after the same targeted, synchronous apoptotic degeneration of layer II/III and V callosal projection neurons (CPN) in this region (Brill et al., 2009). Ultimately, two considerable and critical challenges will be to (1) control such recruitment in a directed manner; (2) assess true functional integration of recruited adult-born neurons. Taken together, these results demonstrate that endogenous neural precursors can be induced to differentiate into CNS neurons in a region-specific manner and at least partially replace neuronal populations that do not normally undergo neurogenesis. It appears that these results are generalizable to at least several classes of projection neurons, including the clinically relevant striatal medium spiny neurons and corti‑ cospinal motor neurons. Microenvironmental factors appear capable of supporting and instructing the neuronal differentiation and the axon extension of adult-born neurons derived from endogenous precursors. 9.5 Molecular Controls over Development of Neuronal Subtypes The mammalian neocortex is a complex, highly organized, six-layered structure that contains hundreds of different neuronal cell types and a diverse range of glia (Rallu et al., 2002; Molyneaux et al., 2007; Kishi et al., 2009). However, the mecha‑ nisms by which such a rich neuronal diversity is produced in the CNS are poorly understood. Therefore, understanding molecular controls over development of individual neuronal subtypes from neural precursors is critical toward the goal of cellular repair of the adult neocortex. Gene expression analysis in the brain is generally complicated by the coexis‑ tence of many different cell types, resulting in high background noise and the masking of small differences in cell type-specific gene expression. The relative lack of approaches for efficient isolation of pure neuronal populations, and the diffi‑ culties involved with analysis of minute amounts of mRNA, have previously hampered progress toward understanding the molecular development of neuronal subtypes. However, recently, the rapid emergence of microarray technologies, as well as development of approaches for isolation of neuronal subtypes have 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex 179 provided for a synergistic advance in the field. DNA microarrays (also known as gene chips or gene arrays) are a collection of DNA spots representing individual genes, arranged on a chip, and used for monitoring expression levels of thousands of genes simultaneously, thus allowing comparison of thousands of genes between different neuronal populations. 9.5.1 Molecular Controls over Development of Corticospinal Motor Neurons and Other Forebrain Neuronal Populations Our laboratory has begun to uncover molecular controls over the development of corticospinal motor neurons (CSMN; upper motor neurons; residents of layer V of the cerebral cortex) and other forebrain projection neurons. CSMN degeneration is a key component of motor neuron degenerative diseases, including amyotrophic lateral sclerosis (ALS), and CSMN injury contributes critically to the loss of motor function in spinal cord injury. The anatomical and morphological development of CSMN have been extensively characterized, but the genetic mechanisms that control their development were previously unknown. Importantly, understanding the molecular controls over CSMN circuitry development (and that of other key neuronal populations) might enable future strategies for therapeutic repair or modulation in disease. To uncover the gene regulation programs of mouse CSMN development and other forebrain projection neurons, our lab developed and established approaches using comparative microarray analysis of stage-specific gene expression by CSMN purified by fluorescence-activated cell sorting (FACS) (Arlotta et al., 2005). Like other forebrain projection neurons, there were previously no specific markers for CSMN that could be utilized to isolate them. However, CSMN extend axons to the spinal cord, so they can be retrogradely labeled by injecting an appropriate retrograde label in the spinal cord. Our lab developed an approach to retrogradely label CSMN with fluorescent latex microspheres, dissociate labeled cortex, and isolate the labeled CSMN by FACS (Arlotta et al., 2005). In addition to CSMN, we initially also purified two distinct cortical projection neuron subtypes, CPN and corticotectal projection neurons (CTPN). CPN are interhemi‑ spheric callosal projection neurons, a subset of which share lamina V location with CSMN, offering insight into genes that are involved in cell type specification rather than lamina specification by comparing between CSMN and CPN. CTPN are subcerebral layer V projection neurons extending axons to the tectum instead of the spinal cord, sharing many overlapping early developmental gene regulation programs with CSMN. Comparison between CSMN and CTPN offered the opportunity for the identification of genes unique to CSMN among other highly related layer V subcerebral projection neurons. Using these FACS-purified cell populations at multiple developmental stages, comparative molecular-genetic analysis was performed using microarrays between CSMN, CPN, and CTPN mRNA expression. We identified genes specifically expressed in CSMN 180 N. Kishi et al. (Arlotta et al., 2005), CPN (Arlotta et al., 2005; Molyneaux et al., 2009), and more recently other projection neurons (e.g., corticothalamic projection neurons (CThPN), corticostriatal projection neurons (CStrPN), among other sub-populations). One of the identified CSMN-specific genes is COUP-TF1 interacting protein 2 (Ctip2, also known as Bcl11b) (Arlotta et al., 2005). Ctip2 had been identified by Kominami and colleagues to have a critical role in the immune system, controlling T cell subtype specification and survival in the developing thymus (Wakabayashi et al., 2003), but its expression or function in the CNS had not been investigated. We found that CTIP2 is expressed postmitotically at high levels only in neocortical subcerebral projection neurons (“subcerebral projection neurons” project from cell bodies in layer V of neocortex to targets caudal to the cerebrum – spinal cord and brainstem). CTIP2 is expressed in CSMN, CTPN, and all subcerebral projection neurons, but not in CPN, indicating that CTIP2 is expressed at high levels specifi‑ cally in subcerebral projection neurons, which extend axons outside the cerebrum. In Ctip2 mutant mice, CSMN axons exhibit defects in fasciculation, outgrowth, and pathfinding, resulting in failure of CSMN to connect to the spinal cord (Arlotta et al., 2005). We more recently identified that Ctip2 in the Gsh2 developmental domain plays a totally different and critical role in the proper differentiation of striatal medium spiny neurons and the patch-matrix cytoarchitectural organization of the striatum (Arlotta et al., 2008). The second published example of a CSMN-specific gene is Forebrain embryonic zinc finger-like (Fezl, more recently renamed Fez family member 2, Fezf 2) (Chen et al., 2005a,b; Molyneaux et al., 2005). Fezf 2 is expressed in a subset of ventricular zone (VZ) progenitors, and by all subcerebral projection neurons, including CSMN and CTPN. Unlike Ctip2, which is expressed in postmitotic subcerebral projection neurons after they reach their positions in the developing cortical plate, Fezf 2 is expressed in VZ and subventricular zone progenitors of subcerebral neurons, and expression continues in mature subcerebral neurons, giving the first suggestion that Fezf 2 is centrally involved in the birth and early neuron subtype-specific specifica‑ tion of CSMN. Importantly, in Fezf 2 mutant mice, the entire population of CSMN, CTPN, and other subcerebral projection neurons is never born in the neocortex. In addition, both anterograde and retrograde labels confirm the total absence of the corticospinal tract in Fezf 2 mutant mice, indicating that no other neurons compen‑ sate to form such circuitry. Gain-of-function analysis using in vivo electroporation showed that mis-expression of the single gene Fezf 2 in progenitors that normally generate superficial layer cortical neurons results in a progenitor fate-shift, producing an accessory layer of Ctip2-expressing neurons that send axons toward the spinal cord, further reinforcing that Fezf 2 plays a critical instructive role in the specification of subcerebral projection neurons (now viewed in the field as a “master gene” for cortical neurons projecting subcerebrally). This work also identified the first examples of postmitotic regulators of neuron fate and identity. As an example of postmitotic critical regulators of the develop‑ ment of CSMN and other corticofugal neurons (neurons that send their axons beyond the cortex and include subcerebral projection neurons as a subset), we (Lai et al., 2008) identified that Sox5, which encodes an SRY-box transcriptional 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex 181 regulator, postmitotically controls the sequential generation of distinct corticofugal projection neuron populations and their diversity. Sox5 loss-of-function leads to earlier-born corticofugal neuron subtypes (in particular subplate neurons, cortico‑ thalamic neurons) inappropriately differentiating into subcerebral projection neurons (in particular, CSMN). A series of gain-of-function and genetic rescue experiments identify that SOX5 represses suites of downstream genes including CTIP2, and therefore the onset of differentiation of CSMN, thereby allowing the earlier generation of the other corticofugal neuron types before SOX5 is progres‑ sively downregulated. Interestingly, we recently identified that SOX6, another SOX transcriptional regulator, and SOX5 are mutually exclusively expressed in dorsal (cortical pallial) vs. ventral (subpallial) telencephalic progenitors, parcellating pallial from subpallial telencephalic progenitors and ensuring their sharp molecular distinction and sharp differences in partial fate restriction (Azim et al., 2009a). Beyond the scope of this review, SOX6 also controls the sequential generation and appropriate differentiation of cortical interneurons, thus regulating cortical interneuron diversity (Azim et al., 2009a; Batista-Brito et al., 2009). An example of another cortical postmitotic regulator that acts by setting and sharpening spatial areas and boundaries, and thereby regulates acquisition of precise neuronal identity, is the transcription factor basic helix-loop-helix family, member e22 (Bhlhb5) (Joshi et al., 2008). In collaboration with Lin Gan’s Lab at the University of Rochester, we identified that Bhlhb5 regulates precise subcerebral projection neuron development. Bhlhb5 is postmitotically expressed in cortical layers II-V, with an initial high caudo-medial to low rostro-lateral gradient that becomes refined into a sharp boundary between sensory and motor cortices. In the absence of Bhlhb5 function, the molecular identity of projection neurons in specific areal domains is abnormal, affecting the development of somatosensory and caudal motor cortex. CSMN differentiation is particularly disrupted, as the CSMN axonal tract (corticospinal tract (CST)) fails to form appropriately. A series of loss-of-function and other analyses demonstrated that Bhlhb5 controls the post‑ mitotic areal identity of CSMN and other projection neurons, executing appropriate subtype-specific differentiation programs. Additional examples highlight the progressive refinement of precise neuron identity from less precisely generated early postmitotic neurons, e.g. LIMhomeodomain-related genes Lmo4 and Clim1 (Azim et al., 2009b). During early differentiation, Lmo4 and Clim1 are expressed in both presumptive subcerebral projection neurons and CPN in layer V, co-localizing with a number of key subtype identity-controlling transcription factors. During mid to late differentiation, this overlapping expression gradually diminishes, and Lmo4 and Clim1 adopt their largely cell type-specific expression pattern in CPN and subcerebral projection neurons, respectively. Only small subsets of neurons maintain overlapping expres‑ sion. The progressive sharpening of molecular expression boundaries further reinforces that important postmitotic events occur during the sequential acquisition of distinct cortical projection neuron subtype identities, and suggests important transcriptional regulatory roles of these LIM-HD-related genes during neuronal differentiation. 182 N. Kishi et al. 9.5.2 Molecular Controls over Development of Callosal Projection Neurons CPN are projection neurons that extend their axons across the corpus callosum interhemispherically. CPN are primarily located in layer II/III, with a smaller subset in layer V and fewer in layer VI. CPN are involved in transfer and bilateral integra‑ tion of cortical information, and abnormal CPN development and dysfunction has been centrally implicated in autism (Egaas et al., 1995; Piven et al., 1997; Vidal et al., 2006; Freitag et al., 2009; McAlonan et al., 2009). Recently, two groups characterized the transcription factor special AT-rich sequence binding protein 2 (Satb2) as a critical postmitotic control over CPN speci‑ fication as a broad population, across cortical layers (Alcamo et al., 2008; Britanova et al., 2008). SATB2 is a DNA binding protein that regulates chromatin organiza‑ tion and gene expression, and is expressed in cortical neurons that extend their axons across the corpus callosum in the developing brain. Loss of Satb2 function results in abnormal differentiation of presumptive CPN toward subcerebral projec‑ tion neuron fate, as indicated by the abnormal adoption of anatomical and molecular characteristics of subcerebral projection neurons by some of these neurons, including Ctip2 expression. Additionally, Satb2 was shown to function at least in part by directly repressing Ctip2 expression (Alcamo et al., 2008; Britanova et al., 2008). Therefore, Satb2 interacts with the Fezf 2 – Ctip2 pathway to suppress subcerebral projection neuron identity and control CPN differentiation (Chen et al., 2008). Our lab analyzed the molecular development of CPN in parallel to that of CSMN (Arlotta et al., 2005). From these data, we used in situ hybridization and immuno‑ cytochemistry to confirm and identify a number of genes differentially expressed by CPN during their development (Molyneaux et al., 2009). Some of these genes appear specific to all CPN, whereas others discriminate between CPN of the deep layers and the upper layers. Further, a subset of genes finely subdivides CPN within individual layers and appear to label CPN subpopulations that have not been described previously using anatomical and morphologic criteria. These results demonstrate the heterogeneity of this broad population of callosal projection neurons, and identify what appears to be a combinatorial program of candidate controls over the specification and development of diverse and precise CPN sub‑ types, each with distinct interhemispheric (and often collateral) connectivity. 9.5.3 Molecular Analysis of Excitatory and Inhibitory Neurons in the Adult Forebrain Additional complementary work toward understanding cortical neuron molecular genetic heterogeneity has been performed in the adult CNS by Nelson and colleagues, who analyzed major neuronal classes in the adult forebrain using 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex 183 microarrays (Sugino et al., 2006). They isolated various adult neuronal subtypes by retrograde labeling with a fluorescent tracer, or using fluorescently labeled neurons from transgenic mice. In these transgenic mice, specific neuronal subtypes are visualized with fluorescent proteins, such as GFP and YFP driven by the promoters Thy1, Gad1, and Gad2. THY1 is an immunoglobulin superfamily member that is expressed by excitatory projection neurons in many parts of the nervous system, as well as by several non-neuronal cell types, including thymocytes. GAD1/2 are glutamic acid decarboxylase 1 or 2, and are expressed in distinct subpopulations of inhibitory GABAergic interneurons. Using these transgenic mice, they defined 12 adult neuronal populations characterized by location, neurotransmitters, and electrophysiological properties, and analyzed gene expression profiles by micro array. Based on similarity of gene expression, they classified these 12 populations, showing that this molecular taxonomy almost parallels the traditional classification criteria (e.g. GABAergic neurons vs. glutamatergic pyramidal neurons, neocortex vs. hippocampus vs. amygdala). These approaches will help in classifying highly heterogeneous neuronal populations at molecular levels. 9.6 Conclusions There is now consensus that neurogenesis occurs constitutively in the olfactory bulb and hippocampal dentate gyrus in adult mammalian brains. The discoveries of adult neurogenesis in the olfactory bulb/SVZ and the dentate gyrus of the hippocampus are important both for understanding normal CNS function (in particular, regarding learning and novelty, see e.g. Magavi et al., 2005; Aimone et al., 2006; Kee et al., 2007), and from the point of view of CNS repair. Adult brain neurogenesis and precursor/“stem cell” biology are, together, highly relevant, because the generation, precise differentiation, connectivity, and functional integration of new neurons is now known to occur and contribute functionally in the adult mammalian brain. Recent studies show that even non-neurogenic regions of the brain can be induced to generate new neurons in relatively small numbers from endogenous progenitors, by de-repression of progenitors, providing a neurogenic microenvironment, and combinations of cell-intrinsic transcriptional controls and cell-extrinsic regulation and survival support. In the future, it will be critical toward therapeutic strategies to focus further on understanding molecular mechanisms that will be both instructive for and supportive of specific neuron subtype specification, differentiation, axon extension, circuit integration, and survival. This field is at the relative beginning of understanding the complex interplay between diverse adult neural precursors’ developmental potential, and their com‑ plex differentiation controls and local microenvironmental regulation. Progress over the past decade has shown that it is possible to induce new neurons even in the adult brain. Progress over the coming decades in the relevant developmental and regenerative biologies offer the potential for significant advances toward directed repair of the adult CNS. 184 N. Kishi et al. Acknowledgements This review was updated, expanded in scope, integrated, and modified from previously published review articles for different readerships, with only minor or no modification of some subsections (Emsley et al., 2005; Sohur et al., 2006; Kishi et al., 2009) and with some information integrated from a much more extensive review of cortical neuron subtype specifi‑ cation (Molyneaux et al., 2007). This work was partially supported by grants from the National Institutes of Health (NS45523, NS49553, NS41590), the Harvard Stem Cell Institute, the Spastic Paraplegia Foundation, the ALS Association, and the International Rett Syndrome Foundation (IRSF) to J.D.M. N.K. was supported by fellowships from the Japan Society for the Promotion of Science and the Rett Syndrome Research Foundation (now IRSF). U.S.S. was partially supported by fellowships from the CP Repair Research Fund, and the Edward R. and Anne G. Lefler Center. J.G.E. was partially supported by a Heart and Stroke Foundation of Canada Fellowship, a grant from the Paralyzed Veterans of America/Travis Roy Foundation, and a grant from the Children’s Neurobiological Solutions Foundation to J.D.M. We thank Bradley Molyneaux, Paola Arlotta, Sara Shnider, and Eiman Azim for contributions to subsections of the manuscript to which they also contributed written material. References Aimone JB, Wiles J, Gage FH (2006) Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9:723–727. Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK (2008) Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57:364–377. Altman J (1969) Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137:433–457. Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–335. Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2:287–293. Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD (2005) Neuronal subtypespecific genes that control corticospinal motor neuron development in vivo. Neuron 45:207–221. Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD (2008) Ctip2 controls the differen‑ tiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci 28:622–632. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endog‑ enous precursors in the adult brain after stroke. Nat Med 8:963–970. Azim E, Jabaudon D, Fame RM, Macklis JD (2009a) SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci 12:1238–1247. Azim E, Shnider SJ, Cederquist GY, Sohur US, Macklis JD (2009b) Lmo4 and Clim1 progres‑ sively delineate cortical projection neuron subtypes during development. Cereb Cortex 19 Suppl 1:i62–i69. Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G (2009) The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron 63:466–481. Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascon S, Erdelyi F, Szabo G, Parras C, Guillemot F, Frotscher M, Berninger B, Hevner RF, Raineteau O, Gotz M (2009) Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci 12:1524–1533. 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex 185 Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, Sestan N, Molnar Z, Tarabykin V (2008) Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57:378–392. Chen J, Magavi SS, Macklis JD (2004) Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci USA 101:16357–16362. Chen B, Schaevitz LR, McConnell SK (2005a) Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA 102:17184–17189. Chen JG, Rasin MR, Kwan KY, Sestan N (2005b) Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci USA 102:17792–17797. Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK (2008) The Fezf 2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci USA 105:11382–11387. Egaas B, Courchesne E, Saitoh O (1995) Reduced size of corpus callosum in autism. Arch Neurol 52:794–801. Ehninger D, Kempermann G (2003) Regional effects of wheel running and environmental enrich‑ ment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex 13:845–851. Emsley JG, Mitchell BD, Kempermann G, Macklis JD (2005) Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol 75:321–341. Freitag CM, Luders E, Hulst HE, Narr KL, Thompson PM, Toga AW, Krick C, Konrad C (2009) Total brain volume and corpus callosum size in medication-naive adolescents and young adults with autism spectrum disorder. Biol Psychiatry 66:316–319. Fricker-Gates RA, Shin JJ, Tai CC, Catapano LA, Macklis JD (2002) Late-stage immature neocortical neurons reconstruct interhemispheric connections and form synaptic contacts with increased efficiency in adult mouse cortex undergoing targeted neurodegeneration. J Neurosci 22:4045–4056. Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438. Gould E, Reeves AJ, Graziano MS, Gross CG (1999a) Neurogenesis in the neocortex of adult primates. Science 286:548–552. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999b) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260–265. Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E (1999c) Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA 96:5263–5267. Gould E, Vail N, Wagers M, Gross CG (2001) Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA 98:10910–10917. Guo F, Ma J, McCauley E, Bannerman P, Pleasure D (2009) Early postnatal proteolipid promoterexpressing progenitors produce multilineage cells in vivo. J Neurosci 29:7256–7270. Joshi PS, Molyneaux BJ, Feng L, Xie X, Macklis JD, Gan L (2008) Bhlhb5 regulates the post‑ mitotic acquisition of area identities in layers II–V of the developing neocortex. Neuron 60:258–272. Kaplan MS (1981) Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol 195:323–338. Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adultgenerated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10:355–362. Kishi N, Sohur US, Molyneaux BJ, Arlotta P, Macklis JD (2009) Analysis of molecular-genetic controls over development of neuronal subtypes. In: Encyclopedia of neuroscience (Binder M, Hirokawa N, Windhorst U, eds). New York: Springer-Verlag. Koketsu D, Mikami A, Miyamoto Y, Hisatsune T (2003) Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J Neurosci 23:937–942. 186 N. Kishi et al. Kornack DR, Rakic P (2001) Cell proliferation without neurogenesis in adult primate neocortex. Science 294:2127–2130. Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD (2008) SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron 57:232–247. Lois C, Alvarez-Buylla A (1993) Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA 90:2074–2077. Magavi SS, Leavitt BR, Macklis JD (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405:951–955. Magavi SS, Mitchell BD, Szentirmai O, Carter BS, Macklis JD (2005) Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J Neurosci 25:10729–10739. McAlonan GM, Cheung C, Cheung V, Wong N, Suckling J, Chua SE (2009) Differential effects on white-matter systems in high-functioning autism and Asperger’s syndrome. Psychol Med 39:1885–1893. McKay R (1997) Stem cells in the central nervous system. Science 276:66–71. Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD (2005) Fezl is required for the birth and specification of corticospinal motor neurons. Neuron 47:817–831. Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD (2007) Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci 8:427–437. Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD (2009) Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci 29:12343–12354. Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M (2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110:429–441. Palmer TD, Ray J, Gage FH (1995) FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci 6:474–486. Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM (2002) Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 52:802–813. Piven J, Saliba K, Bailey J, Arndt S (1997) An MRI study of autism: the cerebellum revisited. Neurology 49:546–551. Rallu M, Corbin JG, Fishell G (2002) Parsing the prosencephalon. Nat Rev Neurosci 3:943–951. Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710. Richards LJ, Kilpatrick TJ, Bartlett PF (1992) De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci USA 89:8591–8595. Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD (2008) PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11:1392–1401. Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F (2000) Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 25:481–492. Sheen VL, Arnold MW, Wang Y, Macklis JD (1999) Neural precursor differentiation following transplantation into neocortex is dependent on intrinsic developmental state and receptor competence. Exp Neurol 158:47–62. Shin JJ, Fricker-Gates RA, Perez FA, Leavitt BR, Zurakowski D, Macklis JD (2000) Transplanted neuroblasts differentiate appropriately into projection neurons with correct neurotransmitter and receptor phenotype in neocortex undergoing targeted projection neuron degeneration. J Neurosci 20:7404–7416. Snyder EY, Yoon C, Flax JD, Macklis JD (1997) Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci USA 94:11663–11668. 9 Adult Neurogenesis and Neuronal Subtype Specification in the Neocortex 187 Sohur US, Emsley JG, Mitchell BD, Macklis JD (2006) Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci 361:1477–1497. Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB (2006) Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci 9:99–107. van der Kooy D, Weiss S (2000) Why stem cells? Science 287:1439–1441. Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, Williamson PC, Rajakumar N, Sui Y, Dutton RA, Toga AW, Thompson PM (2006) Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biol Psychiatry 60:218–225. Wakabayashi Y, Watanabe H, Inoue J, Takeda N, Sakata J, Mishima Y, Hitomi J, Yamamoto T, Utsuyama M, Niwa O, Aizawa S, Kominami R (2003) Bcl11b is required for differentiation and survival of alphabeta T lymphocytes. Nat Immunol 4:533–539. Wang Y, Sheen VL, Macklis JD (1998) Cortical interneurons upregulate neurotrophins in vivo in response to targeted apoptotic degeneration of neighboring pyramidal neurons. Exp Neurol 154:389–402. Chapter 10 Culturing Adult Neural Stem Cells: Application to the Study of Neurodegenerative and Neuropsychiatric Pathology Seiji Hitoshi, Tod Kippin, and Derek van der Kooy Abstract Stem cells exist in many, perhaps all, adult tissues and serve the common function of a reservoir of precursors that can mediate cell turnover or repair damage. Among tissue-specific stem cell populations, neural stem cells are particularly amenable to study because they are readily isolated and cultured in simple culture systems that allow demonstration of cell-autonomous self-renewal and multipotentiality. Here, we describe the most widely employed culture system, the neurosphere assay, used to reveal these cardinal properties of neural stem cells which respond to specific growth factors and proliferate in serum-free media to form clonal sphere colonies. Although several pitfalls and limitations exist, the neurosphere assay can provide detailed information about kinetics and behavior of neural stem cells when utilized under appropriate conditions. Further, the neurosphere assay together with histochemical analysis using nucleotide analog labeling may be applied to estimate the number and cellular kinetics of neural stem cells in the adult brain under physiological and pathological conditions. S. Hitoshi (*) Division of Neurobiology and Bioinformatics, National Institute for Physiological Sciences, 5-1 Higashiyama Myodaiji, Okazaki, Aichi 444-8787, Japan and Department of Physiological Sciences, School of Life Sciences, Graduate University for Advanced Studies, Kanagawa 240-0193, Japan e-mail: [email protected] T. Kippin Department of Psychology, University of California, Santa Barbara, CA, USA and The Neuroscience Research Institute, University of California, Santa Barbara, CA, USA D. van der Kooy Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945‑2_10, © Springer 2011 189 190 S. Hitoshi et al. 10.1 Introduction Tissue-specific stem cells are relatively quiescent in the niche that provides optimal environmental conditions to maintain the stem cells undifferentiated. Isolation and culture of tissue-specific stem cells have enabled us to understand the basic properties of these cells and even provided an avenue for ex vivo expansion to establish exogenous pools of stem cells that may be useful to promote repair of damaged tissue via transplantation. However, procedures for culturing stem cells largely depend on the tissue of origin and the cardinal features of stem cells, self-renewal and multipotentiality, which are not always demonstrated at the single cell level in the in vitro culture systems. This is particularly critical for the study of stem cells as their in vivo anatomical niche often contains a host of heterogeneous precursors but only a fraction of which act as bona fide stem cells. Hematopoietic stem cells have a long and extensive history of study, with the technology to study these tissue-specific stem cells far exceeding those derived from other tissues. For instance, hematopoietic stem cells can be isolated by a FACS technique using several surface markers in combination (for example, Lineage marker−, Sca-1+, c-Kit+, Thy-1low) or using a fluorescent dye, in which the stem cells are enriched in a side population after incubation with Hoechst33342 (Kondo et al. 2003). The self-renewal and multipotential capabilities of the isolated hematopoietic stem cells are tested by transplanting single stem cells into host animals that are depleted of bone marrow by irradiation and the demonstration of reconstruction of hematopoietic system. Although transient amplification of isolated hematopoietic stem cells has been reported, “long-term” hematopoietic stem cells that can reconstitute bone marrow in the host by serial transplantation are lost after a substantial period of culture. This is probably because current culture systems lack critical factors or cell–cell interaction signals or both that are provided by surrounding cells in the niche. However, these defects are, at least in part, overcome by the overexpression of Hox genes because, for example, overexpression of HoxB4 genes drastically enhances the ex vivo retention of long-term hematopoietic stem cells (Antonchuk et al. 2002; Kondo et al. 2003). The FACS technology used to isolate hematopoietic stem cells has been applied to the isolation of neural stem cells and has achieved some success (Rietze et al. 2001; Murayama et al. 2002), although the absolute purity of neural stem cells has not been achieved by this method. In contrast to the hematopoietic stem cells, neural-derived cells with stem cell attributes are relatively easily isolated as floating neurosphere colonies from embryonic and adult rodent brains in serum-free media for more than several weeks (or perhaps indefinitely) while maintaining their self-renewal and multipotential capabilities at the clonal level (Reynolds et al. 1992; Reynolds and Weiss 1992). However, care must be paid to the culture procedures and interpretation of results because inadequate techniques may result in aberrant neurosphere formation and has led to confusion regarding the attributes of neurosphere-forming cells, which will be described in the following sections. 10 Culturing Adult Neural Stem Cells 191 10.2 Procedures of the Neurosphere Assay 10.2.1 Culture Media The neurosphere culture is extremely sensitive to the purity of culture media, and therefore, usage of disposable plastic laboratory wares and commercially available liquid materials for all procedures is strongly recommended. As originally described (Reynolds et al. 1992), chemically-defined serum-free media (SFM) is used for neural stem cell culture. Because the insulin concentration that is optimal for culturing neural stem cells is much higher (25 mg/ml) than that used for neuronal cell culture and commercial media (for example, 5 mg/ml in Neurobasal media supplemented with N2 Supplement; Invitrogen Gibco), SFM has to be made in our laboratory as follows: For 100 ml SFM Water (W3500; Sigma) 10 × Dulbecco’s modified Eagle’s Medium (DMEM)/F-12 Dissolve one package each of DMEM (12100-046; Invitrogen Gibco) and F-12 (21700-075; Invitrogen Gibco) in 200 ml of water and filter sterilize. Store at 4°C. 10 × Hormone Mix See below. 1 M HEPES (15630-080; Invitrogen Gibco) 7.5% sodium bicarbonate (S8761; Sigma) 30% glucose Dissolve 30 g of glucose (S6152; Sigma) in 100 ml of water and filter sterilize. Store at 4°C. 200 mM l-glutamine (15630-080; Invitrogen Gibco) Filter sterilize and store at 4°C for no more than 3 days. For 200 ml 10 × Hormone Mix Water (W3500; Sigma) 10 × DMEM/F-12 1 M HEPES (15630-080; Invitrogen Gibco) 7.5% sodium bicarbonate (S8761; Sigma) 30% glucose In 20 ml water, dissolve the followings: Putrescine (P5780; Sigma) Apo-transferrin (T2252; Sigma) Insulin (I5500; Sigma) Predissolved in 2 ml of 0.1 N hydrochloric acid (210-4; Sigma). Progesterone (P6149; Sigma) Dissolve 1 mg Progesterone in 1.59 ml of 95% ethanol and store 20 ml aliquots at −20°C. Selenium (S9133; Sigma) Dissolve 1 mg Selenium in 1.93 ml water and store 20-ml aliquots at −20°C. Filter sterilize, aliquot into 10 ml/tube and store at −20°C. 75 ml 10 ml 10 ml 500 ml 1.5 ml 2 ml 1 ml 150 ml 20 ml 1 ml 3 ml 4 ml 19.25 mg 200 mg 50 mg 20 ml 20 ml (continued) 192 S. Hitoshi et al. Growth factor preparation FGF-2 (human recombinant, F0291; Sigma) 10 ng/ml For a stock solution, reconstitute 25 mg FGF-2 in 500 ml of 10 × Hormone Mix or 5% bovine albumin solution (A4628; Sigma) and store 10- to 20-ml aliquots at −20°C. Heparin (2 mg/ml, H3149; Sigma) should be added at usage. EGF (from mouse submaxillary glands, E4127; Sigma) 20 ng/ml For a stock solution, reconstitute 100 mg EGF in 1,000 ml of 10 × Hormone Mix or 5% bovine albumin solution and store 10- to 20-ml aliquots at −20°C. After the growth factors are diluted in SFM to make a working media, the media should be used within the day. SFM with the same contents has recently been available from StemCell Technologies [NeuroCult NSC basal medium (05700) and NeuroCult NSC proliferation supplement (05701)]. 10.2.2 Culturing Neural Stem Cells from Embryonic Brains The embryonic brain structure changes drastically along development and dissection procedures should be modified so that minimal volume of brain tissue containing neural stem cells is excised in order to minimize the level of contamination with nonstem cells or cells with transient stem cell properties (Seaberg et al. 2005). The protocol to dissect brains of mouse embryos at 14.5 days postcoitum (E14.5), when neural stem cells reside in the ventricular zone of dorsal and ventral forebrain, is shown below (Fig. 10.1). Further, tissue from the cortex and ganglionic eminence Fig. 10.1 Dissection procedures of embryonic brains. LGE lateral ganglionic eminence, MGE medial ganglionic eminence, CGE caudal ganglionic eminence, Ctx cortex 10 Culturing Adult Neural Stem Cells 193 should be collected separately because these regions (and other regions) contain neural stem cells that exhibit distinct gene expression profiles along both the dorsoventral and rostrocaudal axes (Hitoshi et al. 2002b). 1. Dissection procedures A. Timed-pregnant mice at 14.5 gestational age are killed by deep anesthesia and cervical dislocation, and embryos are excised into fresh phosphatebuffered saline supplemented with 0.6% glucose and 100 unit/ml penicillin/streptomycin (15140-122; Invitrogen Gibco) (PBS-G). B,C. Heads of the embryos are decapitated into a new dish containing PBS-G and a cut made along the midline in the epidermis and calvarium. D. Make a cut in the cortex (Ctx) as shown in Fig. 10.1 and excise cortical tissue. Remove the meninges from the cortex with a fine forceps. E,F. By flipping the cortex, ganglionic eminence becomes visible. Excise only the bulging tissue so that the mantle zone is excluded from the culture. The “heart-shaped” ganglionic eminence consists of medial, lateral and caudal ganglionic eminences (MGE, LGE and CGE) and these three regions can be cultured separately. 2. The tissue is collected in a 14-ml polypropylene round-bottom tube (BD Falcon 352029; BD Biosciences) and mechanically dissociated in 1 ml of SFM, using a fire-polished narrow-bored glass pipette, into a single cell suspension. Viability of the cells after trituration (typically 15–20 times) should be more than 85%. Plastic tips (200 ml) and a Gilson pipette may be used to dissociate cells but may result in slightly lower cell viability. In addition, 200-ml plastic tips from some companies preferentially absorb neurosphere-forming cells even though the tips are wetted beforehand. 3. Viable cells are counted by trypan blue exclusion and plated at 10 cells/ml in 500 ml SFM containing FGF-2 or EGF or both in a 24-well plate (Falcon 353047; BD Biosciences). When the culture is scaled up or down, cell density to the volume of media (10 cells/ml) and to the surface area of the dish (5 cells/cm2) should be kept constant in order to ensure clonally-derived colonies. Floating sphere colonies with a diameter of more than 0.1 mm at 7 days in vitro are considered to be neural stem cell-derived neurospheres because these colonies consistently exhibit the properties of self-renewal and multipotentiality. 10.2.3 Culturing Neural Stem Cells from Adult Brains Neural stem cells that generate neurospheres in vitro reside in the subependymal zone surrounding the medial (septal side) and lateral (striatal side) portions of the lateral ventricles in the adult brain (Reynolds and Weiss 1992), as well as from the subependymal zone along the entire ventricular axes (Weiss et al. 1996). Dentate gyrus of the hippocampus is considered to be another region where neural stem 194 S. Hitoshi et al. Fig. 10.2 Dissection procedures of adult brains. Sep septum, Str striatum, Hipp hippocampus cells produce new neurons throughout the lifespan (Temple and Alvarez-Buylla 1999; Gage 2000); however, cells from the dentate gyrus of adult mice or rats do not yield neurospheres under the typical culture conditions described below (Seaberg and van der Kooy 2002). In one study, cells from the dentate gyrus were reported to form neurospheres but required brain-derived neurotrophic factor (BDNF) in addition to FGF-2 and EGF to proliferate in vitro (Bull and Bartlett 2005). Thus, proliferation capability and growth factor dependency of hippocampal neural stem cells may be distinct between species. The protocol to dissect and excise striatal subventricular zone of adult mouse brains is shown below (Fig. 10.2). Connections between cells and myelin formation are so dense that enzymatic digestion is necessary to isolate single cells. 1. Dissection procedures A. Adult mice are killed by deep anesthesia and/or cervical dislocation, and brains are aseptically taken from the skull into oxygenized artificial cerebrospinal fluid (aCSF) that consists of 124 mM NaCl, 5 mM KCl, 1.3 mM MgCl2, 2 mM CaCl2, 26 mM NaHCO3, 0.18% glucose, and 10 Culturing Adult Neural Stem Cells 195 100 unit/ml penicillin/streptomycin. Cut along the midline and divide brain into hemispheres. B,C,D. Make a cut along the corpus callosum by a fine spring scissors and flip the cortex so that the lateral portion of the lateral ventricles is visible. Cut off the cortex. E. Dorsal view of the right hemisphere after removal of the cortex, in which striatum (Str), septum (Sep) and hippocampus (Hipp) are seen. F,G. Entire subependyma of the anterior lateral ventricle with gray color is excised from the striatum, avoiding inclusion of white tissue to minimize the contamination of myelin, since myelin debris suppresses the neurosphere formation. 2. The subependyma tissue is cut into several small pieces and collected in a 15-ml polypropylene conical tube (BD Falcon 352096; BD Biosciences). The tissue is subjected to the enzyme digestion [1.33 mg/ml trypsin (T1005; Sigma), 0.67 mg/ ml hyaluronidase (H6254; Sigma) and 0.2 mg/ml kynurenic acid (H3375; Sigma) in 1 ml of oxygenized aCSF but with high Mg2+ (3.2 mM) and low Ca2+ (0.1 mM), filter sterilized] for 1 h at 37°C with gentle shaking (100 rpm). 3. The enzyme digestion is stopped by adding trypsin inhibitor (109878; Roche; at a final concentration of 0.7 mg/ml) or fetal bovine serum (final 1%) and gently triturated 10 times by a fire-polished glass pipette. At this step, tissue pieces become invisible. 4. Centrifuge the cell suspension at 270 × g (~1,200 rpm in most swing rotor centrifuges) for 5 min and discard the supernatant. Resuspend the cells in 1 ml of SFM and repeat the centrifugation as above. Discard the supernatant and resuspend the cells in 1 ml of SFM containing growth factors. Triturate cells 10–15 times by a fire-polished narrow-bored glass pipette into single cells. Plastic tips (200 ml) and a Gilson pipette may be used to dissociate cells but may result in slightly lower cell viability. In addition, 200-ml plastic tips from some companies preferentially absorb neurosphere-forming cells even though the tips are wetted beforehand. 6. Viable cells are counted by trypan blue exclusion and plated at 5 cells/ml in 500 ml SFM containing FGF-2 and EGF in a 24-well plate (Falcon 353047; BD Biosciences). Because adult neural stem cells proliferate more rapidly than embryonic ones, number of neurospheres with a diameter > 0.08 mm is counted 5–6 days after plating. 10.2.4 Self-Renewal Capability and Differentiation Potential of Neural Stem Cells The self-renewal of neural stem cells is defined as they go through a cell cycle either with a symmetric cell division that produces two stem cells (self-renewal with expansion) or with an asymmetric cell division that produces one daughter stem cell 196 S. Hitoshi et al. and one nonstem progeny cell (self-renewal without expansion). While it is very difficult to test the in vivo self-renewal capability of neural stem cells in the adult brain, their in vitro self-renewal capability can be estimated by a simple passaging culture method. Because primary neurospheres are clonally derived from single neural stem cells, the number of secondary neurospheres reflects the number of symmetric divisions the original neural stem cell undergoes during the primary neurosphere formation. Therefore, counting and averaging secondary neurosphere numbers derived from sufficient numbers of single primary neurospheres of equivalent size or cell number are employed to estimate the expansion capability of cultured neural stem cells. Nevertheless, due to the laborious work of these procedures, passaging primary neurospheres in bulk is often used despite this technique resulting in potential biases due to the size of and cell numbers in primary neurospheres. 1. Pick up floating (or lightly attached to the dish) round neurospheres with a diameter of ~0.15 mm under an inverted microscope into 100 ml of SFM containing growth factors. 2. Triturate the neurosphere by pipetting 30 times using 200-ml plastic tips and the Gilson pipette into single cells. Typically, 1,000–1,500 viable cells are recovered from single neurospheres. 3. To avoid high cell density in the culture, plate one-tenth of the cell suspension in 160 ml of SFM containing growth factors in a 96-well plate. Passaged neurospheres tend to grow more rapidly and to attach to each other more readily than do primary neurospheres. Therefore, the cell density should be kept below 2 cells/ml (1 cells/cm2) and the number of neurospheres should be counted 5–6 days after the plating. When appropriately cultured, more than 95% of adult brain-derived neurospheres with a diameter > 0.08 mm can be passaged at least twice. Even more extensive passaging procedures (5 times or more) may be necessary to ensure that initial primary neurosphere-forming cells are bona fide neural stem cells (Louis et al. 2008), although it has yet to be experimentally demonstrated to what extent the in vitro self-renewal capability correlates with in vivo selfrenewal potential of the stem cell. The multipotentiality within neural lineage of neural stem cells can be demonstrated by simply plating single neurospheres under differentiation conditions. If neuronal and glial differentiation is simultaneously observed from single neurospheres then the original neural stem cell that clonally generated the neurosphere is considered multipotent. 1. Prepare a cover glass (10 mm diameter) by extensive wash and autoclaving. Overlay the cover glass with 100 ml of poly-ethylenimine (50% w/v, P3143; Sigma) at a final concentration of 0.1% in 0.15 M borate buffer (pH 8.4) for 1 h at room temperature. Alternatively, the same differentiation protocol can be employed using tissue culture plates as described for the neurosphere-formation assay. 2. Wash the cover glass 5 times with PBS. Coat the cover glass further with Matrigel basement membrane matrix (354234; BD Biosciences) at 0.6 mg/ml in SFM for 1 h at 37°C. 10 Culturing Adult Neural Stem Cells 197 3. Wash the cover glass twice with SFM and overlay 100 ml of 10% Knockout Serum Replacement (10828-028; Invitrogen Gibco)/SFM. 4. Pick up a floating (or lightly attached to the dish) round neurosphere and put it onto the cover glass. 5. After 7 days, differentiated cells are ready for immunostaining. Typically, O4 antibody (mouse monoclonal IgM) is added to the culture and incubated for 30 min at 37°C. After a brief wash with PBS, the cells are fixed by 4% paraformaldehyde. The cover glass is washed 3 times with PBS, blocked in 10% normal goat serum/PBS for 1 h at room temperature, and then incubated with anti-bIII tubulin (mouse monoclonal IgG) and anti-GFAP (rabbit polyclonal IgG) antibodies. After washing with PBS, appropriate fluorescent dye-conjugated secondary antibodies are applied. Finally, the cells are counter-labeled with the nuclear stain Hoechst 33342 (1 mg/ml; B2261; Sigma). 10.2.5 Confusion and Pitfalls Regarding the Neurosphere Assay The experimental demonstration of the self-renewal and multipotential capabilities depends critically on the clonal generation of primary neurospheres from single neural stem cells, which is evidenced by mixing experiments. For example, when subependymal cells from wild-type and those from GFP-expressing mice are mixed and cultured at appropriately low cell densities, neural stem cells from each cell source yield ubiquitously GFP-negative and ubiquitously GFP-positive neurospheres (Morshead et al. 2003; Young et al. 2007). However, the clonality of neurospheres has recently been challenged (Singec et al. 2006; Jessberger et al. 2007), showing that fusion between neurospheres is not a rare event even at low cell densities. The inconsistency between studies may be due to differences of culture conditions; passaged neurospheres tend to grow faster and to adhere much more easily to each other, and vibration of the dish, heterogeneity of temperature distribution of the media or usage of nonadhesive culture dish facilitates the movement of growing neurospheres and subsequent fusion. Indeed, neurosphere cultures using a semi-solid culture system that suppresses the motion of neurospheres or agarose gel coating to each cell suggest that fusion, if any, has little effect on estimating the number of neurospheres (Golmohammadi et al. 2008; Coles-Takabe et al. 2008). Indeed, movement of plates in order to study growing spheres under the microscope (Singec et al. 2006; Jessberger et al. 2007) greatly increases the probability of nonclonal neurospheres (Coles-Takabe et al. 2008). The in vivo relevance of neural stem cell-derived neurospheres remains to be verified conclusively. Specifically, it is unknown how many of the primary neurospheres are derived from bona fide neural stem cells that are maintained throughout the lifespan of the animals. The earliest isolation of neurosphere-forming cells during development is from neural tissue at E5.5; however, these neural stem cells rely on LIF for neurosphere formation with the earliest isolation of LIF-independent neural stem cells occurring at E8.5 (Hitoshi et al. 2004). Interestingly, LIF-dependent 198 S. Hitoshi et al. neurospheres can be derived directly in the absence of extrinsic signals from embryonic stem cells and passaging these cells produces LIF-independent neurospheres in vitro suggesting an intrinsic, default developmental program for generating neural stem cells (Smukler et al. 2006). Subsequently, two distinct populations of neurosphere-forming cells arises which are FGF2-responsive and then EGFresponsive exhibiting distinct proliferation kinetics from E11 through E17 when the neurosphere-forming cell population becomes predominantly responsive to either FGF2 or EGF (Martens et al. 2000). Nevertheless, the total number of neurosphereforming cells in the embryonic brain far exceeds that in the adult brain indicating that there are many embryonic cells which exhibit neural stem cells characteristics when isolated in vitro but are not bona fide life-long neural stem cells. Initially self-renewing and multipotent neurosphere-forming cells derived from many regions of the postnatal day 1 brain rapidly lose multipotentiality during in vitro passaging, whereas neurosphere-forming cells from the anterior lateral ventricle at the same age retain self-renewal and multipotentiality across several passages (Seaberg et al. 2005). Self-renewing and multipotential neurosphere-forming cells are only derived from the subependyma of the entire ventricular axis of the adult brain (Weiss et al. 1996) and these cells retain these characteristics during extensive passaging indicating that these are bona fide neural stem cells. However, there is a continuing controversy regarding the characteristics of neurosphere-forming cells cultured in the presence of EGF. It has been suggested that the majority of, if not all, EGFresponsive, neurosphere-forming cells in the adult brain are transit-amplifying cells (type C cells indicated by Dlx2 expression), which are progeny of quiescent neural stem cells (type B cells indicated by GFAP expression) (Doetsch et al. 2002). However, interpretation of these results is complicated because some GFAP-positive B cells in the subependyma also express Dlx2 (C. Morshead, personal communication). Perhaps a small portion of EGF-responsive (transit-amplifying) cells may retain sufficient self-renewal capability to generate neurospheres that are cultured through passaging procedures. It is possible that EGF-responsive neural stem cells in the adult brain may divide faster than FGF2-responsive ones (cell cycle times of more than 15 days) (Morshead et al. 1998) and may be more sensitive to anti-proliferating reagents used in the study by Doetsch et al. (2002). Alternatively, while most transit-amplifying cells differentiate and migrate out of the subependyma or die, a small number of neurosphere-initiating, transit-amplifying cells retain their stemness and may once again become quiescent neural stem cells. Regardless of the existence of EGF-responsive neural stem cells in the adult brain, it should be noted that addition of growth factors in the culture modifies the behavior of neural precursor cells and long-term passaging results in transformation with the cells exhibiting cell-autonomous changes in proliferating ability and gene expression (Morshead et al. 2002). Be that as it may, there are a constant number of neurosphere-forming cells that can be derived from the anterior lateral ventricle subependyma between 2 and 8 months of age followed by a gradual reduction in this population during senescence (Enwere et al. 2004; Kippin et al. 2004, 2005b; Maslov et al. 2004), with similar age-dependent declines in the number of long-term nucleotide analog-retaining 10 Culturing Adult Neural Stem Cells 199 cells (Kippin et al. 2005b) or double nucleotide incorporation (Maslov et al. 2004) and reduced olfactory bulb neurogenesis (Enwere et al. 2004; Kippin et al. 2005b), suggesting that in old age there is reduction in the overall number of neural stem cells. These data qualify the longevity characteristic indicating that stem cell function may be constant during adulthood with a subpopulation of stem cells retained during senescence. However, it is unclear if this decline is mediated stochastically or if there are intrinsic pre-existing differences between the cells that lose neurosphere-forming ability compared to those that retain it or if it is due to cell-autonomous or niche-mediated factors. 10.2.6 Application of the Neurosphere Assay The neurosphere assay can be used to study direct effects of neuro- and psychomimetic factors on neural stem cells in which the factors are simply added to the culture (Table 10.1). However, the interpretation of results may not be simple. Effects of an exogenously added factor should be determined by primary neurosphere formation, passaging procedures, and differentiation assay. When the number of primary neurospheres increases in the presence of a factor, the factor may augment the survival of neural stem cells, enhance their self-renewal, or even reverse the fate of differentiating neural progenitors back to self-renewing neural stem cells. Thus, to determine the nature of the effects on the neurosphere culture system, assessment of self-renewal employing clonal passaging procedures is indispensable. When single primary neurospheres grown in the presence of the factor generate more secondary neurospheres than are cultured in the absence of the factor, the factor is regarded as facilitating symmetric divisions in the primary neurosphere, that is, enhancing the self-renewal of the sphere-initiating neural stem cell. The neurosphere culture and subsequent differentiation assay could be easily applied to a high-throughput screening of reagents that may modify the size of the neural stem cell pool and neurogenesis in the adult mammalian brain. Table 10.1 Effects of neuro- and psycho-mimetic reagents on neurospheres Primary Secondary Reagents neurospheres neurospheres Differentiation References N.D. N.D. Heo et al. (2007) ↑ Ab, oligomeric Lópes-Toledano and Shelanski (2004) Dopamine ↓ ↓ N.D. Kippin et al. (2005) Olanzapine N.D. N.D. No effects Councill et al. (2006) Serotonin ↑ No effects N.D. Hitoshi et al. (2007) Antidepressants No effects N.D. N.D. Hitoshi et al. (2007) Mood stabilizers ↑ ↑ No effects Higashi et al. (2008) No effects N.D. N.D. Higashi et al. (2008) Anticonvulsants w/o mood stabilizing action 200 S. Hitoshi et al. In designing and interpreting experimental applications of pharmacological substances to culture systems, several characteristics of the interaction between neural stem cells and the compound must be accounted for. The pharmacological binding site and mechanism of action should be considered according to basic pharmacological principles. Neurospheres express a wide variety of receptors for neurotransmitters (e.g., Councill et al. 2006; Kippin et al. 2005; Melchiorri et al. 2007), hormones (e.g., Brännvall et al. 2002; Hitoshi et al. 2007; Wang et al. 2008) , as well as retrograde transmitter molecules (e.g., Aguado et al. 2005; Torroglosa et al. 2007). Thus, the employment of direct agonists to stimulate these receptors can be highly informative; however, investigations of the effects of psychotropic drugs require carefully designed experiments. For instance, the employment of antagonists or indirect agonists alone may lead to misleading negative results and may require additional receptor stimulation to fully understand the effects of these drugs on the neurosphere assay system. Further, it is important to determine whether the receptors of interest are present on neural stem cells or other precursors which can be achieved by sorting for the receptor of interest (e.g., immunostaining followed by FACS) and establishing that neurosphere-forming cells express these receptors. Given that the majority of neurosphere cells are not neural stem cells (e.g., lack self-renewal and/or multipotentiality), it is important to determine which cells are impacted by pharmacological manipulations through the employment of clonal passaging. For instance, effects on neural stem cell symmetric division will alter numbers of passaged neurospheres but not necessarily the number of neurospheres in the directly manipulated culture. Meticulous application of sound cellular and pharmacological techniques are critical in determining the effects of drugs on neural stem cells and their progeny in vitro that will be predictive of the in vivo effects on these cells. 10.3 Application of the Neurosphere Assay to Understanding In Vivo Neural Stem Cell Function Consistent with the general function of tissue-specific stem cells, neural stem cells within the ventricular system are involved in turnover of neurons in the adult brain, although in a limited fashion within two neurogenically-privileged brain regions, the olfactory bulbs and hippocampus. The utility of the neurosphere assay was established early in the process of identifying the in vivo properties of neural stem cells. The lateral ventricle subependymal zone contains at least two pools of proliferating precursors, one proliferating rapidly and the other proliferating slowly. Ablation of the rapidly proliferating precursors by high dose tritiated thymidine or Ara-C is followed by a gradual repopulation of these cells (Morshead et al. 1994; Doetsch et al. 1999). Moreover, neurosphere-forming cells are spared following a single high-dose of tritiated thymidine exposure indicating that they are relatively quiescent under basal conditions but that these cells are recruited into active cell cycle during the repopulation process and their number is reduced if tritiated thymidine is administered again during repopulation (Morshead et al. 1994). Gancylcovir-induced activation of thymidine kinase over-expressed under the 10 Culturing Adult Neural Stem Cells 201 control of the GFAP promoter (GFAP-HSVtk) results in ablation of the neurosphereforming cell both in vitro and in vivo (Imura et al. 2003; Morshead et al. 2003). Further extensive studies have shown migration from the anterior lateral ventricle subependyma to the olfactory bulb (for a recent review, see, e.g., Ortega-Perez et al. 2007) and the GCV-induced ablation of neurosphere-forming cells in the GFAPHSVtk mouse results in abolishment of adult olfactory bulb neurogenesis (Garcia et al. 2004); thus the neurosphere-forming cell acts as a stem cell in the adult nervous system contributing to the addition of new cells to at least one neurogenic region. Although the same manipulation reduces hippocampal neurogenesis, there is controversy about whether the ultimate precursor for neurogenesis in this region exhibits neural stem cell properties or capacity to form neurospheres (see, e.g., Bull and Bartlett 2005; Palmer et al. 1997; Seaberg et al. 2002). The estimation of pool size of neural stem cells in the adult brain has been achieved by a number of methods including using the neurosphere assay and immunohistochemical and electromicroscopic-level morphological analyses with each method having a number of benefits and drawbacks. The neurosphere assay has been applied for the estimation of neural stem cell numbers in normal and pathological conditions (Hitoshi et al. 2004; Kippin et al. 2004; Enwere et al. 2004). Since primary neurospheres are derived clonally from single neural stem cells under appropriate culture conditions, it provides a feasible method to estimate the total number of neural stem cells (i.e., self-renewing and multipotent cells) in the subependyma by counting primary neurospheres that are serially passaged and retain neural multipotentiality. However, the major drawback of this procedure is the reliance on ex vivo methodology which may be impacted by dissection and dissociation procedures or the application of culture conditions which may not be optimal for the cells of interest. For instance, differences in growth factor dependency of neurosphere formation have been reported for subependymal cells derived from the forebrain versus more posterior regions (Weiss et al. 1996), as well as from different species (Ray and Gage 2006). Alternatively, incorporation and long-term retention of nucleic acid analogs indexes the slowly dividing cells in the subependyma, which estimates neural stem cell proliferation. Although many of the long-term nucleotide analog-retaining cells express markers of neural stem cells (see, e.g., Batista et al. 2006) and incorporate a second nucleotide analog indicating long-term in vivo proliferation (Maslov et al. 2004), due to the lack of specific markers for the bona fide stem cells and basic information of their kinetics, absolute numbers of adult neural stem cells using this method remains elusive. Application of long-term nucleotide analog-retention with the neurosphere assay has generally revealed the same effects on neural stem cells but this combination has also indicated reductions in neural stem cell number accompanied by compensatory increases in their proliferation (Hitoshi et al. 2002a) and reduction in their proliferation with maintenance of the population size (Campbell and Kippin 2010). Thus, the employment of multiple techniques can reveal novel information regarding the in vivo neural stem cell population. Doetsch, Alvarez-Buylla and colleagues (reviewed in, e.g., Alvarez-Buylla et al. 2002; Doetsch 2003) have identified the “B” cells in the subependyma as the neural stem cells which can be quantified by morphological criterion under electron 202 S. Hitoshi et al. microscopy. Although this latter method has been applied to investigate alteration in the proportion of cells (including B cells) within the subependyma, its ability to estimate total neural stem cell numbers is limited by the technical infeasibility of performing analyses of the entire subependyma. Thus, the co-use of multiple methods of neural stem cell population estimation is highly recommended. 10.3.1 Neural Stem Cells and Excitotoxic Damage or Neurodegeneration The neurosphere assay has also been critical in establishing the responsiveness of neural stem cells to adult brain damage. Given that activation of microglia and formation of glia scaring are well documented, it is expected that a variety of forms of brain insult will increase proliferation around the site of injury. However, increased proliferation is also observed in subependyma following a variety of forms of damage (see, for reviews, Lie et al. 2004; Picard-Riera et al. 2004). Moreover, the numbers of neurosphere-forming cells in the subependyma of the lateral ventricles increases following stroke (Zhang et al. 2004) or hypoxiaischemia (Yang and Levison 2006). Similarly, spinal trauma induces expansion of neurosphere-forming cells in the spinal cord (Xu et al. 2006). Although activation of neural stem cells does not result in restoration of damaged neurons in the adult brain, there is some evidence that this activation is involved in minimizing the extent of stroke-induced damage (Won et al. 2006). Recently, our group examined the pool of neurosphere-forming cells during the course of pathology in a transgenic mouse model of Huntington’s disease and found that the onset of behavioral symptoms is associated with induction of a cell nonautonomous expansion of neurosphere-forming cells (Batista et al. 2006). These findings mirror those based on the postmortem histochemical analyses of tissue from deceased Huntington’s patients indicating increased volume of the subependymal zone with expansion of “B”-cells (putatively the neural stem cells) (Curtis et al. 2005). In our study, we observed that the increased expansion of neurosphere-forming cells in vivo, once induced, is accompanied by increased expansion in vitro which is maintained in a cell-autonomous and clonal fashion indicating the factors in the disease state are capable of “reprogramming” neural stem cells. This latter finding suggests that the consequent changes in neural stem cell function induced during disease may be amenable to molecular study even following passaging in vitro which may reveal novel mechanisms of stem cell regulation. 10.3.2 Neural Stem Cells and Psychoses The etiology of schizophrenia and the mechanism underlying its treatment by antipsychotic drugs are largely unknown. However, there is a well-established literature demonstrating abnormalities in brain morphology in patients with schizophrenia 10 Culturing Adult Neural Stem Cells 203 (see, e.g., McCarley et al. 1999). Recently, Reif et al. (2006) reported reduced proliferation in adult neurogenic regions in schizophrenics. Further, gene expression profiling of postmortem brain tissue from schizophrenics reveals abnormalities in a number of cell proliferation genes (reviewed in Toro and Deakin 2007) and a number of genes identified in association with increased incidence of disrupted adult neurogenesis (reviewed in Reif et al. 2007). Thus, there are multiple lines of clinical evidence suggesting that schizophrenia is associated with disruption of neural stem cells. To investigate this issue, we have examined neural stem cells in the prenatal stress model of psychoses. Similar to schizophrenic patients, prenatally-stressed rodents exhibit hyper-responsiveness to novelty and stimulant drugs as well as abnormalities in dopamine and glutamate neurotransmission in limbo-corticostriatal circuits (Ary et al. 2007; Kippin et al. 2008; Koenig et al. 2005). Moreover, prenatally-stressed rodents also exhibit a permanent reduction in the number of neurosphere-forming cells (Kippin et al. 2004) as well as reduced adult neurogenesis (Coe et al. 2003; Lemaire et al. 2000). Conversely, antipsychotic drugs, especially typical antipsychotic drugs, alter adult brain morphology (primarily increasing striatal volume and decreasing ventricular volume) in both schizophrenic populations and in laboratory animals (reviewed in Jeste et al. 1998), and in some studies, antipsychotic treatmentrelated increases in tissue volume are associated with positive treatment outcome (Gur et al. 1998; Lieberman et al. 2001). We have recently demonstrated that chronic haloperidol produces alterations in ventricular morphology that are associated with increased numbers of neurosphere-forming cells in the adult rat subependyma (Kippin et al. 2005). Although the molecular mechanism by which antipsychotic drugs achieve clinical efficacy is not completely known, all antipsychotic drugs antagonize dopamine D2 receptors. Briefly, the minimum clinically effective doses of antipsychotics are associated with relatively consistent levels (approximately 70–80%) of D2R occupancy in the striatum of patients regardless of whether typical or atypical antipsychotic drugs are employed (Kapur et al. 1999; Nyberg and Farde 2000). Consistent with the suggestion that a threshold of D2R occupancy is critical for the efficacy of antipsychotic drugs, we only observed changes in neural stem cell number following treatments that established plasma drug levels sufficient to produce greater than 70% D2R occupancy, indicating that the antipsychotic drug-induced changes in neural stem cell number may contribute to the clinical efficacy of these drugs. We also found that this haloperidol-induced expansion of neural stem cells is mediated by central dopamine D2 receptors (D2R) as evidenced by lack of haloperidol-induced neural stem cell expansion in D2R null mice and lack of NSC expansion following chronic treatment with a peripheral D2R antagonist (domperidone) in rats. High dose treatment with haloperidol also increased the numbers of rapidly-proliferating progenitors and adult-born neurons and glia (lower haloperidol doses were not examined on these latter measures). These results suggest that haloperidol expands the neural stem cell pool producing increased cytogenesis that contributes to alterations in brain morphology which possibly mediates effects observed clinically. Thus, we have generated converging evidence for a relation between neural stem cell function and both the exhibition of psychoses in the prenatal 204 S. Hitoshi et al. stress animal model and the control of psychotic symptoms by antipsychotic drugs. However, the casual link between the adult neural stem cell pool and psychoses, as well as whether or not specific disruptions of adult neural stem cell function are capable of inducing abnormalities in behavior, remains to be determined. 10.3.3 Neural Stem Cells and Depression Similar to the current state of knowledge regarding schizophrenia, the causes of depression and the molecular/cellular bases of antidepressant drug actions are not entirely understood. Further, unipolar mood disorders are widely associated with abnormalities of brain morphology involving reduced tissue volume, particularly in the hippocampus, and increased ventricular volume (for reviews, see Sheline 2003; Videbech and Ravnkilde 2004). Given the close association between depression and stress exposure and that stress exposure can reduce both hippocampal volume and adult neurogenesis, it has been widely posited that stress-induced reductions in neurogenesis are causative to the development of depressive symptoms (see, e.g., Banasr and Duman 2007; Sahay et al. 2007; Zhao et al. 2008). Recently, we have demonstrated that the forced swim model of depression (which induces a number of behavioral alterations which are typically referred to as “learned helplessness”) decreases the number of adult neurosphere-forming cells in the adult mouse brain (Hitoshi et al. 2007), indicating that stress effects on neural stem cells may contribute to the manifestation of the behavioral alterations in depression models. Consistent with the structural abnormalities identified in depressed patients, there is emerging evidence that antidepressant drugs can increase brain tissue volumes, particularly in the hippocampus (see, e.g., Kasper and McEwen 2008) suggesting that the action of these drugs may also involve increased production of adult-born cells. Consistent with this hypothesis, Santarelli et al. (2003) reported that the behavioral effects of antidepressant drugs in mice were dependent upon the production of adult neurogenesis; however, this effect was not observed in a different strain of mouse (Holick et al. 2008). Nevertheless, our group has recently observed that treatment with fluoxetine restores the number of neurosphere-forming cells in the subependymal zone of mice exposed to chronic forced swim (Hitoshi et al. 2007). These findings stand in apparent contrast to early reports on the regional-specific effects of antidepressant drugs, including fluoxetine, on proliferation in the adult brain (e.g., Malberg et al. 2000); however, it is important to point out that, in the earlier studies, drug treatment was examined only in nonstressed animals. Thus, subject variables likely constitute critical factors in determining the impact of psychotropic drugs on neural stem cells and their progeny, and understanding the mechanisms underlying psychiatric medications may require employment of appropriate animal models in addition to drug exposure. 10 Culturing Adult Neural Stem Cells 205 10.4 Concluding Remarks The neurosphere assay has been employed by hundreds of labs in order to study neural stem cells in vitro. As with all assays, its correspondence to the in vivo cell it attempts to measure is under continual examination. This has led to improvements in the neurosphere assay, as well as the development of additional measures of neural stem cell function. Nevertheless, the ability of the neurosphere assay to rigorously quantify numbers of neural stem cells, self-renewal of neural stem cells and their differentiation potential has made this assay extremely useful in modern neuroscience and stem cell biology research. References Aguado T, Monory K, Palazuelos J et al (2005) The endocannabinoid system drives neural progenitor proliferation. FASEB J 19:1704–1706 Alvarez-Buylla A, Seri B, Doetsch F (2002) Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull 57:751–758 Antonchuk J, Sauvageau G, Humphries RK (2002) HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109:39–45 Banasr M, Duman RS (2007) Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets 6:311–320 Batista CM, Kippin TE, Willaime-Morawek S et al (2006) A progressive and cell non-autonomous increase in striatal neural stem cells in the Huntington’s disease R6/2 mouse. J Neurosci 26:10452–10460 Brännvall K, Korhonen L, Lindholm D (2002) Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci 21:512–520 Bull ND, Bartlett PF (2005) The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci 25:10815–10821 Campbell JC, Kippin TE (2010) The interaction of neural stem cells and chronic alcohol consumption. In Drug Addiction and Adult Neurogenesis. M.F. Olive (ED). Research Signpost/ Transworld Research Network Publishers Coe CL, Kramer M, Czéh B et al (2003) Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry 54:1025–1034 Coles-Takabe BLK, Brain I, Purpura KA et al (2008) Don’t look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells 26:2938–2944 Councill JH, Tucker ES, Haskell GT et al (2006) Limited influence of olanzapine on adult forebrain neural precursors in vitro. Neurosci 140:111–122 Curtis MA, Waldvogel HJ, Synek B et al (2005) A histochemical and immunohistochemical analysis of the subependymal layer in the normal and Huntington’s disease brain. J Chem Neuroanat 30:55–66 Doetsch F (2003) The glial identity of neural stem cells. Nat Neurosci 6:1127–1134 Doetsch F, Petreanu L, Caille I et al (2002) EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36:1021–1034 Enwere E, Shingo T, Gregg C et al (2004) Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci 24:8354–8365 Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438 Golmohammadi MG, Blackmore DG, Large B et al (2008) Comparative analysis of the frequency and distribution of stem and progenitor cells in the adult mouse brain. Stem Cells 26:979–987 206 S. Hitoshi et al. Heo C, Chang K-A, Choi HS et al (2007) Effects of the monomeric, oligomeric, and fibrillar Ab42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. J Neurochem 102:493–500 Higashi M, Maruta N, Bernstein A et al (2008) Mood stabilizing drugs expand the neural stem cell pool in the adult brain through activation of Notch signaling. Stem Cells 26:1758–1767 Hitoshi S, Alexson T, Tropepe V et al (2002a) Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev 16:846–858 Hitoshi S, Tropepe V, Sirard C et al (2002b) Neural stem cell lineages are regionally specified, but not committed, within distinct compartments of the developing brain. Development 129:233–244 Hitoshi S, Seaberg RM, Koscik C et al (2004) Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling. Genes Dev 18:1806–1811 Hitoshi S, Maruta N, Higashi M et al (2007) Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J Neurosci Res 85:3574–3585 Holick KA, Lee DC, Hen R et al (2008) Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology 33:406–417 Imura T, Kornblum HI, Sofroniew MV (2003) The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci 23:2824–2832 Jessberger S, Clemenson Jr GD, Gage FH (2007) Spontaneous fusion and non-clonal growth of adult neural stem cells. Stem Cells 25:871–874 Kasper S, McEwen BS (2008) Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs 22:15–26 Kippin TE, Cain SW, Masum Z et al (2004) Neural stem cells show bidirectional experience-dependent plasticity in the perinatal mammalian brain. J Neurosci 24:2832–2836 Kippin T, Kapur S, van der Kooy D (2005) Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci 25:5815–5823 Kippin TK, Szumlinski KK, Kapasova Z et al (2008) Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology 33:769–782 Koenig JI, Elmer GI, Shepard PD et al (2005) Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res 156:251–261 Kondo M, Wagers AJ, Manz MG et al (2003) Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol 21:759–806 Lemaire V, Koehl M, Le Moal M et al (2000) Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 97:11032–11037 Lie DC, Song H, Colamarino SA et al (2004) Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol 44:399–421 Lim DA, Huang YC, Alvarez-Buylla A (2007) The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurg Clin N Am 18:81–92 Lópes-Toledano MA, Shelanski ML (2004) Neurogenic effect of b-amyloid peptide in the development of neural stem cells. J Neurosci 24:5439–5444 Louis SA, Rietze RL, Deleyrolle L et al (2008) Enumeration of neural stem and progenitor cells in the neural colony forming cell assay. Stem Cells 26:988–996 Melchiorri D, Cappuccio I, Ciceroni C et al (2007) Metabotropic glutamate receptors in stem/ progenitor cells. Neuropharmacology 53:473–480 Morshead CM, Craig CG, van der Kooy D (1998) In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development 125:2251–2261 Morshead CM, Benveniste P, Iscove NN et al (2002) Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nature Med 8:268–273 Morshead CM, Garcia D, Sofroniew MV et al (2003) The ablation of glial fibrillary acidic proteinpositive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci 18:76–84 10 Culturing Adult Neural Stem Cells 207 Murayama A, Matsuzaki Y, Kawaguchi A et al (2002) Flow cytometric analysis of neural stem cells in the developing and adult mouse brain. J Neurosci Res 69:837–847 Ortega-Perez I, Murray K, Lledo PM (2007) The how and why of adult neurogenesis. J Mol Histol 38:555–562 Palmer TD, Takahashi J, Gage FH (1997) The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci 8:389–404 Picard-Riera N, Nati-Oumesmar B, Baron-Van Evercooren A (2004) Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res 6:223–231 Ray J, Gage FH (2006) Differential properties of adult rat and mouse brain-derived neural stem/ progenitor cells. Mol Cell Neurosci 31:560–573 Reif A, Schmitt A, Fritzen S et al (2007) Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci 257:290–299 Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710 Reynolds BA, Tetzlaff W, Weiss S (1992) A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci 12:4565–4574 Rietze RL, Valcanis H, Brooker GF et al (2001) Purification of a pluripotent neural stem cells from the adult mouse brain. Nature 412:736–739 Sahay A, Drew MR, Hen R (2007) Dentate gyrus neurogenesis and depression. Prog Brain Res 163:697–722 Seaberg RM, van der Kooy D (2002) Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci 22:1784–1793 Seaberg RM, Smukler SR, van der Kooy D (2005) Intrinsic differences distinguish transiently neurogenic progenitors from neural stem cells in the early postnatal brain. Dev Biol 278:71–85 Sheline YI (2003) Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 54:338–352 Singec I, Knoth R, Meyer RP et al (2006) Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Method 3:801–806 Smukler SR, Runciman SB, Xu S et al (2006) Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J Cell Biol 172:79–90 Temple S, Alvarez-Buylla A (1999) Stem cells in the adult mammalian central nervous system. Curr Biol 9:135–141 Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C et al (2007) Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells 25:88–97 Tropepe V, Sibilia M, Ciruna BG et al (1999) Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol 208:166–188 Videbech P, Ravnkilde B (2004) Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966 Wang JM, Liu L, Brinton RD (2008) Estradiol-17beta-induced human neural progenitor cell proliferation is mediated by an estrogen receptor beta-phosphorylated extracellularly regulated kinase pathway. Endocrinology 149:208–218 Weiss S, Dunne C, Hewson J et al (1996) Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16:7599–7609 Won Sj, Kim SH, Xie L et al (2006) Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exp Neurol 8:250–259 Xu Y, Kitada M, Yamaguchi M et al (2006) Increase in bFGF-responsive neural progenitor population following contusion injury of the adult rodent spinal cord. Neurosci Lett 397:174–179 Yang Z, Levison SW (2006) Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience 139:555–564 Young KM, Fogarty M, Kessaris N et al (2007) Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci 27:8286–8296 Index A Addiction, 53, 79–86 Adrenocorticotropic factor, 100 Adrenocorticotropic hormone (ACTH), 100 Aged brain, 137 Aging, 53, 77–79 a-linolenic acid, 152 Allostasis, 54–55 Alzheimer disease, 17 Amygdala, 3, 11, 18 Animal model, 113, 117, 121 Antidepressant, 100, 102–104, 204 Antipsychotic, 203 Anxiety, 79, 84 Ara-C, 200 Arousal, 56–57 a-synuclein, 30 B Basic helix-loop-helix family, member e22 (Bhlhb5), 181 Bcl11b, 180, 181 bIII-Tubalin, 161 B-FABP, 156 b-FGF, 160 Brain-derived neurotrophic factor (BDNF), 32, 104, 140, 154 Brain plasticity, 3 Bromodeoxyuridine (BrdU), 39–41, 45, 150 C CA1, 154 Callosal projection neurons (CPN), 177, 180–182 Calretinin (CR), 8 cAMP-response element binding protein (CREB), 103, 104, 161–164 Caspases, 138 Caudate nucleus, 13 Cell division asymmetric, 195 symmetric, 195 Cell proliferation, 39–41, 44, 45 Cerebral cortex, 141 Choline acetyl transferase (ChAT), 13 Clim1, 181 Clonality, 197 Coriticotectal projection neurons (CTPN), 179, 180, 182 Coriticothalamic projection neuron (CThPN), 180 Corticospinal motor neurons (CSMN), 179–182 Corticosterone, 57, 59, 74–76, 78–79, 86 Corticostriatal projection neurons (CStrPN), 180 COUP-TF1 interacting protein 2 (Ctip2), 180–182 CPN. See Callosal projection neuron CR. See Calretinin CREB. See cAMP-response element binding protein CSMN. See Corticospinal motor neuron CThPN. See Coriticothalamic projection neuron CTPN. See Coriticotectal projection neuron D DARPP-32, 137 Default, 198 Dentate gyrus (DG), 38–47 Depression, 204 Differences individual, 57, 72, 74–75, 78–80, 85–86 sex, 69, 70, 74 strain, 69, 72 T. Seki et al. (eds.), Neurogenesis in the Adult Brain II: Clinical Implications, DOI 10.1007/978‑4‑431‑53945-2, © Springer 2011 209 210 Differentiation, 143 Docosahexaenoic acid (DHA), 151 Dopamine, 60, 84 Dopaminergic neuron, 8, 23 Doublecortin, 135, 161 E Eicosapentaenoic acid (EPA), 151 Electoroconvulsive therapy (ECT), 104 Endothelial cells, 139 Enriched environment, 18 Epilepsy, 37–47 F FABP7, 156 Fez family member 2 (Fezf2), 180, 182 Fluorescence-activated cell sorting (FACS), 190 Forebrain embryonic zinc finger-like (Fezl), 180 Fusion, 197 Index Hypothalamic-pituitary-adrenal axis (HPA axis), 54, 57, 59–60, 74–75, 78–79, 84–86, 100, 101 I Inflammation, 138–139 Insulin-like growth factor-1 (IGF-1), 135 Interleukin, 75–76 Interleukin-1beta (IL-1b), 101 K Kainic acid, 38 L Lewy body, 23 Linoleic acid, 152 Lipofuscin, 10 Lmo4, 181 Long-term potentiation (LTP), 155 G GABAergic neurons, 13 Gancyclovir, 200 GCL. See Granule cell layer Glial cell line-derived neurotrophic factor (GDNF), 144 Glucocorticoid hormone, 100 Glucocorticoids, 100 Glutamate neurotransmission, 101 GPR40, 159–161 G protein-coupled receptor (GPCR), 159 Granule cell layer (GCL), 157 Granulocyte colony-stimulating factor (G-CSF), 32, 143 M Matrix metalloproteinase–9 (MMP–9), 140 Mature-BDNF, 154 Memory, 77–79 1-methyl–4-phenyl–1,2,3,6-tetrahydropyri‑ dine (MPTP), 26 Microglia, 138 Migration, 143 Model, 134 Monocyte chemoattractant protein-1 (MCP-1), 140 Mood/affect disorders, 18 Mossy fiber sprouting, 38, 43 Motor recovery, 142 Multipotentiality, 196 H Hilar basal dendrite (HBD), 41, 42 Hippocampus, 38–41, 43, 45, 46, 112–114, 117–119, 121 Homeostasis, 54 5-HT. See Serotonin 5-HT1A, 103 5-HT2A, 103 5-HT2C, 103 Human, 141 Human postmortem materials, 8 Huntington disease, 17 Hydrocephalus, 17 N NA. See Noradrenaline Neural progenitor, 39, 40, 44 Neural stem cell (NSC), 38–41 Neuroblast migration, 138 Neurodegeneration, 202 Neurodevelopmental theory, 119 Neurogenerative diseases, 3 Neurogenesis, 37–47, 109–122 Neurogenesis-modifying drugs, 143 Neuronal migration, 43 Neuronal subtype, 173, 175, 178, 183 Neurosphere assay, 191 Index Neurotrophin, 164 Neurotrophin-3 (NT-3), 164 Nitric oxide, 76 N-methyl-d-aspartate (NMDA), 158 Noradrenaline (NA), 102 Notch, 135 n-3 PUFA, 163 NSC. See Neural stem cell Nucleus accumbens, 13 O Olfactory peduncle, 16 Olfactory tubercle, 16 P Parkinson’s disease, 17, 23 Parvalbumin (PV), 10, 137 pCREB, 161 Peroxisome proliferator-activated recptor (PPAR), 155 Phospholipase C (PLC), 160 Pilocarpine, 38, 39, 44 Piriform cortex, 3, 10, 11 Polysialylated-neural cell adhesion molecule (PSA-NCAM), 150 Polyunsaturated fatty acid (PUFA), 149–166 Post-traumatic stress disorder (PTSD), 77, 85 Predictablility, 56 Prepulse inhibition (PPI), 112–117 Protein kinase C (PKC), 163 Psycho-mimetic factor, 199 Psychosis, 202 PUFA-GPR40 signaling, 149–166 Putamen, 13 PV. See Parvalbumin R Retinoid X receptor (RXR), 158 Retrovirus, 42 Road-map towards the clinic, 144 S Schizophrenia, 18, 109–122, 202 SDF-1a. See Stromal-derived factor 1a SE. See Status epilepticus Seizure, 37–47 Seizure focus, 38 Self-renewal, 195 Semi-solid, 197 Serotonin (5-HT), 102, 103 211 Serum-free media (SFM), 191 SGZ. See Subgranular zone Side population, 190 Somatostatin (SS), 13 Sox5, 180, 181 Sox6, 181 Special AT-rich sequence binding protein 2 (Satb2), 182 Squirrel monkeys, 3 Status epilepticus (SE), 39–47 Stem cell hematopoietic, 190 neural, 189 Stress, 18, 100–103 acute, 69–70 chronic, 57, 69, 72 footshock, 70 physical, 57, 69 postnatal, 73–74 predator, 70 prenatal, 73–74, 85–86 psychological, 57, 70 sleep deprivation, 71 syndrome, 54 Striatum, 3, 13 Stromal-derived factor 1a (SDF-1a), 140 Subependymal zone, 193, 198 Subgranular zone (SGZ), 157 Substantia nigra, 23 Subventricular zone (SVZ), 40, 175–177, 183 Survival, 143 Synapses, 142 Synaptic connectivity, 142 T T cells, 139 Temporal lobe epilepsy, 38 Temporal migratory stream, 10 Thymidine kinase, 200 Transit-amplifying cell, 198 Tritiated thymidine, 200 Tumor necrosis factor-a (TNF-a), 135 Tyrosine hydroxylase, 24 Tyrosine kinase receptor type 2 (TrkB), 165 V Vascular endothelial growth factor (VEGF), 104 Vascular remodeling, 139 Vasculature, 76 Ventricular zone, 192