* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Practice Problem - HCC Southeast Commons
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Discodermolide wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
George S. Hammond wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Homoaromaticity wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
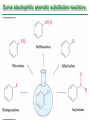
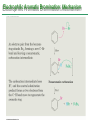
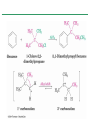
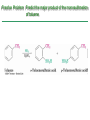
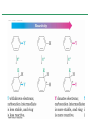
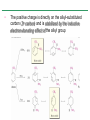
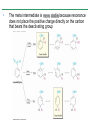
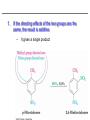
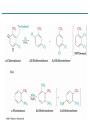
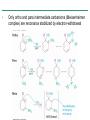
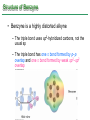
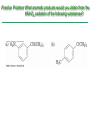
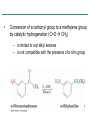
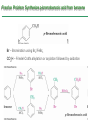
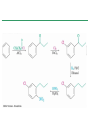
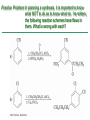
Chapter 16 Introduction • Electrophilic aromatic substitution is the most common reaction of aromatic compounds – It replaces a proton (H+) on an aromatic ring with another electrophile (E+) – It leads to the retention of the aromatic core Some electrophilic aromatic substitution reactions 1. Bromination of Aromatic Rings • Benzene is a site of electron density – Its 6 electrons are in a cyclic conjugated system – Its 6 electrons are sterically accessible to other reactants because they are located above or below the plane • Benzene acts as an electron donor (a Lewis base or nucleophile) – It reacts with electron acceptors (Lewis acids or electrophiles) – Benzene’s electrons participate as a Lewis base in reactions with Lewis acids • Bromination of benzene occurs in two steps: – Step 1: The electrons act as a nucleophile toward Br2 (in a complex with FeBr3) to form a nonaromatic carbocation intermediate – Step 2: The resonance-stabilized carbocation intermediate loses H+ to regenerate the aromatic ring Electrophilic Alkene Addition Electrophilic Aromatic Bromination • Aromatic rings are less reactive toward electrophiles than alkenes – Unlike alkenes, benzene does not react rapidly with Br2 in CH2Cl2 – For bromination, benzene requires FeBr3 as a catalyst to polarize the bromine reagent and make it more electrophilic • Step 1: The electrons act as a nucleophile and attack the polarized Br2 (in a complex with FeBr3) to form a nonaromatic carbocation intermediate – It is a slow, rate-limiting step (high DG‡) – The carbocation is doubly allylic (nonaromatic) and has three resonance forms – The carbocation intermediate is not aromatic and is high in energy (less stable than benzene) – Step 1 is endergonic, has a high DG‡ and is a slow reaction • Step 2: The resonance-stabilized carbocation intermediate loses H+ to regenerate the aromatic ring and yield a substitution product in which H+ is replaced by Br+ – It is similar to the 2nd step of an E1 reaction – The carbocation intermediate transfers a H+ to FeBr4- (from Br- and FeBr3) – This restores aromaticity (in contrast with addition in alkenes) – Step 2 is exergonic, has a low DG‡ and is a fast reaction Electrophilic Aromatic Bromination: Mechanism Why is there electrophilic aromatic substitution rather than addition? – Substitution reaction retains the stability of the aromatic ring and is exergonic Addition Loss of aromaticity Endergonic Substitution Retention of aromaticity Exergonic Practice Problem: Monobromination of toluene gives a mixture of three bromotoluene products. Draw and name them. 2. Other Aromatic Substitutions • The reaction with bromine involves a mechanism that is similar to many other reactions of benzene with electrophiles – The cationic intermediate was first proposed by G. W. Wheland and is often called the Wheland intermediate Electrophilic Aromatic Substitution • An electrophilic aromatic substitution reaction involves two steps: – reaction of an electrophile E+ with an aromatic ring – loss of H+ from the resonance-stabilized carbocation intermediate to regenerate the aromatic ring • The same general mechanism is used by other aromatic substitutions including: Chlorination Iodination Nitration Sulfonation F is too reactive for monofluorination Aromatic Chlorination • Benzene ring reacts with Cl2 in the presence of FeCl3 catalyst to yield chlorobenzene – It requires FeCl3 to polarize Cl2 (make it more electrophilic) Aromatic Iodination • Benzene ring reacts with I2 in the presence of an oxidizing agent (H2O2 or CuCl2) to yield iodobenzene – Iodine must be oxidized to form a more powerful electrophilic I+ species (with Cu2+ or peroxide) Aromatic Nitration • Benzene ring reacts with a mixture of concentrated nitric and sulfuric acids (HNO3 and H2SO4) to yield nitrobenzene – The combination of nitric acid and sulfuric acid produces NO2+ (nitronium ion), an electrophile – The electrophile NO2+ is produced when HNO3 is protonated by H2SO4 and loses H2O – NO2+ reacts with benzene to give a carbocation intermediate which loses H+ to yield nitrobenzene – Aromatic nitration is useful in the pharmaceutical industry because the nitro-substituted product can be reduced by Fe or SnCl2 to yield arylamine Aromatic Sulfonation • Benzene ring reacts with fuming sulfuric acid (a mixture of H2SO4 and SO3) to yield benzenesulfonic acid – The reactive electrophile is either HSO3+ or neutral SO3 depending on reaction conditions – The reactive electrophile is either sulfur trioxide SO3 or its conjugate acid HSO3+ – The reaction occurs via the Wheland intermediate (carbocation) – The reaction is reversible (Sulfonation is favored in strong acid; desulfonation, in hot, dilute aqueous acid) – Aromatic sulfonic acids are useful as intermediates in the synthesis of dyes and pharmaceuticals. • Example: Sulfadrugs A sulfadrug – Aromatic sulfonic acids undergo alkali fusion reaction • Heating with NaOH at 300 ºC followed by neutralization with acid replaces the SO3H group with an OH • Example: Synthesis of p-cresol Practice Problem: How many products might be formed on chlorination of o-xylene (o-dimethylbenzene), m-xylene, and p-xylene? Practice Problem: When benzene reacts with D2SO4, deuterium slowly replaces all six hydrogens in the aromatic ring. Explain. 3. Alkylation of Aromatic Rings: The Friedel-Crafts Reaction • Benzene ring reacts with an alkyl chloride in the presence of AlCl3 catalyst to yield an arene – Alkylation was first reported by Charles Friedel and James Crafts in 1877 • Friedel-Crafts alkylation – is an electrophilic aromatic substitution in which the electrophile is a carbocation, R+. – AlCl3 catalyst promotes the formation of the alkyl carbocation, R+, from the alkyl halide, RX – The Wheland (carbocation) intermediate forms – Alkylation is the attachment of an alkyl group to benzene; R+ substitutes for H+ Friedel-Crafts Alkylation Reaction: Mechanism Limitations of the Friedel-Crafts Alkylation 1. Only alkyl halides can be used (F, Cl, Br, I) – Aryl halides and vinylic halides do not react (their carbocations are too high in energy to form) 2. No reaction occurs if the aromatic ring has an amino group or a strongly electron-withdrawing group substituent – Amino groups react with AlCl3 catalyst in an acidbase reaction 3. It is difficult to control the reaction. Multiple alkylations can occur because the first alkylation is activating – Polyalkylation is often observed 4. Carbocation rearrangements occur during alkylation, particularly when a 1o alkyl halide is used – Catalyst, temperature and solvent affect the amount of rearrangement Rearranged Unrearranged Carbocation rearrangements of Friedel-Crafts alkylation – are similar to those that occur during electrophilic additions to alkenes – can involve hydride (H:-) or alkyl shifts More Stable Limitations of the Friedel-Crafts Alkylation 1. Only alkyl halides can be used (F, Cl, Br, I) 2. No reaction occurs if the aromatic ring has an amino group or a strongly electron-withdrawing group substituent 3. It is difficult to control the reaction. Multiple alkylations can occur because the first alkylation is activating 4. Carbocation rearrangements occur during alkylation, particularly when a 1o alkyl halide is used Practice Problem: The Friedel-Crafts reaction of benzene with 2chloro-3-methylbutane in the presence of AlCl3 occurs with carbocation rearrangement. What is the structure of the product? Practice Problem: Which of the following alkyl halides undergo Friedel-Crafts reaction without rearrangement? Explain. a. CH3CH2Cl b. CH3CH2CH(Cl)CH3 c. CH3CH2CH2Cl d. (CH3)3CCH2Cl e. Chlorocyclohexane Practice Problem: What is the major monosubstitution product from Friedel-Crafts reaction of benzene with 1chloro-2-methylpropane in the presence of AlCl3? 4. Acylation of Aromatic Rings: The Friedel-Crafts Reaction • Benzene ring reacts with a carboxylic acid chloride, RCOCl, in the presence of AlCl3 catalyst to yield an acylbenzene – Acylation is the attachment of an acyl group,-COR, to benzene; RCO+ substitutes for H+ • Friedel-Crafts acylation – is an electrophilic aromatic substitution in which the reactive electrophile is a resonance-stabilized acyl cation, RCO+. – AlCl3 catalyst promotes the formation of the acyl cation, RCO+, from the acyl chloride, RCOCl – The acyl cation, RCO+, does not rearrange; it is resonance-stabilized – The Wheland (carbocation) intermediate forms Friedel-Crafts Acylation Reaction: Mechanism – The mechanism of Friedel-Crafts acylation is similar to Friedel-Crafts alkylation • In Friedel-Crafts acylation, there is no carbocation rearrangement nor multiple substitution – No carbocation rearrangement: The acyl cation, RCO+, does not rearrange because it is resonance-stabilized by interaction of the vacant orbital on C with lone pair of electrons on O – No multiple substitution: Acylated benzene is less reactive than nonacylated benzene Practice Problem: Identify the carboxylic acid chloride that might be used in a Friedel-Crafts acylation reaction to prepare each of the following acylbenzenes 5. Substituent Effects in Substituted Aromatic Rings A substituent present on an aromatic ring affects: • the reactivity of the aromatic ring • the orientation of the reaction Substituents affect the reactivity of the aromatic ring Substituents may • activate the ring, make it (much) more reactive than benzene or • deactivate the ring, make it (much) less reactive than benzene Substituents affect the orientation of the reaction Substituents present on the ring determine the position of the 2nd substitution: ortho, meta, and para Classification of Substituent Effect Substituents can be classified as: • • • ortho- and para-directing activators, ortho- and para-directing deactivators, and meta-directing deactivators The directing effects of the groups correlate with their reactivities: • • • All meta-directing groups are strongly deactivating Most ortho- and para-directing groups are activating Halogens are unique being ortho- and para-directing but weakly deactivating Origins of Substituent Effects Reactivity and orientation in electrophilic aromatic substitutions are controlled by an interplay of inductive effects and resonance effects: – Inductive effect - withdrawal or donation of electrons through a s bond – Resonance effect - withdrawal or donation of electrons through a bond Inductive Effects Inductive effects - withdrawal or donation of electrons through a s bond due to electronegativity and polarity of bonds in functional groups • Halogens, C=O, CN, and NO2 groups inductively withdraw electrons through s bond connected to ring • Alkyl groups inductively donate electrons • Halogens, C=O, CN, and NO2 inductively withdraw electrons through s bond connected to ring • Alkyl groups inductively donate electrons Resonance Effects Resonance effect - withdrawal or donation of electrons through a bond due to the overlap of a p orbital on the substituent with a p orbital on the aromatic ring • C=O, CN, and NO2 groups withdraw electrons from the aromatic ring by resonance • Halogen, OH, alkoxyl (OR), and amino substituents donate electrons to the aromatic ring by resonance • C=O, CN, and NO2 groups withdraw electrons from the aromatic ring by resonance – electrons flow from the ring to the substituents, placing a positive charge in the ring • C=O, CN, and NO2 groups withdraw electrons from the aromatic ring by resonance placing a positive charge in the ring – Effect is greatest at ortho and para – Z is more electronegative than Y • Halogen, OH, alkoxyl (OR), and amino substituents donate electrons to the aromatic ring by resonance – electrons flow from the substituents to the rings placing a negative charge in the ring • Halogen, OH, alkoxyl (OR), and amino substituents donate electrons to the aromatic ring by resonance placing a negative charge in the ring – Effect is greatest at ortho and para – Y has a lone pair of electrons Contrasting Effects: Inductive vs Resonance • When the two effects act in opposite direction, the strongest effects dominate. – Halogens have electron-withdrawing inductive effects due to electronegativity – Halogens have electron-donating resonance effects due to lone-pair electrons – Resonance interactions are generally weaker, affecting orientation. Thus, halogens deactivate the ring Practice Problem: Predict the major product of the monosulfonation of toluene. Practice Problem: Write resonance structures for nitrobenzene to show the electron-withdrawing resonance effect of the nitro group. Practice Problem: Write resonance structures for chlorobenzene to show the electron-donating resonance effect of the chloro group. Practice Problem: Predict the major products of the following reactions a. Mononitration of bromobenzene b. Monobromination of nitrobenzene c. Monochlorination of phenol d. Monobromination of aniline 6. An Explanation of Substituent Effects • Activating groups donate electrons to the ring, stabilizing the Wheland intermediate (carbocation) – OH, OR, NH2 and R • Deactivating groups withdraw electrons from the ring, destabilizing the Wheland intermediate – CN, C=O, NO2 and X • An electron-withdrawing group makes the ring more electron-poor (eg. CN and Cl) • An electron-donating group makes the ring more electron-rich (eg. CH3 and NH2) Practice Problem: Rank the compounds in each group in order of their reactivity to electrophilic substitution a. Nitrobenzene, phenol, toluene, benzene b. Phenol, benzene, chlorobenzene, benzoic acid c. Benzene, bromobenzene, benzaldehyde, aniline Practice Problem: Explain why Friedel-Crafts alkylations often give polysubstitution but Friedel-Crafts acylations do not Practice Problem: Would you expect trifluoromethylbenzene to be more reactive or less reactive than toluene toward electrophilic substitution? Explain. Ortho- and Para-Directing Activators: Alkyl Groups • Alkyl groups are activating – They have an electron-donating inductive effect • Alkyl groups are ortho and para directors – The ortho and para intermediates are the most stabilized (lower in energy) – The positive charge is directly on the alkylsubstituted carbon (3o carbon) and is stabilized by the inductive electron-donating effect of the alkyl group • The positive charge is directly on the alkyl-substituted carbon (3o carbon) and is stabilized by the inductive electron-donating effect of the alkyl group Ortho- and Para-Directing Activators: OH and NH2 • OH, OR and NH2 groups are activating – They have a strong electron-donating resonance and a weak electron-withdrawing inductive effect • OH, OR and NH2 groups are ortho and para directors – The ortho and para intermediates are the most stabilized (lower in energy) – The positive charge is stabilized by resonance donation of an electron pair from O or N • The ortho and para intermediates are more stable because of resonance donation of an electron pair from O or N Practice Problem: Acetanilide is less reactive than aniline toward electrophilic substitution. Explain. Ortho- and Para-Directing Deactivators: Halogens • Halogens are deactivating – They have a strong electron-withdrawing inductive and a weak electron-donating resonance effect • Halogens are ortho and para directors – The ortho and para intermediates are the most stabilized (lower in energy) – Halogens stabilize the positive charge by resonance donation of a lone pair of electrons • The ortho and para intermediates are more stable because of resonance donation of an electron pair from X Meta-Directing Deactivators • All meta-directing groups are strongly deactivating – They have electron-withdrawing inductive and resonance effects that reinforce each other – The ortho and para intermediates are destabilized – The positive charge of the carbocation intermediate in ortho and para attack is directly on the carbon that bears the deactivating group and resonance cannot produce stabilization • The meta intermediate is more stable because resonance does not place the positive charge directly on the carbon that bears the deactivating group Summary of Substituent Effects in Aromatic Substitution Practice Problem: Draw resonance structures for the intermediates from reaction of an electrophile at the ortho, meta, and para positions of nitrobenzene. Which intermediates are most stable? 7. Trisubstituted Benzenes: Additivity of Effects Three rules for the additive effects of two different groups: 1. If the directing effects of the two groups are the same, the result is additive 2. If the directing effects of two groups oppose each other, the more powerful activating group determines the principal outcome 3. The position between the two groups in metadisubstituted compounds is unreactive 1. If the directing effects of the two groups are the same, the result is additive – It gives a single product 2. If the directing effects of two groups oppose each other, the more powerful activating group determines the principal outcome – It usually gives mixtures of products 3. The position between the two groups in metadisubstituted compounds is unreactive – The reaction site is too hindered – To make aromatic rings with three adjacent substituents, it is best to start with an orthodisubstituted compound Practice Problem: What product would you expect from bromination of p-methylbenzoic acid? Practice Problem: At what positions would you expect electrophilic substitution to occur in the following substances? Practice Problem: Show the major product(s) from reaction of the following substances with (i) CH3CH2Cl, AlCl3 and (ii) HNO3, H2SO4 8. Nucleophilic Aromatic Substitution • Nucleophilic aromatic substitution is a reaction that aryl halides with electron-withdrawing substituents undergo – It replaces a halide ion (X-) on an aromatic ring with another nucleophile (Nu-) Nucleophilic Aromatic Substitution • A nucleophilic aromatic substitution reaction occurs in two steps by the addition/elimination mechanism: – Step 1: Addition of the nucleophile (Nu-) to the electron-deficient aryl halide, forming a resonance stabilized carbanion intermediate (Meisenheimer complex) – Step 2: Elimination of a halide ion (X-) from the carbanion intermediate to regenerate the aromatic ring Nucleophilic Aromatic Substitution: Mechanism • Nucleophilic aromatic substitution occurs ONLY if the aryl halide has an electron-withdrawing substituent in ortho and/or para position – The more such substituents, the faster the reaction – Only ortho and para electron-withdrawing substituents can stabilize the anion intermediate through resonance • Only ortho and para intermediate carbanions (Meisenheimer complex) are resonance stabilized by electron-withdrawal • A nucleophilic aromatic substitution reaction is neither an Sn1 nor an Sn2 reaction: – Not Sn1: Aryl cations are unstable for dissociation to occur – Not Sn2: Backside displacement is sterically blocked Electrophilic vs Nucleophilic Aromatic Substitution • Electrophilic Aromatic Substitution • Nucleophilic Aromatic Substitution – is favored by electrondonating groups – is favored by electronwithdrawing groups – involves a carbocation intermediate – involves a carbanion intermediate – replaces a H with an electrophile – replaces a leaving group with a nucleophile • Electron-donating groups • Electron-withdrawing groups – favor electrophilic aromatic substitution – favor nucleophilic aromatic substitution – stabilize carbocation intermediate – stabilize carbanion intermediate – are ortho-para directors in electrophilic reaction – are ortho-para directors in nucleophilic reaction but meta-directors in electrophilic substitution Practice Problem: Propose a mechanism for the reaction of 1chloroanthraquinone with methoxide ion to give the substitution product 1-methoxyanthraquinone. Use curved arrows to show the electron flow in each step. 9. Benzyne • Aryl halides without electron-withdrawing substituents undergo substitution with a benzyne intermediate – Phenol is prepared on an industrial scale by treatment of chlorobenzene with dilute aqueous NaOH at 340°C under high pressure • The synthesis of phenol occurs in two steps by the elimination/addition mechanism rather than addition/elimination: – Step 1: Elimination of a HX from halobenzene in an E2 reaction catalyzed by a strong base, forming a highly reactive benzyne intermediate – Step 2: Addition of a nucleophile (Nu-) to the benzyne intemediate Evidence for Benzyne as an Intermediate • Bromobenzene with 14C only at C1 gives substitution product with label scrambled between C1 and C2 – The reaction proceeds through a symmetrical intermediate in which C1 and C2 are equivalent – The intermediate must be benzyne • Trapping experiments further demonstrate that benzyne was the intermediate – Benzyne is too reactive to be isolated and thus can be intercepted in a Diels-Alder reaction Structure of Benzyne • Benzyne is a highly distorted alkyne – The triple bond uses sp2-hybridized carbons, not the usual sp – The triple bond has one bond formed by p–p overlap and one bond formed by weak sp2–sp2 overlap Practice Problem: Treatment of p-bromotoluene with NaOH at 300oC yields a mixture of two products, but treatment of m-bromotoluene with NaOH yields a mixture of three products. Explain 10. Oxidation of Aromatic Compounds There are two reactions of alkylbenzene side chains: • Oxidation of Alkylbenzene Side Chains • Bromination of Alkylbenzene Side Chains Aromatic ring activates neighboring benzylic (C-H) position toward oxidation Oxidation of Alkylbenzene Side Chains • Alkyl side chains can be oxidized to carboxyl groups, -CO2H, by strong oxidizing agents such as KMnO4 and Na2Cr2O7 – The alkyl side chains must have a C-H next to the ring – This converts an alkylbenzene into a benzoic acid, Ar-R Ar-CO2H • The mechanism of side-chain oxidation involves reaction of C-H next to the ring to form intermediate benzylic radicals – t-butylbenzene is inert (no benzylic H’s) Practice Problem: What aromatic products would you obtain from the KMnO4 oxidation of the following substances? Bromination of Alkylbenzene Side Chains • Reaction of an alkylbenzene with N-bromo-succinimide (NBS) and benzoyl peroxide (radical initiator) introduces Br into the side chain – Bromination occurs exclusively in the benzylic position Mechanism of NBS (Radical) Reaction • Abstraction of a benzylic hydrogen atom generates an intermediate benzylic radical – – – This reacts with Br2 to yield product and Br· Br· radical cycles back into reaction to carry on chain Br2 is produced from reaction of HBr with NBS • Bromination occurs exclusively in the benzylic position because the benzylic radical intermediate is resonance-stabilized – The benzylic radical is stabilized by overlap of its p orbital with the ring p electron system Practice Problem: Refer to Table 5.3 for a quantitative idea of the stability of a benzyl radical. How much stable (in kJ/mol) is the benzyl radical than a primary alkyl radical? How does a benzyl radical compare in stability to an allyl radical Practice Problem: Styrene, the simplest alkenylbenzene, is prepared commercially for use in plastics manufacture by catalytic dehydrogenation of ethylbenzene. How might you prepare styrene from benzene? 11. Reduction of Aromatic Compounds There are two reduction reactions: • Catalytic hydrogenation of Aromatic Rings • Reduction of Aryl Alkyl Ketones Catalytic hydrogenation of Aromatic Rings • Reduction of an aromatic ring requires more powerful reducing conditions (H2/Pt at high pressure or rhodium catalysts) • Aromatic rings are inert to catalytic hydrogenation under conditions that reduce alkene double bonds – It is possible to selectively reduce an alkene double bond in the presence of an aromatic ring Reduction of Aryl Alkyl Ketones • Aromatic ring activates neighboring carbonyl group toward reduction • Aryl alkyl ketone is converted into an alkylbenzene by catalytic hydrogenation over Pd catalyst • Conversion of a carbonyl group to a methylene group by catalytic hydrogenation (C=O CH2) – is limited to aryl alkyl ketones – is not compatible with the presence of a nitro group Practice Problem: Show how you would prepare diphenylmethane (Ph)2CH2, from benzene and an appropriate acid chloride 12. Synthesis of Trisubstituted Benzenes • These syntheses require planning and consideration of alternative routes 1. Compare the target and the starting material 1. Consider reactions that efficiently produce the outcome. 1. Look at the product and think of what can lead to it • A synthesis combines a series of proposed steps to go from a defined set of reactants to a specified product Synthesis as a Tool for Learning Organic Chemistry • In order to propose a synthesis, one must be familiar with reactions: – – – – What they begin with What they lead to How they are accomplished What the limitations are • The order in which reactions are carried is critical in the synthesis of substituted aromatic rings – The introduction of a new substituent is strongly affected by the directing effects of other substituents Practice Problem: Synthesize p-bromobenzoic acid from benzene Br – Bromination using Br2/FeBr3 CO2H – Friedel-Crafts alkylation or acylation followed by oxidation Practice Problem: Propose a synthesis of 4-chloro-1-nitro-2propylbenzene from benzene Cl – Chlorination using Cl2/FeCl3 NO2 – Nitration using HNO3/H2SO4 CH2CH2CH3 – Friedel-Crafts acylation followed by reduction Practice Problem: Propose syntheses of the following substances from benzene: a. m-Chloronitrobenzene b. m-Chloroethylbenzene c. p-Chloropropylbenzene Practice Problem: In planning a synthesis, it is important to know what NOT to do as to know what do. As written, the following reaction schemes have flaws in them. What is wrong with each? Chapter 16