* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Senna (Cassia angustifolia)
Survey
Document related concepts
Transcript
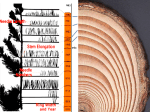
The wonderful world of polyketides This one helps you “go” From Senna leaf and fruit, Cassia angustifolia (Leguminosae/Fabaceae). Senna is a stimulant laxative that acts on the wall of the large intestine, increasing peristaltic movement. Ansamycin antibiotics are produced by cultures of Amycolatopsis mediterranei Rifamycin SV (below ) is an anti-tuberculosis drug that works against Gram-positive bacteria by inhibiting bacterial RNA synthesis - it binds to DNA-dependent bacterial RNA polymerase. Starter unit acetyl CoA + Organisms that produce polyketides make highly reactive poly-β-keto chains, which must be stabilized by association with groups on the enzyme surface until chain assembly is complete and then cyclizations occur. Polyketide formation: folding is the key to ring formation: 1. Aldol or Claisen reaction forms the ring 2. Enolization produces phenol a metabolite from the mold Alternaria tenuis Multiple aromatic rings can result from longer poly-b-keto chains formation of a depside occurs through esterification of two phenolic acid units A depside from cranberries Turner, et al, 2007 Formation of anthrones and anthraquinones: 1. chain folds in half 2. aldol condensation, dehydration, enolization sequence forms the rings 3. oxidation of hydroxyl groups occurs after “Emetics” or laxatives found in Senna (Cassia angustifolia), Cascara (Rhamnus purshianus) “Sennosides” have sugars attached to the ring skeleton Assembly of hypericin (St. John’s Wort – Hypericum perforatum) St. John’s Wort Hypericum perforatum Senna (Cassia angustifolia) Some common themes in polyketides Alkylations occur adjacent to oxygenated C and prior to cyclization by aromatization Radical coupling often joins phenolic rings. Ex: griseofulvin, an antifungal Produced by cultures of Penicillium griseofulvum and is effective for difficult skin infections. It’s absorbed from the gut and concentrated in keratin, so it is used orally to control dermatophytes. Works by disruption of the mitotic spindle, inhibiting fungal mitosis. Oxidative cleavage, lactone and acetal formation results in major skeletal modifications Patulin is a potent carcinogen produced by Penicillium patulum, a common contaminant on apples. Products made from contaminated fruit may contain dangerous levels of patulin, so juices, etc. are routinely screened for patulin content (max of 50 μg kg−1). Common reactions in polyketide biosynthesis • • • • Aldol/Claisen condensations cyclization Enolization aromatic ring Radical coupling join two pieces together Aromatic cleavage open ring & allow for twisting & reconfiguration of C skeleton • Lactone or acetal formation produces a new ring Poison Ivy / Oak (Toxicodendron radicans or Rhus radicans; T. toxicaria) contains urushiols – part fatty acid, part polyketide Family: Anacardiaceae Phenol gets oxidized to a quinone, which is the active form, produces allergic rxn. The origins of aflatoxins (Aspergillus flavus & Aspergillus parasiticus) Aflatoxin – producing fungi grow mainly on peanuts, corn, rice, pistachio nuts Targets the liver, which becomes enlarged due to fat deposition, cell necrosis, etc. Recent dog food contamination resulted in several pet deaths Aflatoxin B1 is carcinogenic - after epoxidization by CYP450 it can intercalate DNA, causing mutations. Cannabis sativa Cannabaceae THC (tetrahydrocannibinol) works by mimicking the natural molecules that bind to endogenous cannabinoid receptors (analgesia): CB1 in the brain (mood, memory motor control, pain, appetite) CB2 in immune/reproductive system cells alpha-linolenic acid (20%–25%) Ether linkage necessary heat, light inactive psychoactive aromatic ring dec. activity inactive Structure-activity studies of natural products: Many plants produce a range of compounds having similar structures with a few modifications in substituents, side chains, sugars attached. Comparison of the relative bioactivities of these molecules allows determination of which structural features are necessary for activity Important for turning drug leads into synthetic derivatives with increased activity Two origins of methyl groups on polyketide skeletons Methylation using SAM is more common in fungi Actinomycetes (e.g. Streptomyces) are filamentous bacteria that gain methyls by incorporation of propionate via methylmalonyl-CoA as a building block. There are a wide variety of macrolides produced by Streptomyces spp., most of which have some antibiotic activity. Many are readily identified by names ending in “mycin”. Note: Streptococcus are a separate genus Tetracyclines: produced from polyketide chain through series of aldol condensations, enolizations, and oxidations, similar to biosynthesis of anthrones. They have broad-spectrum activity against a variety of Gram-positive and Gram-negative bacteria. produced by Streptomyces Bacterial resistance to tetracyclines has developed in pathogens such as Pneumococcus, Staphylococcus, Streptococcus, and E.coli. Mechanism of resistance: decreased cell permeability, and membrane-embedded transport proteins that export the tetracycline out of the cell before it can exert its effect. Still effective for infections caused by Chlamydia, Mycoplasma, Brucella, Rickettsia, and chronic bronchitis due to Haemophilus influenzae. produced by fungi Gibberella, Fusarium spp. that cause fruit & root rot Macrolide ring formation and modification: The macrolide antibiotics are a large family of compounds, many with antibiotic activity, - characterized by a macrocyclic lactone ring (12, 14, or 16 membered) - the poly-b-keto chain undergoes reductions, dehydrations, during chain extension, so the molecule will not undergo cyclizations to fully aromatic structure. Macrolide antibiotics Erythromycins (Saccharopolyspora erythreus) target Gram-positive bacteria such as MRSA (methicillinresistant Staph. aureus) from Streptomyces antibioticus Mode of action: “hijacks” replication by binding to 50S subunit of bacterial ribosome, blocking translocation of growing peptidyl RNA Several different linkers are used in building polyketide chain Spiramycins: Produced by Streptomyces ambofaciens Used to treat toxoplasmosis, a feline disease caused by protozoan Toxoplasma gondii Larger polyene antifungals Amphotericin is commonly used to treat Candida infections (with azoles) Works by binding to ergosterol, a sterol in the fungal membrane Disrupts membrane causing pores to form, cells leak vital nutrients & electrolytes Used to treat yeasts & Cryptococcus, and also to reduce mold growth on surfaces Compounds were isolated from a MeOH/CH2Cl2 extract of the sponge (3 kg) and found to kill HCT-116 colon cancer cells at sub uM concentrations. IC50 = 8 ng/mL for (2) Hurgholide A inhibited Candida at 31 ug/mL How do they work? These macrolides kill cells by disrupting the actin cytoskeleton. One dimeric molecule binds simultaneously to two molecules of G-actin, forming a tertiary complex and inhibiting polymerization. The Swinholides also cause the filamentous actin strands to break. All caused loss of cellular microfilaments at nM concentrations. Cells treated with swinholide I collapsed and formed neuron-like structures. Compounds 2 & 3 were isolated after several rounds of column chromatography in mg quantities. Many marine toxins are complex polyethers with origins in the acetate pathway The brevetoxins are potent neurotoxins that bind to sodium channels , keeping them open. Brevenal (below) is a functional antagonist to brevetoxin, inhibiting its activity in all assays. Brevenal reduces brevetoxin binding to rat brain synaptosomes, blocks brevetoxin-induced bronchoconstriction in sheep, and reduces brevetoxin toxicity in fish. Biosynthetic studies have shown that fragments from the citric acid cycle and a 4C starter unit from mevalonate are involved in generating C skeleton. Some of the methyls originate from methionine. Karenia brevis is a marine dinoflagellate known for production of several different families of bioactive ladder-frame polyether compounds. They contain similar trans-fused, ladder-shaped, cyclic ether ring systems; with ring sizes (5 to 9membered rings), number of rings (4, 5, 6, 10, and 11), and side chains vary among the different families. Algal blooms by this organism cause red tide in Florida. Red tide off the coast of San Diego, caused by the dinoflagellate Lingulodinium polyedrum Tamulamides A and B bind to the same sites as the toxins but do not cause toxic effects at nM concentrations.