* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Aromatic Compounds
Marcus theory wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Hydroformylation wikipedia , lookup
George S. Hammond wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
Petasis reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Macrocyclic stereocontrol wikipedia , lookup
Homoaromaticity wikipedia , lookup
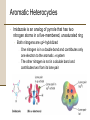
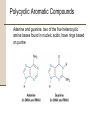
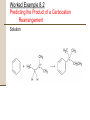
Chapter 8 Aromatic Compounds © 2006 Thomson Higher Education Early Days of Organic Chemistry Aromatic Compounds • Formerly used to describe fragrant substances such as benzaldehyde (from cherries, peaches, and almonds), toluene (from Tolu balsam), and benzene (from coal distillate) • Now used to refer to the class of compounds that contain six-membered benzene-like rings with three double bonds Present Days of Organic Chemistry Aromatic Compounds • Many naturally occurring compounds are aromatic in part • Steroidal hormone estrone • • • Analgesic morphine Many synthetic drugs are aromatic in part • Antianxiety agent diazepem (Valium) Benzene • Found to cause bone marrow depression • Leads to leukopenia, or lowered white blood cell count, on prolonged exposure 8.1 Naming Aromatic Compounds Aromatic substances have acquired nonsystematic names • Nonsystematic names are discouraged but allowed by IUPAC • • • Common name for methylbenzene is toluene Common name for hydroxybenzene is phenol Common name for aminobenzene is aniline Naming Aromatic Compounds Monosubstituted Benzenes • Systematically named in same manner as other hydrocarbons • – Benzene used as parent name • • • C6H5Br is bromobenzene C6H5NO2 is nitrobenzene C6H5CH2CH2CH3 is propylbenzene Naming Aromatic Compounds Arenes • Alkyl-substituted benzenes • Named depending on the size of the alkyl group • Alkyl substituent smaller than the ring (6 or fewer carbons), named as an alkyl substituted benzene • Alkyl substituent larger than the ring (7 or more carbons), named as a phenyl-substituted alkane Phenyl • Derived from the Greek pheno (“I bear light”) • Michael Faraday discovered benzene in 1825 from the oily residue left by illuminating gas used in London street lamps • Used for the –C6H5 unit when the benzene ring is considered as a substituent Naming Aromatic Compounds Benzyl • Used for the C6H5CH2– group Naming Aromatic Compounds Disubstituted benzenes • Named using one of the prefixes 1. ortho- (o-) • 2. meta- (m-) • 3. Ortho-disubstituted benzene has two substituents in a 1,2 relationship Meta-disubstituted benzene has its substituents in a 1,3 relationship para- (p-) • Para-disubstituted benzene has its substituents in a 1,4 relationship Naming Aromatic Compounds Benzenes with more than two substituents • Named by numbering the position of each so that the lowest possible numbers are used • The substituents are listed alphabetically when writing the name Any of the monosubstituted aromatic compounds in Table 8.1 can serve as a parent name, with the principal substituent (-OH in phenol or –CH3 in toluene) attached to C1 on the ring 8.2 Structure and Stability of Benzene Benzene • Benzene is unsaturated • Benzene is much less reactive than typical alkene and fails to undergo the usual alkene reactions • • Cyclohexene reacts rapidly with Br2 and gives the addition product 1,2-dibromocyclohexane Benzene reacts only slowly with Br2 and gives the substitution product C6H5Br Structure and Stability of Benzene A quantitative idea of benzene’s stability is obtained from heats of hydrogenation • Benzene is 150 kJ/mol (36 kcal/mol) more stable than might be expected for “cyclohexatriene” Structure and Stability of Benzene Carbon-carbon bond lengths and angles in benzene • • All carbon-carbon bonds are 139 pm in length • Intermediate between typical C-C single bond (154 pm) and typical double bond (134 pm) Benzene is planar • All C-C-C bond angles are 120° • All six carbon atoms are sp2-hybridized with p orbital perpendicular to the plane of the ring Structure and Stability of Benzene All six carbon atoms and all six p orbitals in benzene are equivalent • Each p orbital overlaps equally well with both neighboring p orbitals, leading to a picture of benzene in which the six p electrons are completely delocalized around the ring • Benzene is a hybrid of two equivalent forms • • Neither form is correct by itself The true structure of benzene is somewhere in between the two resonance forms Structure and Stability of Benzene If six p atomic orbitals combine in a cyclic manner, six benzene p molecular orbitals result • The three lower-energy molecular orbitals, denoted y1, y2, and y3, are bonding combinations • y2 and y3 have the same energy and are said to be • degenerate The three higher-energy molecular orbitals, denoted y4*, y5*, and y6*, are antibonding combinations • y4* and y5* have the same energy and are said to be degenerate Structure and Stability of Benzene • y3 and y4* have nodes passing through ring carbon atoms, thereby no p electron density on these carbons • The six p electrons occupy the three bonding molecular orbitals and are delocalized over the entire conjugated system 8.3 Aromaticity and the Hückel 4n + 2 Rule Benzene and other benzene-like aromatic molecules share similar characteristics: • Benzene is cyclic and conjugated • Benzene is unusually stable, it is 150 kJ/mol (36 kcal/mol) more stable than might be expected • Benzene is planar and has the shape of a regular hexagon. All bond angles are 120º, all carbon atoms are sp2-hybridized, and all carbon-carbon bond lengths are 139 pm • Benzene undergoes substitution reactions that retain the cyclic conjugation rather than electrophilic addition reactions that would destroy the conjugation • Benzene is a resonance hybrid whose structure is intermediate between two line-bond structures Aromaticity and the Hückel 4n + 2 Rule The Hückel 4n + 2 rule • Theory devised in 1931 by the German physicist Erich Hückel • • • A molecule is aromatic only if it has a planar, monocyclic system of conjugation and contains a total of 4n + 2 p electrons, where n is an integer (n = 0, 1, 2, 3,…) Only molecules with 2, 6, 10, 14, 18,… p electrons can be aromatic Molecules with 4n p electrons (4, 8, 12, 16,…) can not be aromatic, said to be antiaromatic because delocalization of their p electrons would lead to their destablization Aromaticity and the Hückel 4n + 2 Rule Examples of the Hückel 4n + 2 rule • Cyclobutadiene • Contains four p electrons • The p electrons are localized into two double bonds rather than delocalized around the ring • • • • Antiaromatic Highly reactive Shows none of the properties associated with aromaticity Not prepared until 1965 Aromaticity and the Hückel 4n + 2 Rule • Benzene • Contains six p electrons (4n + 2 = 6 when n = 1) • Aromatic Aromaticity and the Hückel 4n + 2 Rule • Cyclooctatetraene • Contains eight p electrons • The p electrons are • • • • localized onto four double bonds rather than delocalized around the ring Not aromatic The molecule is tub-shaped rather than planar It has no cyclic conjugation because neighboring p orbitals do not have the necessary parallel alignment for overlap Resembles an open-chain polyene in its reactivity Aromaticity and the Hückel 4n + 2 Rule Energy Levels of Cyclic Conjugated Molecules (4n + 2 Electrons) • • • • • • There is always a single lowest-lying MO, above which the MOs come in degenerate pairs When electrons fill the various molecular orbitals, one pair of electrons fills the lowest-lying orbital and two pairs of electrons fill each of the n successive energy levels – a total of 4n + 2. Any other number would leave a bonding energy level partially unfilled Energy levels of the six benzene p molecular orbitals The lowest-energy MO, y1, occurs single and contains a pair of electrons y2 and y3, are degenerate, and it takes two pairs of electrons to fill them The result is a stable six-pelectron aromatic molecule with filled bonding orbitals 8.4 Aromatic Heterocycles Heterocyclic compounds can also be aromatic Heterocycle • A cyclic compound that contains atoms of two or more different elements in its ring, usually carbon along with nitrogen, oxygen, or sulfur • Pyridine is much like benzene in its p electron structure • A six-membered heterocycle with nitrogen in its ring • Each of the five sp2-hybridized carbons has a p orbital perpendicular to the plane of the ring and each p orbital contains one p electron Aromatic Heterocycles • The nitrogen atom is also sp2-hybridized and has one electron in a p orbital, bringing the total to six p electrons • The nitrogen lone pair electrons are in an sp2 orbital in the plane of the ring and are not involved with the aromatic p system Aromatic Heterocycles • Pyrimidine is much like benzene in its p electron structure • Has two nitrogen atoms in a six-membered, unsaturated ring 2 • Both nitrogens are sp -hybridized, and each contributes one electron to the aromatic p system Aromatic Heterocycles • Pyrrole is a five membered heterocycle with six p electrons • • • Aromatic Each of the sp2-hybridized carbons contributes one p electron The sp2-hybridized nitrogen atom contributes the two electrons from its lone pair, which occupies a p orbital Aromatic Heterocycles • Imidazole is an analog of pyrrole that has two nitrogen atoms in a five-membered, unsaturated ring • Both nitrogens are sp2-hybridized • One nitrogen is in a double bond and contributes only one electron to the aromatic p system • The other nitrogen is not in a double bond and contributes two from its lone pair Aromatic Heterocycles Nitrogen atoms have different roles depending on the structure of the molecule • In pyridine and pyrimidine, the nitrogen atoms are both in double bonds and contribute only one p electron to the aromatic sextet, like a carbon atom in benzene does • In pyrrole, the nitrogen atom is not in a double bond and contributes two p electrons (the lone pair) to the aromatic sextet • In imidazole, both a double-bonded “pyridine-like” nitrogen that contributes one p electron and a “pyrrole-like” nitrogen that contributes two p electrons are present in the same molecule Aromatic Heterocycles Pyrimidine and imidazole rings are important in biological chemistry • Pyrimidine is the parent ring system in cytosine, thymine, and uracil, three of the five heterocycle amine bases found in nucleic acids • An aromatic imidazole ring is present in histidine, one of the twenty amino acids found in proteins Worked Example 8.1 Accounting for the Aromaticity of a Heterocycle Thiophene, a sulfur-containing heterocycle, undergoes typical aromatic substitution reaction rather than addition reactions. Why is thiophene aromatic? Worked Example 8.1 Accounting for the Aromaticity of a Heterocycle Strategy • Recall the requirements for aromaticity • A planar, cyclic, conjugated molecule with 4n + 2 p electrons • See how requirements for aromaticity apply to thiophene Worked Example 8.1 Accounting for the Aromaticity of a Heterocycle Solution • Thiophene is the sulfur analog of pyrrole • The sulfur atom is sp2-hybridized and has a lone pair of electrons in a p orbital perpendicular to the plane of the ring • Sulfur also has a second lone pair of electrons in the ring plane 8.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds • Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke Polycyclic Aromatic Compounds All polycyclic aromatic hydrocarbons can be represented by a number of different resonance forms • Naphthalene has three • Naphthalene shows many of the chemical properties associated with aromaticity • Heat of hydrogenation measurements show an aromatic stablilization energy of approximately 250 kJ/mol (60 kcal/mol) • Naphthalene reacts slowly with electrophiles to give substitution products rather than double-bond addition products Polycyclic Aromatic Compounds Aromaticity of Naphthalene Naphthalene has a cyclic, conjugated p electron system, with p orbital overlap both around the ten-carbon periphery of the molecule and across the central bond • 10 is a Hückel number (4n + 2 when n = 2) so there is p electron delocalization and consequent aromaticity in naphthalene • Polycyclic Aromatic Compounds There are many heterocyclic analogs of naphthalene • Quinoline, isoquinoline, indole, and purine • Quinoline, isoquinoline, and purine all contain pyridinelike nitrogens that are part of a double bond and contribute one electron to the aromatic p system • Indole and purine contain pyrrole-like nitrogens that contribute two p electrons Polycyclic Aromatic Compounds Biological molecules that contain polycyclic aromatic rings • The amino acid tryptophan contains an indole ring and the anti-malarial drug quinine contains a quinoline ring Polycyclic Aromatic Compounds • Adenine and guanine, two of the five heterocyclic amine bases found in nucleic acids, have rings based on purine 8.6 Reactions of Aromatic Compounds: Electrophilic Substitution Electrophilic aromatic substitution • A process in which an electrophile (E+) reacts with an aromatic ring and substitutes for one of the hydrogens • The most common reaction of aromatic compounds • This reaction is characteristic of all aromatic rings • The ability of a compound to undergo electrophilic substitution is a good test of aromaticity Reactions of Aromatic Compounds: Electrophilic Substitution • Many substituents can be introduced onto an aromatic ring through electrophilic substitution reactions • • • • • • Halogen (-Cl, -Br, -I) Nitro group (-NO2) Sulfonic acid group (-SO3H) Hydroxyl group (-OH) Alkyl group (-R) Acyl group (-COR) Reactions of Aromatic Compounds: Electrophilic Substitution Electrophilic alkene addition • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons • of the double bond and forms a bond to one carbon, leaving a positive charge at the other carbon The carbocation intermediate then reacts with the nucleophilic Cl- ion to yield the addition product Reactions of Aromatic Compounds: Electrophilic Substitution One difference between electrophilic aromatic substitution reactions and electrophilic alkene addition reactions is that aromatic rings are less reactive toward electrophiles than alkenes are • Br2 in CH2Cl2 solution reacts instantly with most alkenes but does not react with benzene at room temperature Reactions of Aromatic Compounds: Electrophilic Substitution Electrophilic aromatic substitution reaction begins in a similar way to electrophilic alkene addition reaction • FeBr3 catalyst is needed for bromination of benzene to occur • • FeBr3 polarizes Br2 molecule making it more electrophilic Polarization makes FeBr4-Br+ species that reacts as if it were Br+ • The polarized Br2 molecule reacts with the nucleophilic benzene ring to yield a nonaromatic carbocation intermediate which is doubly allylic and has three resonance forms Reactions of Aromatic Compounds: Electrophilic Substitution The intermediate carbocation in electrophilic aromatic substitution is more stable than a typical alkyl carbocation because of resonance but much less stable than the starting benzene ring Comparison of alkene addition and aromatic substitution • Instead of adding Br- to give an addition product, the carbocation intermediate loses H+ from the bromine-bearing carbon • • • • If addition occurred, the 150 kJ/mol stabilization energy of the aromatic ring would be lost and the overall reaction would be endergonic When substitution occurs, the stability of the aromatic ring is retained and the reaction is exergonic Loss of H+ restores aromaticity to ring The net effect is the substitution of H+ by Br+ Reactions of Aromatic Compounds: Electrophilic Substitution The mechanism of the electrophilic bromination of benzene • The reaction occurs in two steps and involves a resonance-stabilized carbocation intermediate Reactions of Aromatic Compounds: Electrophilic Substitution Aromatic Halogenation • Electrophilic substitution reactions can introduce halogens into aromatic rings • Aromatic rings react with Cl2 in the presence of FeCl3 catalyst to yield chlorobenzenes • • Reaction mechanism just like Br2 in the presence of FeBr3 Reaction used in the synthesis of numerous pharmaceutical agents such as the antianxiety agent diazepam (Valium) Reactions of Aromatic Compounds: Electrophilic Substitution Fluorine is too reactive to give mono-fluorinated products Iodine itself is unreactive toward aromatic rings • An oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to a more powerful electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the I+ to yield a substitution product Reactions of Aromatic Compounds: Electrophilic Substitution Electrophilic aromatic halogenations occur in the biosynthesis of numerous naturally occurring molecules, particularly those produced by marine organisms • Thyroxine, synthesized in the thyroid gland in humans, is a thyroid hormone involved in regulating growth and metabolism Reactions of Aromatic Compounds: Electrophilic Substitution Aromatic Nitration • Aromatic rings can be nitrated with a mixture of concentrated nitric and sulfuric acids • The electrophile is the nitronium ion, NO2+ which is generated from HNO3 by protonation and loss of water • • The nitronium ion reacts with benzene to yield a carbocation intermediate, and loss of H+ The product is a neutral substitution product, nitrobenzene Reactions of Aromatic Compounds: Electrophilic Substitution Aromatic nitration • Does not occur naturally • Important in the laboratory • The nitro-substituted product can be reduced by reagents such as iron or tin metal or to yield an arylamine, ArNH2 • Attachment of an amino group to an aromatic ring by the two-step nitration-reduction sequence is a key part of the industrial synthesis of many dyes and pharmaceutical agents Reactions of Aromatic Compounds: Electrophilic Substitution Aromatic Sulfonation • Aromatic rings can be sulfonated in the laboratory by reaction with fuming sulfuric acid, a mixture of H2SO4 and SO3 • The reactive electrophile is either HSO3+ or neutral SO3 • Substitution occurs by the same two-step mechanism seen for bromination and nitration • Aromatic sulfonation does not occur naturally • Aromatic sulfonation is widely used in the preparation of dyes and pharmaceutical agents • The sulfa drugs, such as sulfanilamide, were among the first clinically useful antibiotics Reactions of Aromatic Compounds: Electrophilic Substitution • The mechanism of electrophilic sulfonation of an aromatic ring Reactions of Aromatic Compounds: Electrophilic Substitution Aromatic Hydroxylation • Direct hydroxylation of an aromatic ring to yield a hydroxybenzene (a phenol) • • Difficult and rarely done in the laboratory Occurs much more freely in biological pathways • Hydroxylation of p-hydroxyphenyl acetate to give 3,4dihydroxyphenyl acetate • The reaction is catalyzed by p-hydroxyphenylacetate-3hydroxylase and requires molecular O2 plus the coenzyme reduced flavin adenine dinucleotide (FADH2) Reactions of Aromatic Compounds: Electrophilic Substitution • Mechanism of the electrophilic hydroxylation of phydroxyphenyl acetate by reaction of FAD hydroperoxide • The hydroxylating species is an OH+ equivalent that arises by protonation of FAD hydroperoxide, RO-OH + H+ ROH 8.7 Alkylation and Acylation of Aromatic Rings Alkylation • The introduction of an alkyl group onto the benzene ring • Called the Friedel-Crafts reaction after its discoverers • Among the most useful electrophilic aromatic substitution reactions in the laboratory • The reaction is carried out by treating the aromatic compound with an alkyl chloride, RCl, in the presence of AlCl3 to generate a carbocation electrophile, R+ • • Aluminum chloride catalyzes the reaction by helping the alkyl halide to dissociate Loss of H+ completes the reaction Alkylation and Acylation of Aromatic Rings Mechanism of the Friedel-Crafts alkylation reaction • The electrophile is a carbocation, generated by AlCl3-assisted dissociation of an alkyl halide Alkylation and Acylation of Aromatic Rings Friedel-Crafts alkylation has several limitations 1. Only alkyl halides can be used • Aromatic (aryl) halides and vinylic halides do not react because aryl and vinylic carbocations are too high in energy to form under Friedel-Crafts conditions • Vinylic means that a substituent is attached directly to a double bond, C=C-Cl Alkylation and Acylation of Aromatic Rings 2. Friedel-Crafts reactions do not succeed on aromatic rings that are substituted either by a strongly electron-withdrawing group such as carbonyl (C=O) or by an amino group (-NH2, NHR, -NR2) • The presence of a substituent group already on a ring can have a dramatic effect on that ring’s subsequent reactivity toward further electrophilic substitution Alkylation and Acylation of Aromatic Rings • • A skeletal rearrangement of the alkyl carbocation electrophile sometimes occurs during a Friedel-Crafts reaction, particularly when a primary alkyl halide is used Carbocation rearrangements occur either by hydride shift or alkyl shift • Alkylation of benzene with 1-chloro-2,2dimethylpropane yields (1,1dimethylpropyl)benzene Alkylation and Acylation of Aromatic Rings An aromatic ring is acylated by reaction with a carboxylic acid chloride, RCOCl, in the presence of AlCl3 • An acyl group is substituted onto an aromatic ring • • The reactive electrophile is a resonance-stabilized acyl cation • An acyl cation is stabilized by interaction of the vacant orbital on carbon with lone-pair electrons on the neighboring oxygen Because of stabilization, no carbocation rearrangement occurs during acylation Alkylation and Acylation of Aromatic Rings Aromatic alkylations occur in numerous biological pathways • The carbocation electrophile is typically formed by dissociation of an organo diphosphate • A diphosphate group is a common structural feature of many biological molecules It can be expelled as a stable diphosphate ion The dissociation of an organo diphosphate in a biological reaction is typically assisted by complexation to a divalent metal cation such as Mg2+ to help neutralize charge • • Alkylation and Acylation of Aromatic Rings Biosynthesis of phylloquinone, or vitamin K1, the human bloodclotting factor • The key step that joins the 20-carbon phytyl side chain to the aromatic ring is an electrophilic substitution reaction Worked Example 8.2 Predicting the Product of a Carbocation Rearrangement The Friedel-Crafts reaction of benzene with 2chloro-3-methylbutane in the presence of AlCl3 occurs with a carbocation rearrangement. What is the structure of the product? Worked Example 8.2 Predicting the Product of a Carbocation Rearrangement Strategy • A Friedel-Crafts reaction involves initial formation of a carbocation, which can rearrange by either a hydride shift or an alkyl shift to give a more stable carbocation • Draw the initial carbocation, assess its stability, and see if the shift of a hydride ion or an alkyl group from a neighboring carbon will result in an increased stability • • The initial carbocation is a secondary one that can rearrange to a more stable tertiary one by a hydride shift Use the more stable tertiary carbocation to complete the Friedel-Crafts reaction Worked Example 8.2 Predicting the Product of a Carbocation Rearrangement Solution 8.8 Substituent Effects in Electrophilic Substitutions Substituent effects in the electrophilic substitution of an aromatic ring • Substituents affect the reactivity of the aromatic ring • • • Some substituents activate the ring, making it more reactive than benzene Some substituents deactivate the ring, making it less reactive than benzene Relative rates of aromatic nitration Substituent Effects in Electrophilic Substitutions • Substituents affect the orientation of the reaction • • The three possible disubstituted products – ortho, meta, and para – are usually not formed in equal amounts The nature of the substituent on the ring determines the position of the second substitution Substituent Effects in Electrophilic Substitutions Substituents can be classified into three groups • • • ortho- and para-directing activators ortho- and para-directing deactivators meta-directing deactivators • There are no meta-directing activators All activating groups are ortho- and para- directing All deactivating groups other than halogen are meta-directing The halogens are unique in that they are deactivating but orthoand para-directing Substituent Effects in Electrophilic Substitutions Activating and Deactivating Effects • The common characteristic of all activating groups is that they donate electrons to the ring • • • Makes the ring more electron-rich Stabilize the carbocation intermediate Lower activation energy • The common characteristic of all deactivating groups is that they withdraw electrons from the ring • • • Makes the ring more electron-poor Destabilizes the carbocation intermediate Raising the activation energy for its formation Substituent Effects in Electrophilic Substitutions Electrostatic potential maps of benzene, phenol (activated), chlorobenzene (weakly deactivated), and benzaldehyde (more strongly deactivated) • The –OH substituent makes the ring more negative (red) • The –Cl makes the ring less negative (orange) • The –CHO makes the ring still less negative (yellow) Substituent Effects in Electrophilic Substitutions The electron donation or electron withdrawal may occur by either an inductive effect or a resonance effect • Inductive effect • • Due to an electronegativity difference between the ring and the attached substituent Resonance effect • Due to overlap between a p orbital on the ring and an orbital on the substituent Substituent Effects in Electrophilic Substitutions Orienting Effects: Ortho and Para Directors • The ortho and para intermediates are more stable than the meta intermediate because they have more resonance forms Substituent Effects in Electrophilic Substitutions Any substituent that has a lone pair of electrons on the atom directly bonded to the aromatic ring allows an electrondonating resonance interaction to occur • • Additional electron-donating resonance interaction lowers the energy of the hybrid Electron-donating substituent increases electrophilicity of ortho and para positions and acts as an ortho and para director Substituent Effects in Electrophilic Substitutions Orienting Effects: Meta Directors Substituent Effects in Electrophilic Substitutions Any substituent that has a positively polarized atom (d+) directly attached to the ring increases the activation energy leading to the intermediate hybrid for ortho and para substitutions • • • Substitution at ortho or para position gives a higher energy intermediate Meta substitution avoids higher energy intermediate and has a lower activation energy Approaching electrophile is directed to the meta positions Substituent Effects in Electrophilic Substitutions A Summary of Substituent Effects in Electrophilic Substitutions Worked Example 8.3 Predicting the Product of an Electrophilic Aromatic Substitution Reaction Predict the major product of the sulfonation of toluene. Worked Example 8.3 Predicting the Product of an Electrophilic Aromatic Substitution Reaction Strategy • Identify the substituent present on the ring • Decide whether the substituent is ortho- and para-directing or meta-directing • According to Figure 8.15 an alkyl substituent is ortho- and para-directing • Sulfonation of toluene will give primarily a mixture of o-toluenesulfonic acid and p-toluenesulfonic acid Worked Example 8.3 Predicting the Product of an Electrophilic Aromatic Substitution Reaction Solution 8.9 Oxidation and Reduction of Aromatic Compounds Alkyl substituents on aromatic rings containing a benzylic hydrogen react readily with common laboratory oxidizing agents such as aqueous KMnO4 or Na2Cr2O7 and are converted into carboxyl groups • Net conversion of an alkylbenzene into a benzoic acid Ar-R • Ar-CO2H Oxidation of butylbenzene into benzoic acid Oxidation and Reduction of Aromatic Compounds • The mechanism of side-chain oxidation involves reaction of a C-H bond at the position next to the aromatic ring (the benzylic position) to form an intermediate benzylic radical • Benzylic radicals are stabilized by resonance and thus form more readily than typical alkyl radicals Oxidation and Reduction of Aromatic Compounds Side chain oxidations occur in various biosynthetic pathways • The neurotransmitter norepinephrine is biosynthesized from dopamine by a benzylic hydroxylation reaction • • Radical reaction Reaction catalyzed by the copper-containing enzyme dopamine b-monooxygenase Oxidation and Reduction of Aromatic Compounds Aromatic ring • Activates a neighboring (benzylic) C-H position toward oxidation • Activates a neighboring carbonyl group toward reduction • An aryl alkyl ketone prepared by Friedel-Crafts acylation of an aromatic ring can be converted into an alkylbenzene by catalytic hydrogenation over a palladium catalyst • Propiophenone reduced to propylbenzene by catalytic hydrogenation is 8.10 An Introduction to Organic Synthesis: Polysubstituted Benzenes There are many reasons for carrying out laboratory synthesis of an organic molecule • • • In the pharmaceutical industry, new molecules are designed and synthesized in the hope that some might be useful drugs In the chemistry industry, syntheses are done to devise more economical routes to known compounds In biochemistry laboratories molecules synthesized to probe enzyme mechanisms An Introduction to Organic Synthesis: Polysubstituted Benzenes Planning a successful multistep synthesis of a complex molecule requires knowledge of the uses and limitations of numerous organic reactions The trick to planning an organic synthesis is to work backward, often referred to as the retrosynthetic direction • Keep starting material in mind and work backward to it • Look at the final product and determine possible immediate precursors of that product • Work backward one step at a time An Introduction to Organic Synthesis: Polysubstituted Benzenes Examples of synthetic planning using polysubstituted aromatic compounds as the targets • Electrophilic substitution on a disubstituted benzene ring is governed by the same resonance and inductive effects that affect monosubstituted rings • Must consider the additive effects of two groups An Introduction to Organic Synthesis: Polysubstituted Benzenes If the directing effects of the two groups reinforces each other, the situation is straightforward 1. • In p-nitrotoluene both the methyl and the nitro group direct further substitution to the same position (ortho to the methyl = meta to the nitro). A single product is thus formed on electrophilic substitution An Introduction to Organic Synthesis: Polysubstituted Benzenes 2. If the directing effects of the two main groups oppose each other, the more powerful activating group has the dominant influence • Nitration of p-methylphenol yields primarily 4-methyl2-nitrophenol because –OH is a more powerful activator than –CH3 An Introduction to Organic Synthesis: Polysubstituted Benzenes 3. Further substitution rarely occurs between the two groups in a meta-disubstituted compound because this site is too hindered • Aromatic rings with three adjacent substituents must therefore be prepared by some other route • The substitution of an ortho-disubstituted compound Worked Example 8.4 Synthesizing a Polysubstituted Benzene Propose a synthesis of 4-bromo-2-nitrotoluene from benzene. Worked Example 8.4 Synthesizing a Polysubstituted Benzene Strategy Draw the target molecule Identify the substituents 1. 2. • Recall how each group can be introduced separately 3. • 4. The three substituents on the ring are a bromine, a methyl group, and a nitro group A bromine can be introduced by bromination with Br2/FeBr3, a methyl group can be introduced by FriedelCrafts alkylation with CH3Cl/ AlCl3, and a nitro group can be introduced by nitration with HNO3/H2SO4 Then plan retrosynthetically Worked Example 8.4 Synthesizing a Polysubstituted Benzene Solution • The final step will involve introduction of one of the three groups – bromine, methyl, or nitro • Three possibilities: Worked Example 8.4 Synthesizing a Polysubstituted Benzene • Immediate precursors of p-bromotoluene • Toluene • • Because the methyl group would direct bromination to the ortho and para positions Bromobenzene • Because Friedel-Crafts methylation would yield a mixture of ortho and para products Worked Example 8.4 Synthesizing a Polysubstituted Benzene • The immediate precursor of toluene • Benzene, which could be methylated in a Friedel-Crafts reaction • The immediate precursor of bromobenzene • Benzene, which could be brominated • Two valid routes possible from benzene to 4-bromo-2- nitrotoluene Worked Example 8.5 Synthesizing a Polysubstituted Benzene Propose a synthesis of 4-chloro-2propylbenzenesulfonic acid from benzene. Worked Example 8.5 Synthesizing a Polysubstituted Benzene Strategy Draw the target molecule 2. Identify the substituents • The three substituents on the ring are chlorine, a propyl group, and a sulfonic acid group 3. Recall how each of the three can be introduced • A chlorine can be introduced by chlorination using Cl2/FeCl3, a propyl group can be introduced by FriedelCrafts acylation with CH3CH2COCl/ AlCl3 followed by reduction with H2/Pd, and a sulfonic acid group can be introduced by sulfonation with SO3/H2SO4 4. Then plan retrosynthetically 1. Worked Example 8.5 Synthesizing a Polysubstituted Benzene Solution • The final step will involve introduction of one of the three groups – chlorine, propyl, or sulfonic acid • Three possibilities: Worked Example 8.5 Synthesizing a Polysubstituted Benzene • The immediate precursors to m-chloropropylbenzene • Because the two substituents have a meta relationship, the first substituent placed on the ring must be a meta director so that the second substitution will take place at the proper position • Because primary alkyl groups such as propyl cannot be introduced directly by Friedel-Crafts alkylation, the precursor of m-chloropropylbenzene is probably mchloropropiophenone, which could be catalytically reduced Worked Example 8.5 Synthesizing a Polysubstituted Benzene • The immediate precursor of m-chloropropiophenone • • Propiophenone, which could be chlorinated in the meta position The immediate precursor of propiophenone • Benzene which could undergo Friedel-Crafts acylation with propanoyl chloride and AlCl3 Worked Example 8.5 Synthesizing a Polysubstituted Benzene • The final synthesis is a four-step route from benzene: