chapter2
... • there are four sets of eclipsed C-H interactions and one flagpole interaction • a boat conformation is less stable than a chair conformation by 27 kJ (6.5 kcal)/mol ...
... • there are four sets of eclipsed C-H interactions and one flagpole interaction • a boat conformation is less stable than a chair conformation by 27 kJ (6.5 kcal)/mol ...
Nucleophilic ring opening of aziridines
... bearing N-(S)-CHMePh chirality were used, optically active amines 19 could be synthesized in excellent diastereoselectivity in the case of 2-isopropylidene 16. The application of this multi-component method was demonstrated in asymmetric synthesis of 2-substituted piperidines leading to (S)-coniine ...
... bearing N-(S)-CHMePh chirality were used, optically active amines 19 could be synthesized in excellent diastereoselectivity in the case of 2-isopropylidene 16. The application of this multi-component method was demonstrated in asymmetric synthesis of 2-substituted piperidines leading to (S)-coniine ...
Chapter 1 Structure and Bonding
... Hyperconjugation makes substituted alkenes more stable by stabilizing p-orbitals cis alkenes are less stable than trans alkenes because of steric crowding cis cycloalkenes are more stable than trans for the small rings ...
... Hyperconjugation makes substituted alkenes more stable by stabilizing p-orbitals cis alkenes are less stable than trans alkenes because of steric crowding cis cycloalkenes are more stable than trans for the small rings ...
synthetic.applicatio..
... dependent for aziridine esters (2S,3R)-1 and (2R,3S)-4, but was also influenced by solvent (Scheme 2).11 The retention product always predominated for trisubstituted aziridine esters 1 with CH2Cl2 giving the best selectivity. For the tetrasubstituted aziridine esters 4, use of hexane as solvent with ...
... dependent for aziridine esters (2S,3R)-1 and (2R,3S)-4, but was also influenced by solvent (Scheme 2).11 The retention product always predominated for trisubstituted aziridine esters 1 with CH2Cl2 giving the best selectivity. For the tetrasubstituted aziridine esters 4, use of hexane as solvent with ...
NAME - HCC Learning Web
... The conjugate acid to (CH3)3N: is (CH3)3NH+. An electron pair donor substance considers being a Lewis acid. The stronger the acid, the weaker the conjugate base. ...
... The conjugate acid to (CH3)3N: is (CH3)3NH+. An electron pair donor substance considers being a Lewis acid. The stronger the acid, the weaker the conjugate base. ...
Ethers and Epoxides
... Naming these heterocyclic compounds depends on the ring size and number of oxygens. (It can be confusing at first…) ...
... Naming these heterocyclic compounds depends on the ring size and number of oxygens. (It can be confusing at first…) ...
108B Carbohydrate Activity KEY
... 6. Draw Haworth projections and the chair conformation for the following aldohexoses using the backbone structures from #5. Consult Fig 25.3 of McMurry; memorize the structure of DGlucose for the final exam. Left or right side on Fischer projection ...
... 6. Draw Haworth projections and the chair conformation for the following aldohexoses using the backbone structures from #5. Consult Fig 25.3 of McMurry; memorize the structure of DGlucose for the final exam. Left or right side on Fischer projection ...
1990-Spring-Exam-2-student
... a) Given a chair-like ring (with -O- in place of a -CH2-) and all the axial and equatorial bonds shown, complete that conformation with the substituent’s of (I) in their proper location and orientation, then draw the second chair conformation that is in equilibrium with the first. H ...
... a) Given a chair-like ring (with -O- in place of a -CH2-) and all the axial and equatorial bonds shown, complete that conformation with the substituent’s of (I) in their proper location and orientation, then draw the second chair conformation that is in equilibrium with the first. H ...
Factors influencing ring closure through olefin metathesis – A
... or radical species. Common rings such as 5–7 membered ones are easily available by these methods. However, formation of medium or large rings by these methods either proceeds with low yields or does not proceed at all due to unfavourable enthalpic and entropic factors. In recent years, olefin metath ...
... or radical species. Common rings such as 5–7 membered ones are easily available by these methods. However, formation of medium or large rings by these methods either proceeds with low yields or does not proceed at all due to unfavourable enthalpic and entropic factors. In recent years, olefin metath ...
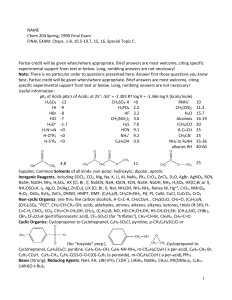
NAME Chem 204 Spring, 1990 Final Exam FINAL EXAM: Chaps. 1
... terpenes in red algae’s. For (b) it is essential that substituent’s be clearly shown as to whether they are axial or equatorial. OH CH3 ...
... terpenes in red algae’s. For (b) it is essential that substituent’s be clearly shown as to whether they are axial or equatorial. OH CH3 ...
Aromatic Compounds
... electron to the aromatic sextet, like a carbon atom in benzene does • In pyrrole, the nitrogen atom is not in a double bond and contributes two p electrons (the lone pair) to the aromatic sextet • In imidazole, both a double-bonded “pyridine-like” nitrogen that contributes one p electron and a “pyrr ...
... electron to the aromatic sextet, like a carbon atom in benzene does • In pyrrole, the nitrogen atom is not in a double bond and contributes two p electrons (the lone pair) to the aromatic sextet • In imidazole, both a double-bonded “pyridine-like” nitrogen that contributes one p electron and a “pyrr ...
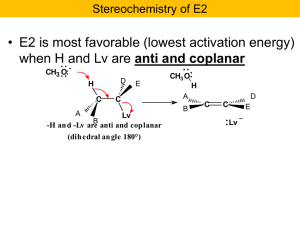
Nucleophilic Substitution and b
... In the more stable chair of the trans isomer, there is no H anti and coplanar with Lv, but there is one in the less stable chair ...
... In the more stable chair of the trans isomer, there is no H anti and coplanar with Lv, but there is one in the less stable chair ...
Molecular orbital approach to substituent effects in amine
... and bulk diffusion of P. Our results for D M M P decomposition on a-Fe2039 show that it is active for D M M P decomposition but deactivates as phosphate forms. Unlike Mo, Pt does not readily form a stable bulk oxide. Thus, removal of P can occur only when gas-phase oxygen is present to drive the for ...
... and bulk diffusion of P. Our results for D M M P decomposition on a-Fe2039 show that it is active for D M M P decomposition but deactivates as phosphate forms. Unlike Mo, Pt does not readily form a stable bulk oxide. Thus, removal of P can occur only when gas-phase oxygen is present to drive the for ...
Phenol - Macmillan Academy
... Electron pair donation takes place from a p orbital on oxygen It increases the electron density of the delocalised system It makes substitution much easier compared to benzene The electron density is the greatest at the 2,4 and 6 positions Substitution takes place at the 2,4 and 6 positions Phenol r ...
... Electron pair donation takes place from a p orbital on oxygen It increases the electron density of the delocalised system It makes substitution much easier compared to benzene The electron density is the greatest at the 2,4 and 6 positions Substitution takes place at the 2,4 and 6 positions Phenol r ...
synthesis, chemistry and optical resol
... [26.10]betweenanenes (13a-c) by a new route of general applicability and some preliminary chemical studies on these conformationally flexible olefins, including the optical resolution and absolute configuration of the [26.10] and [22.10] homologues. Our interest in the aforementioned betweenanenes w ...
... [26.10]betweenanenes (13a-c) by a new route of general applicability and some preliminary chemical studies on these conformationally flexible olefins, including the optical resolution and absolute configuration of the [26.10] and [22.10] homologues. Our interest in the aforementioned betweenanenes w ...
Lecture 4 - Sugars, ring structures
... The most stable form of a sugar in a six membered ring is in the chair formation. The substituent groups on the molecule are either in an axial or equatorial position depending on what gives the most stable molecule. ...
... The most stable form of a sugar in a six membered ring is in the chair formation. The substituent groups on the molecule are either in an axial or equatorial position depending on what gives the most stable molecule. ...
Dicyanomethylenedihydrofuran photorefractive materials
... First observed in inorganic crystals such as LiNbO3 and LiTaO3 in 1969 1, the PR effect is a reversible refractive index modulation process induced by light and an electric field in a material. The PR effect has promising applications including optical data storage, phase conjugation and optical pro ...
... First observed in inorganic crystals such as LiNbO3 and LiTaO3 in 1969 1, the PR effect is a reversible refractive index modulation process induced by light and an electric field in a material. The PR effect has promising applications including optical data storage, phase conjugation and optical pro ...
Aromatic Compounds
... An acyl group, -COR, is substituted onto an aromatic ring • The reactive electrophile is a resonance-stabilized acyl cation • An acyl cation is stabilized by interaction of the vacant orbital on carbon with lone-pair electrons on the neighboring oxygen • Because of stabilization, no carbocation rear ...
... An acyl group, -COR, is substituted onto an aromatic ring • The reactive electrophile is a resonance-stabilized acyl cation • An acyl cation is stabilized by interaction of the vacant orbital on carbon with lone-pair electrons on the neighboring oxygen • Because of stabilization, no carbocation rear ...
Phenol File
... hydrogen is also produced this reaction is similar to that with aliphatic alcohols such as ethanol 2C6H5OH(s) ...
... hydrogen is also produced this reaction is similar to that with aliphatic alcohols such as ethanol 2C6H5OH(s) ...
Aromatic Compounds
... An acyl group, -COR, is substituted onto an aromatic ring • The reactive electrophile is a resonance-stabilized acyl cation • An acyl cation is stabilized by interaction of the vacant orbital on carbon with lone-pair electrons on the neighboring oxygen • Because of stabilization, no carbocation rear ...
... An acyl group, -COR, is substituted onto an aromatic ring • The reactive electrophile is a resonance-stabilized acyl cation • An acyl cation is stabilized by interaction of the vacant orbital on carbon with lone-pair electrons on the neighboring oxygen • Because of stabilization, no carbocation rear ...
Number of students performing at
... (12 pts) Mark as true (T) or false (F) the following statements. Do not explain! • (T) Single bonds are always σ-bonds; • (F) Resonance structures are always in a state of rapid equilibrium; • (F) The gauche conformation of butane is a transition state; • (T) The chair conformation of cyclohexane is ...
... (12 pts) Mark as true (T) or false (F) the following statements. Do not explain! • (T) Single bonds are always σ-bonds; • (F) Resonance structures are always in a state of rapid equilibrium; • (F) The gauche conformation of butane is a transition state; • (T) The chair conformation of cyclohexane is ...
Chapter 9
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
ENGLISH VERSION Exam Organic Chemistry 2
... A benzophenone structure was used as starting material in the synthesis of Meclizine mentioned above. Design a synthetic route to this substance starting from benzene and suitable reagents. The synthesis may proceed in many steps, but indicate possible weaknesses and byproducts in each step. (10p) O ...
... A benzophenone structure was used as starting material in the synthesis of Meclizine mentioned above. Design a synthetic route to this substance starting from benzene and suitable reagents. The synthesis may proceed in many steps, but indicate possible weaknesses and byproducts in each step. (10p) O ...