* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download In this chapter, alkanes, alkenes, alkynes
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Sulfuric acid wikipedia , lookup
George S. Hammond wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Marcus theory wikipedia , lookup
Transition state theory wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Ene reaction wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Acid–base reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Dr. Peter Wipf
Chemistry 0320 - Organic Chemistry 2
Electrophilic Aromatic Substitution
In this chapter, we discussed the concept of aromaticity and the structures of important
members of this class of organic molecules, such as naphthalene, anthracene, phenanthrene,
azulene, [14]annulene, toluene, phenol, aniline, benzoic acid, pyridine, pyrrole, furan, thiophene,
indole, purine, and pyrimidine ring systems.
The main criteria for aromaticity are:
- high chemical stability
- preference of substitution over addition reactions
- ability to sustain an induced ring current (NMR!)
- meet Hückel' s rule [4n+2]
The resonance energy stabilization in benzene amounts to 30 kcal/mol. This can be
calculated experimentally by considering the -orbital energies ("'s & 's"), or measured
experimentally via the enthalpies of hydrogenation. In molecules such as cyclooctatetraene, a
fully delocalized -electron configuration is less stable than four localized double bonds;
cyclooctatetraene is an example of an antiaromatic molecule, and its geometry is distorted in
order to minimize delocalization. Steric hindrance in a molecule such as the [10]annulene, on the
other hand, can offset any energy decreases due to resonance stabilization.
E
H H
Consideration of these aspects as well as careful inspection of all possible resonance
contributors allows us to understand the correlation between electronic configuration and
reactivity & structure of molecules such as cyclopropenyl-, cyclopentadienyl- and
cycloheptatrienyl-anions and -cations, dipotassium cyclooctatetraene, azulene, cyclobutadiene,
and phenanthrene, as well as larger polyunsaturated rings.
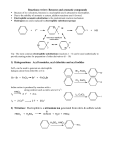
Rather than addition reactions that convert double bonds,
aromatic compounds preferentially undergo substitution
reactions with electrophiles such as halogens (in the
presence of Lewis acids), nitronium ions generated in a
mixture of nitric and sulfuric acids, sulfur trioxide or
concentrated sulfuric acid, carbocations from protonation of
alkenes or alcohols (Friedel-Crafts alkylation), and acylium
cations from the reaction of acid chlorides, anhydrides, or
acids with Lewis acids (Friedel-Crafts acylation).
Br
Br2, FeBr3
The mechanism of electrophilic aromatic
substitution involves (a) the reaction between
the aromatic ring and the electrophile to yield
a pentadienyl cation intermediate (the complex), and, (b) the loss of a proton from
the pentadienyl cation to regenerate the
aromatic ring system.