* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Basal metabolic rate wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Mitochondrion wikipedia , lookup
Phosphorylation wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
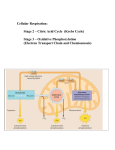
Understanding Applications & Skills Important Words/Phrases Glycolysis Krebs Cycle Electron Transport Chain Oxidation Reduction Redox Phosphorylation Isomerisation Mitochondrion Decarboxylation Introduction You have already learned the basics of cellular respiration. In fact, you knew the formula before you started this course But this is a tremendous oversimplification of the process Now we’re going to learn the details Oxidation and Reduction All chemical reactions either require energy or release it Many reactions can be described using the terms “oxidation” or “reduction” These reactions are often coupled together and are known as “redox” reactions Oxidation and Reduction Oxidation and Reduction To help you remember: OIL RIG (Oxidation Is Losing electrons, Reduction Is Gaining electrons) OR LEO says GER (Losing Electrons is Oxidation, Gaining Electrons is Reduction) Redox and Electron Carriers Electron Carriers NADH and FADH2 Both of these molecules are Nucleic Acids. In their reduced forms they have a tremendous amount of energy that gets released during oxidation FADH2 Oxidized Reduced Overview Cell Respiration can be divided into 4 parts: Glycolysis Pyruvate Oxidation (also called the “linking” reaction) Krebs Cycle (also called the citric acid cycle) The electron transport chain (oxidative phosphorylation) Overview (Continued) Glycolysis takes place in the cytoplasm and is anaerobic The other three parts occur in the mitochondria and require oxygen (aerobic) Each step either produces ATP or reduces NAD or FAD Glycolysis Some Key Points: Starts with Glucose, ends with Pyruvate Two ATP molecules are used, 4 are made Net production is 2 ATP per molecule of glucose Also produces two molecules of NADH Can be divided into 3 General Parts 1. Energy expenditure (steps 1-4) 2. Splitting (steps 4 & 5) 3. Energy production (steps 6-10) Reactions are named according to what happens Glycolysis Step by Step Types of Reactions: Phosphorylation – adding a phosphate group (steps 1 and 3) Isomerase – an isomer is created (step 2, 5 & 8) Redox – the substrate is oxidized while NAD+ is reduced to NADH (6) Dephosphorylation – A phosphate group is removed from the substrate, ATP is produced (7 & 10) Dehydration – Water is removed (9) Decomposition – A large molecule is broken down (4) Glycolysis Odds and Ends Every step is controlled by an enzyme Pyruvate will enter the mitochondria if oxygen is available. If not, it will be reduced to lactate (in animals). This causes NADH to be oxidized Glycolysis 1. Substrate includes Glucose, ATP, ADP, Pi , NAD+ and H+ 2. Main products include Pyruvate, ATP and NADH (H2O) 3. One Glucose molecule yields 2 Pyruvate, 2 ATP and 2 NADH 4. Pyruvate is converted to lactate if oxygen is not available Simulations http://www.science.smith.edu/departments/Biology/Bio231/glycoly sis.html http://highered.mheducation.com/sites/0072507470/student_view0 /chapter25/animation__how_glycolysis_works.html (try quiz!) Linking Reaction (Pyruvate Oxidation) This is a one step reaction, but lots of stuff is happening! It begins in the cytoplasm and ends up in the mitochondria Linking Reaction Three Things are Happening: 1. Pyruvate combines with Coenzyme A to form Acetyl Coenzyme A 2. NAD+ is converted to NADH 3. CO2 is released as a waste product (decarboxylation) Linking Reaction Summary 1. Substrates include pyruvate, coenzyme A, NAD+ & H+ 2. Products include Acetyl Coenzyme A, NADH & CO2 3. Acetyl Coenzyme A is produced in the matrix of the mitochondria Inside the Mitochondria The mitochondrion is an extremely complex organelle Mitochondrion Diagram Labeled Diagram The Krebs/Citric Acid Cycle This is an 8 step process Technically it has no start or finish since it’s a cycle but the point that Acetyl Coenzyme A enters the cycle (where oxaloacetate is converted to citrate) is usually referred to as step #1 step 1 Krebs Cycle Key Points Redox reactions in steps 3, 4, 6 and 8 produce 3 molecules of NADH and 1 molecule of FADH2 ATP is produced in step 5 by the dephosphorylation of Succinyl CoA CO2 is produced as a waste product in steps 3 and 4 (decarboxylation) *when we determine the products that come from one molecule of glucose, we have to realize that two pyruvate molecules were produced so we have two turns of the Krebs cycle per glucose molecule Krebs Cycle Summary Key Substrates Key Products Acetyl Coenzyme A ATP Oxaloacetate NADH NAD+ FADH2 FAD CO2 GDP ADP Krebs Cycle – By the Numbers For each loop of the Krebs Cycle we get: 1 molecule of ATP 3 molecules of NADH 1 molecule of FADH2 But the products of glucose allow for 2 loops of the cycle. So 1 molecule of glucose produces: 2 molecules of ATP 6 molecules of NADH and 2 molecules of FADH2 In the Krebs cycle Recap So if we combine glycolysis, the link reaction and the Krebs cycle what do we have? 4 ATP (net) 10 NADH 2 FADH2 per glucose molecule The Electron Transport Chain So far, a lot of NADH has been made but not much ATP That problem is taken care of in the final step of the respiratory chain; the Electron Transport Chain Electron Transport Chain The Electron Transport Chain (ETC) takes place along the inner membrane of the mitochondria. A series of electron carriers line up in order of their affinity for electrons Electron Transport Chain The reduced NADH molecules contain large amounts of energy and some of this energy is released when the first electron carrier (NADH dehydrogenase) breaks NADH up into NAD+, H+ and 2 electrons. Energy is released as the two electrons get taken up by the carrier and passed on down the line (carrier I to carrier II to carrier III to carrier IV) Each time electrons are transferred, more energy is released ETC Where Does the Energy Go? Each time the energy is released it is used to actively transport protons (H+) out of the matrix into the intermembrane space through pumps that are located in three of the carriers For NADH, 3 H+ ions get pumped out For FADH2, only two H+ ions are pumped out because it gets oxidized by electron carrier II (ubiquinone or q for short) ETC and Chemiosmosis As the H+ gradient increases, there is pressure for them to diffuse back into the matrix. This pressure is based on concentration and electrical gradient. It is called an electrochemical gradient There is another structure adjacent to the electron carriers called ATP synthase. ATP synthase is an enzyme and a membrane channel that H+ ions can diffuse through As they diffuse into the matrix, the kinetic energy they possess is used to combine ADP and P to form ATP ETC (Oxidative Phosphorylation) Since each NADH molecule provides enough energy to pump 3 H+ into the intermembrane space, 3 ATP get made. Similarly, each FADH2 molecule will result in the synthesis of 2 ATP molecules ATP Count Let’s do some Math Each glucose molecule makes 10 NADH 3 x 10 = 30 2 FADH2 2x2=4 4 ATP =4 _____________________________ Total ATP from one molecule of glucose is 38 ATP At least in theory. In reality that number is closer to 30 because it doesn’t work as perfectly as it should What About Oxygen Last Question: What Does Oxygen Do? The final electron carrier, carrier IV (cytochrome oxidase) has a very high affinity for electrons. But it can only hold two electrons. If it isn’t oxidized (i.e. able to lose the electrons) then it won’t be able to accept any more electrons and the ETC will grind to a halt. Fortunately, there is something that has an even higher affinity for electrons than cytochrome oxidase OXYGEN!!! Role of Oxygen Oxygen takes the electrons from carrier IV and then combines with two H+ ions to make a molecule of water As more ATP gets made, more oxygen is needed on a continual basis The availability of Oxygen is usually what limits our ability to make ATP. Now watch this https://www.youtube.com/watc h?v=VER6xW_r1vc