* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Oxacyclopropane (Epoxide) Synthesis: Epoxidation by
Discodermolide wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Ene reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
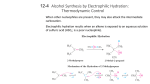
Peroxycarboxylic acids deliver oxygen atoms to double bonds. Peroxycarboxylic acids have the general formula: These compounds react with double bonds because one of the oxygen atoms is electrophilic. The resulting products are an oxacyclopropane and a carboxylic acid. This reaction is referred to as an “epoxidation.” The older common name of an oxacyclopropane was an “epoxide.” Commonly used peroxycaraboxylic acids for this reaction are metachloroperoxybenzoic acid (MCPBA) which is somewhat shock sensitive, and magnesium monoperoxyphthalate (MMPP). The mechanism of this epoxidation reaction involves a cyclic transition state: The peroxycarboxylic acid reactivity with double bonds increases with alkyl substitution, allowing for selective oxidations: Hydrolysis of oxacyclopropanes furnishes the products of anti dihydroxylation of an alkene. Ring opening of oxacyclopropanes with water produces anti vicinal diols. 12-11 Vicinal Syn Dihydroxylation with Osmium Tetroxide The reaction of osmium tetroxide with alkenes yields syn vicinal diols in a two step process: The reaction mechanism involves the concerted addition of the osmium tetroxide to the bond of the alkene: Catalytic amounts of osmium tetroxide in the presence of an oxidizing agent (H2O2) to regenerate the spent osmium tetroxide are often used, due to the expense and toxicity of OsO4. An older reagent for vicinal syn dihydroxylation of alkenes is KMnO4. This reagent is less useful than OsO4 because of its tendency towards overoxidation. The deep purple KMnO4 is converted into a brown precipitate, (MnO2) during the reaction, which can serve as a useful test for the presence of alkenes. 12-12 Oxidative Cleavage: Ozonolysis The mildest reagent capable of breaking both the and bonds in a double bond is ozone, O3. This process is known as “ozonolysis.” Ozone is produced by an electrical discharge in dry oxygen in a instrument called an ozonator. The initial product of the reaction of ozone with an alkene is an ozonide which is then directly reduced to two carbonyl products. 12-13 Radical Additions: Anti-Markovnikov Product Formation Hydrogen bromide can add to alkenes in anti-Markovnikov fashion: a change in mechanism. The reaction products from the treatment of 1-butene with HBr depend upon the presence or absence of molecular oxygen in the reaction mixture: In the presence of oxygen, a radical chain sequence mechanism leads to the antiMarkovnikov product. Small amounts of peroxides (RO-OR) are formed in alkene samples stored in the presence of air (O2). The peroxides initiate the radical chain sequence mechanism, which is much faster than the ionic mechanism operating in the absence of peroxides.