* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Alcohol Synthesis by Electrophilic Hydration
Marcus theory wikipedia , lookup
Elias James Corey wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Stille reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Petasis reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
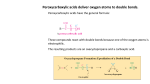
12-4 Alcohol Synthesis by Electrophilic Hydration: Thermodynamic Control When other nucleophiles are present, they may also attack the intermediate carbocation. Electrophilic hydration results when an alkene is exposed to an aqueous solution of sulfuric acid (HSO4- is a poor nucleophile). The addition of water by electrophilic hydration follows Markovnikov’s rule, however carbocation rearrangements can occur because water is a poor nucleophile. The electrophilic hydration process is the reverse of the acid-induced elimination of water (dehydration) of alcohols previously discussed. Alkene hydration and alcohol dehydration are equilibrium processes. All steps are reversible in the hydration of alkenes. The proton serves as a catalyst only: it is regenerated in the reaction. In the absence of protons, alkenes are stable in water. The position of the equilibrium in the hydration reaction can be changed by adjusting the reaction conditions. The reversibility of alkene protonation leads to alkene equilibration. Protonation-deprotonation reactions may interconvert related alkenes and produce an equilibrium mixture of isomers. Under these conditions, a reaction is said to be under thermodynamic control. This mechanism can convert less stable alkenes into their more stable isomers: 12-5 Electrophilic Addition of Halogens to Alkenes Halogen molecules also act as electrophiles with alkenes giving vicinal dihalides. The reaction with bromine results in a color change from red to colorless, which is sometimes used as a test for unsaturation. Halogenations are best carried out at or below room temperature and in inert halogenated solvents (i.e. halomethanes) 12-5 Electrophilic Addition of Halogens to Alkenes Bromination takes place through anti addition. Consider the bromination of cyclohexene. No cis-1,2-dibromocyclohexane is formed. Only anti addition is observed. The product is racemic since the initial attack of bromine can occur with equal probability at either face of the cyclohexene. With acyclic alkenes the reaction is cleanly stereospecific: Cyclic bromonium ions explain the stereochemistry. The polarizability of the Br-Br bond allows heterolytic cleavage when attacked by a nucleophile, forming a cyclic bromonium ion: The bridging bromine atoms serves as the leaving group as the bromonium ion is attacked from the bottom by a Br- ion. In symmetric bromonium ions, attack is equally probable at either carbon atom leading to racemic or meso products. 12-6 The Generality of Electrophilic Addition The bromonium ion can be trapped by other nucleophiles. Bromonation of cyclopentene using water as the solvent gives the vicinal bromoalcohol (bromohydrin). The water molecule is added anti to the bromine atom and the other product is HBr. Vicinal haloalcohols are useful synthetic intermediates. Vicinal haloethers can be produced if an alcohol is used as the solvent, rather than water. Halonium ion opening can be regioselective. Mixed additions to double bonds can be regioselective: The nucleophile attacks the more highly substituted carbon of the bromonium ion, because it is more positively polarized. Electrophilic additions of unsymmetric reagents add in a Markovnikov-like fashion: The electrophilic unit becomes attached to the less substituted carbon of the double bond. Mixtures of products are formed only when the two carbons are not sufficiently differentiated. Reagents of the type A-B, in which A acts as the electrophile, A+, and B the nucleophile, B-, can undergo stereo- and regiospecific addition reactions to alkenes: