* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download O V O O RO OH t-BuOOH, CH2Cl2, Ti(OPr-i)4(cat), 20 oC (L)
Survey
Document related concepts
Cracking (chemistry) wikipedia , lookup
Discodermolide wikipedia , lookup
Elias James Corey wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Ene reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Stille reaction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Transcript
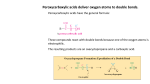
Enantioselective Catalysis Asymmetric Epoxidation. You have already seen in 59-331 that a very common method for converting alkenes to epoxides involves the reaction of the former with peracids. O R O 1 R R2 CH2Cl2 O H O R2 R1 There is a well-known alternative to this, which employs metal complex catalyzed oxidation by a alkyl hydroperoxide. The original method involved a V(O)(OR)3 complex and t-BuOOH; it is selective for allylic alcohols, because the VV alkoxide (shown below) is likely a critical intermediate in the epoxidation. That leaves the OR’s for potential sources of making a chiral catalyst, using some kind of chiral alcohol. O -O V O RO O Other transition metal based compounds (i.e., Mo, Ti) are known for this process, too, and in particular the TiIV catalysts can be made to operate enantioselectively. The overwhelmingly employed set of reagents are: OH t-Bu O O H + Ti( OPr-i)4 + O EtO OEt O (L)-(+)-(R,R)diethyl tartate (or diisopropyl) OH This is particularly useful in that the naturally occurring enantiomer (shown) of diethyl tartrate is pretty cheap (100 g, $65, 2012 dollars), and even the unnatural enantiomer ((S,S)-) isn’t too bad (25 g, $122, 2012 dollars). Furthermore, 3 Å molecular sieves are added to make the reaction reliably catalytic in TiIV and tartrate. Typical loadings of the catalysts are anywhere from 5 mol% TiIV + 6 mol% tartrate to 10 mol% TiIV + 12 mol% tartrate. This process is called the Katsuki-Sharpless epoxidation (older books tend to leave out Katsuki). Here’s a simple example: OH t -BuOOH, CH2Cl2, Ti(OPr-i)4(cat), 20 oC O OH H (L)-(+)-diethyl tartrate(cat) 95% ee (97.5:2.5) er This first thing to notice is that the stereosepecific with respect to alkene geometry; cis- alkene substitutents on the alkene give cis- substituents on the epoxide and trans- gives trans-. The direction of asymmetric induction, is also stereospecific (depending upon the tartrate enantiomer), and a model is useful that is totally empirical. In it, of you lay out the allylic alcohol such that the alcohol is out from and to the right, the natural tartrate based complex attacks from the bottom face, while the unnatural tartrate based reagent attacks from the top face. (D)-(-)-(S,S)-tartrate O "O" unnatural R1 R2 OH R3 R2 R1 O R2 OH H R1 R3 OH R2 natural R1 R2 R1 R2 OH O OH H O "O" (L)-(+)-(R,R)-tartrate In addition, it is possible to accomplish a kinetic resolution on alcohols that have a chiral centre but are racemic (in other words, have one enantiomer react faster than the other), and as a result you can get enantiomerically enriched epoxide and unreacted allylic alcohol (recovered starting material) if the reaction is stopped at ca. 50% conversion. Here’s schematic showing why…. (D)-(-)-(S,S)-tartrate - SLOW (D)-(-)-(S,S)-tartrate - FAST "O" "O" R2 R4 OH R3 R2 R1 H OH R3 R4 H "O" (L)-(+)-(R,R)-tartrate - FAST R1 "O" (L)-(+)-(R,R)-tartrate - SLOW The rate difference between the two (diastereotopic) faces are large to massive (16:1 = 82% ee to 700:1) at -20 oC, often giving excellent results. Ti(OPr-i)4(cat), t -BuOOH, OH OH H (L)-(+)-DET(cat), CH2Cl2 ~ 50% conversion racemic O OH + OH H O H enantiomerically enriched enantiomerically enriched The functional group tolerance turns out to be pretty good. Of the common functional groups, only amines (-NR2), carboxylic acids (-CO2H), thioethers (-SR), phenols (Ar-OH), and phosphines (-PR2) are not tolerated. For carboxylic acids and phenols, there are fairly simple protections available (esters and phenolic ethers, respectively). Not every alkene that is an allylic alcohol undergoes epoxidation under these conditions equally well, however, as there is some steric hindrance issues when too many R groups get in the way of the alcohol function. As a general rule. R1 R1 R2 R2 OH OH R2 R3 OH R5 OH R3 limited # generally good 90% ee R4 OH R3 great great OH R1 too slow a bit of a problem - slowest variable ee's but still 80% ee This was all developed empirically, so the mechanism is proposed rather than unequivocal, but the proposed ‘loaded catalyst’ is R4 R5 E O OR RO O E RO O Ti EO Ti O E O 'loaded catalyst" R2 Comprehensive Organic Synthesis 1991, V 7, Ch. 3.2 O O t-Bu Selected examples: mostly taken from Gawley, Aube, Principles of Asymmetric Synthesis, Elsevier, 2012 Ti(OPr-i)4(cat), O OH OH (-)-DIPT(cat), t -BuOOH, CH2Cl2 55%, 95:5 er O Ti(OPr-i)4(cat), OH OH (+)-DET(cat), t-BuOOH, CH2Cl2 Ti(OPr-i)4(cat), ( )9 OH (-)-DET(cat), t -BuOOH, CH2Cl2 J. Am. Chem. Soc. 1987, 109, 5765. 85%, 97:3 er ( )9 ( )9 O OH (+)-DET(cat), t -BuOOH, CH2Cl2 Ti(OPr-i)4(cat), OH (+)-DET(cat), t -BuOOH, CH2Cl2 O (+)-disparlure 80%, 91% ee (95.5:4.5 er) Ti(OPr-i)4(cat), OH Masamune, Sharpless et al J. Am. Chem. Soc. 1987, 109, 5765. O OH (gypsy moth insect sex pheromone) 77% J. Am. Chem. Soc. 1987, 109, 5765. 96:4 er O OH 95% 95:5 er J. Am. Chem. Soc. 1987, 109, 5765. Asymmetric Dihydroxylations See Kolb, H.C.; VanNieuwenzhe, M. S; Sharpless, K. B. Chem. Rev. 1994, 94, 2483. Recall that alkenes can be converted to 1,2- (vic-) diols by the use of OsO4, followed by a workup that breaks up the osmate ester. It has been known for many years that pyridine accelerates the rate of this reaction; so a reasonable approach to creating an enantioselective dihydroxylation reagent would be to make a chiral pyridine, or at least a chiral amine. Before we get to what would serve appropriately…. There are a couple of problems with this process: i) OsO4 is expensive, so it would be very preferable to use it catalytically, and a have a cheap stoichiometric oxidant bring the OsVI back to OsVIII The best choice for this oxidant is K3Fe(CN)6 with a two phase oxidation (K3Fe(CN)6 is water soluble; OsO4 is the only oxidant in the organic phase. The 2nd best choice is N-methylmorpholine-N-oxide (NMO), which you have O seen before in other contexts as a stoichiometric oxidant. NMO ii) The catalytic cycle has a bottleneck, which is the hydrolysis of the osmate VI ester product to free Os and free diol. This break-up is accelerated by methanesulfonamide, MeSO2NH2, which increases the overall reaction rate by a factor of 50x, except for terminal alkenes. N CH3 O O O S H3C NH2 So what chiral amines (or pyridines)? They are two or three piece combinations of one of two naturally occurring cinchona alkaloid ‘ligands’, link with a tether. The ligands are most commonly… Et Et N RO N (R)(S)- H (S)- OR H (R)- MeO OMe N R = H dihydroquinine (DHQ) N R = H dihydroquinidine (DHQD) These are not actually enantiomers of each other, but rather diastereomers, because the chirality of the C bearing the ethyl is the same in each case ((R)-). For the dihydroxylations, however, they function as the complementary systems to get enantiomeric products; the unofficial term pseudoenantiomers is commonly used. It should also be noticed that it is likely the aliphatic nitrogen atom, and not the aromatic one, that is involved in coordination to Os. So what’s that ‘R’ in the structure? The dihydroxylations are most commonly successful when there are two of these units in the same molecule, and they are hooked together with an ether tether, which is normally one of… Ph N N alk-O O-alk O-alk O-alk O N N alk-O O-alk O-alk N N phthalazine (PHAL) historically the best O alk-O N Ph diphenylpyrimidine (PYR) sterically hindered cases N O O-alk anthraquinone (AQN) 2nd best Ph N Ph DPP similar to PHAL IND best for cisdisubst. alkenes The way the reagent combinations are presented is shown by the following example: using the PHAL spacer with the DHQD ligand is termed (DHQD)2-PHAL. This is sold as AD-mix-β. (DHQ)2-PHAL is sold as AD-mix-α. Like the epoxidation reactions, there is a mnemonic for how each of the reagents direct addition to alkenes. If one puts the alkene in a plane with the largest substituent towards the left and towards the reader. DHQD attacks from the top (or β- face), DHQ from the bottom face (or α- face), such as in: DHQD derivs -face OH HO OH RS RL RS RL RM H OH RS RL OH DHQ derivs -face - - RM H RM H often* (R)-left (R)-right often* (S)-left (S)-right OH Not every alkene is dihydroxylated equally well. The following is a rough schematic; you’ll notice that cis- disubstituted cases are the toughest, and really the only case where the IND spacer is used. 90-99.8% ee PHAL DPP AQN - HO OH great 90-99% ee PHAL AQN DPP 80-97% ee PHAL PYR AQN DPP pretty good 70-97% ee PHAL PYR AQN DPP 20-80% IND moderate 20-97% ee PHAL PYR limited # In general, the higher end of the ranges have RL as aromatic groups, and the lower end has RL as aliphatic groups. The newer spacers (AQN especially) have helped out with aliphatic cases. Typical conditions are as follows. Notice that OsO4 is most often replaced by an osmate salt, K2OsO2(OH)4, a non-volative,w ater soluble form of OsVIII. T = 0-25 oC ligand 1-5 mol% osmate K2OsO2(OH)4 0.2-1 mol% Stoichiometric oxidant K3Fe(CN)6 solvent t-BuOH/H2O And the mechanism? Firstly, it was originally proposed that OsO4 dihydroxylation of alkenes was a [3+2] cycloaddition. This view has been replaced that the initial addition is a [2+2] cycloaddition (reminiscent of a Wittig reaction), followed by a ring expansion/rearrangement. O O [3+2] O O OsO4 + L + alkene Os O O O O L L [2+ 2] nt e m ge n a a rr re O O Os Os O O L The proposed source of enantioselectivity stems from minimization of repulsive steric interactions between osmaoxetane and the benzylic carbon of cinchona alkaloid. Attractive π- stacking interactions between aryl groups on the alkene and the electron poor aromatic of the linker are the proposed source of great selectivity with aryl substituted alkenes. O RS O RM O H minimal repulsion Os RL O H stabilizing -stacking N RO O RS O Os RL O N H (S)- Ar RO (S)- Ar with DHQD good O R H M severe repulsion bad