* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Reactions
Chemical plant wikipedia , lookup
Rate equation wikipedia , lookup
Isotopic labeling wikipedia , lookup
Safety data sheet wikipedia , lookup
Fine chemical wikipedia , lookup
Chemical equilibrium wikipedia , lookup
History of chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Nuclear fusion wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Radical (chemistry) wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
George S. Hammond wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Organic chemistry wikipedia , lookup
Photosynthesis wikipedia , lookup
Process chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Marcus theory wikipedia , lookup
Click chemistry wikipedia , lookup
Ene reaction wikipedia , lookup
Transition state theory wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
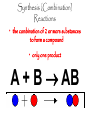
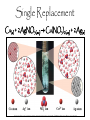
Add to table of contents: Naming Flowchart Chemical nomenclature Naming Ionic Naming acids % comp gum lab Chemical Reactions Classifying reactions Types of Chemical Reactions Pg. 46 Pg. 47 Pg. 48 Pg. 49 Pg. 50 Pg. 51 Pg. 52 Pg. 53 Types of Chemical Reactions • Spontaneous reactions—occur naturally, the process is unaided. • Example: –Decomposition of dead matter = spontaneous endothermic reactions. (absorbs heat energy) –Forest fire = spontaneous exothermic reactions. (releases heat energy) • Non-spontaneous reactions—can only occur when linked to an energy source. • Example: –Striking a match book: Match: head contains KClO3 Book: sand paper strip contains phosphorus. *Need energy (to strike the match for it to light!) Reaction Types Synthesis (Combination) Reactions • the combination of 2 or more substances to form a compound • only one product A + B AB Synthesis (Combination) Reactions • Two or more substances react to form one product • Examples: N2 (g) + 3 H2 (g) 2 NH3 (g) C3H6 (g) + Br2 (l) C3H6Br2 (l) 2 Mg (s) + O2 (g) 2 MgO (s) 2 Mg (s) + O2 (g) 2 MgO (s) Decomposition Reactions • a compound breaks down into 2 or more simpler substances • only one reactant AB A + B Decomposition Reactions • One substance breaks down into two or more substances • Examples: CaCO3 (s) CaO (s) + CO2 (g) 2 KClO3 (s) 2 KCl (s) + O2 (g) 2 NaN3 (s) 2 Na (s) + 3 N2 (g) Combustion Reactions • the burning of any substance in O2 to produce heat A + O2 CO2 +H2O CH4(g) + 2O2(g) CO2(g) + 2H2O(g) Combustion Reactions • Rapid reactions that produce a flame • Most often involve hydrocarbons reacting with oxygen in the air • Examples: CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (g) Whoosh Bottle Demo is a Combustion Reaction 13 Single Replacement • one element replaces another in a compound – metal replaces metal (+) – nonmetal replaces nonmetal (-) A + BC B + AC C. Johannesson Single Replacement Cu(s) + 2AgNO3(aq) Cu(NO3)2(aq) + 2Ag(s) C. Johannesson Double Replacement • ions in two compounds “change partners” • cation of one compound combines with anion of the other AB + CD AD + CB C. Johannesson Double Replacement Pb(NO3)2(aq) + K2CrO4(aq) PbCrO4(s) + 2KNO3(aq) C. Johannesson Practice—Classify the Following Reactions as Synthesis, Decomposition, Single Replacement, or Double Replacement. 3 Mg(s) + 2 FeCl3(aq) 3 MgCl2(aq) + 2 Fe(s) Single Replacement. CO2(g) + H2O(l) H2CO3(aq) Synthesis. 3 KOH(aq) + H3PO4(aq) K3PO4(aq) + 3 H2O(l) Double Replacement. CaCO3 ( s ) CaO(s ) CO 2 ( g ) Decomposition. 18