Mole-mole factor
... A balanced chemical equations tell us: – The formulas and symbols of the reactants and products – The physical state of each substance – If special conditions such as heat are required – The number of molecules, formula units, or atoms of each type of molecule involved in the reaction • Number can b ...
... A balanced chemical equations tell us: – The formulas and symbols of the reactants and products – The physical state of each substance – If special conditions such as heat are required – The number of molecules, formula units, or atoms of each type of molecule involved in the reaction • Number can b ...
Chemical Equilibrium - local.brookings.k12.sd.us
... of the ________ reactants and ________ products are not _____, equal but _______, constant because the rate of _________ formation of the ________ products ____ equal to the ____ rate of _________ formation is _____ of the ________ reactants The Golden Gate Bridge If all other roads leading connects ...
... of the ________ reactants and ________ products are not _____, equal but _______, constant because the rate of _________ formation of the ________ products ____ equal to the ____ rate of _________ formation is _____ of the ________ reactants The Golden Gate Bridge If all other roads leading connects ...
Model-based approach to plant-wide economic control of uid
... industry for the conversion of heavy gasoil to gasoline and diesel. Furthermore, valuable gases such as ethylene, propylene and isobutylene are produced. The performance of the FCC units plays a major role on the overall economics of refinery plants. Any improvement in operation or control of FCC un ...
... industry for the conversion of heavy gasoil to gasoline and diesel. Furthermore, valuable gases such as ethylene, propylene and isobutylene are produced. The performance of the FCC units plays a major role on the overall economics of refinery plants. Any improvement in operation or control of FCC un ...
EVS - RSC - Developments in Microwave Chemistry
... Initially, microwave chemistry was primarily used to carry out analytical processes such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic ...
... Initially, microwave chemistry was primarily used to carry out analytical processes such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic ...
Michelle Tostado WRIT 340 26 February 2013 MWF 11:00 a.m.
... the spirit solution is poured into it. When the inside is toasted, there is an added layer of active carbon that removes unwanted pungent sulfurous compounds [2]. The charring also increased the production of whisky lactones, which introduce fruity flavors and aromas like coconut [2]. The charred b ...
... the spirit solution is poured into it. When the inside is toasted, there is an added layer of active carbon that removes unwanted pungent sulfurous compounds [2]. The charring also increased the production of whisky lactones, which introduce fruity flavors and aromas like coconut [2]. The charred b ...
Chapter 6 Quantities in Chemical Reactions
... When the disengaged gasses are carefully examined, they are found to weigh 113.7 grs.; these are of two kinds, viz. 144 cubical inches of carbonic acid gas, weighing 100 grs. and 380 cubical inches of a very light gas, weighing only 13.7 grs.…and, when the water which has passed over into the bottle ...
... When the disengaged gasses are carefully examined, they are found to weigh 113.7 grs.; these are of two kinds, viz. 144 cubical inches of carbonic acid gas, weighing 100 grs. and 380 cubical inches of a very light gas, weighing only 13.7 grs.…and, when the water which has passed over into the bottle ...
Biomass Program
... The largest use of syngas is for hydrogen production (Wender, 1996). Hydrogen is produced as both a main product and as a by-product. Hydrogen producers often consume the product captively (e.g., ammonia producers, oil refineries, and methanol producers). The total amount of hydrogen consumed worldw ...
... The largest use of syngas is for hydrogen production (Wender, 1996). Hydrogen is produced as both a main product and as a by-product. Hydrogen producers often consume the product captively (e.g., ammonia producers, oil refineries, and methanol producers). The total amount of hydrogen consumed worldw ...
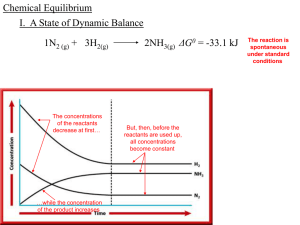
Chemical Equilibrium
... and you need to bail out water. You grab a bucket and begin to bail. After a few minutes, your efforts against the leak keep the water to only about half an inch, but any further bailing doesn’t change the water level; the leak brings in as much water as you bail out. You are at equilibrium. Two oppo ...
... and you need to bail out water. You grab a bucket and begin to bail. After a few minutes, your efforts against the leak keep the water to only about half an inch, but any further bailing doesn’t change the water level; the leak brings in as much water as you bail out. You are at equilibrium. Two oppo ...
Stoichiometery
... Real Chemistry is all about doing chemical reactions. Chemistry is about making or breaking bonds in order to rearrange atoms and make new compounds. ...
... Real Chemistry is all about doing chemical reactions. Chemistry is about making or breaking bonds in order to rearrange atoms and make new compounds. ...
Stoichiometry
... Ex: Calculate the number of grams of oxygen required to react exactly with 4.30 mol of propane, C3H8, in the reaction by the following balanced equation: C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) 5 mol O2 32.0 g O2 4.30 mol C3H8 x _____________ x __________ 1 mol C3H8 1 mol O2 ...
... Ex: Calculate the number of grams of oxygen required to react exactly with 4.30 mol of propane, C3H8, in the reaction by the following balanced equation: C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) 5 mol O2 32.0 g O2 4.30 mol C3H8 x _____________ x __________ 1 mol C3H8 1 mol O2 ...
Chemical Equilibrium - 2012 Book Archive
... equilibrium constants. As such, the equilibrium constant expression for this reaction would simply be ...
... equilibrium constants. As such, the equilibrium constant expression for this reaction would simply be ...
Quantitative Comparison of the Hydrogen Bond
... that the folding of these secondary structure elements may be independent from the rest of the protein. In fact, a peptide consisting of only the first 17 residues adopts a similar β-hairpin structure as this part of the entire protein in the A-state (16), and a peptide comprising the first 35 resid ...
... that the folding of these secondary structure elements may be independent from the rest of the protein. In fact, a peptide consisting of only the first 17 residues adopts a similar β-hairpin structure as this part of the entire protein in the A-state (16), and a peptide comprising the first 35 resid ...
expected output
... To prepare students to act ethically the dangerous chemicals if misused. To produce personal who can run the growing small-scale industries. To prepare for career which requires the knowledge of chemistry in their daily life ...
... To prepare students to act ethically the dangerous chemicals if misused. To produce personal who can run the growing small-scale industries. To prepare for career which requires the knowledge of chemistry in their daily life ...
expected output
... To prepare students to act ethically the dangerous chemicals if misused. To produce personal who can run the growing small-scale industries. To prepare for career which requires the knowledge of chemistry in their daily life. ...
... To prepare students to act ethically the dangerous chemicals if misused. To produce personal who can run the growing small-scale industries. To prepare for career which requires the knowledge of chemistry in their daily life. ...
Chapter 5: Calculations and the Chemical Equation
... One of the most important contribution of chemistry to other areas of sciences providing chemicals or raw materials for building various components of complex structures. Chemists come up with chemical reactions and optimize the conditions at which highest yield of the products could be synthesized. ...
... One of the most important contribution of chemistry to other areas of sciences providing chemicals or raw materials for building various components of complex structures. Chemists come up with chemical reactions and optimize the conditions at which highest yield of the products could be synthesized. ...
Chemical Reactions - 2012 Book Archive
... greater than the total weight of 50 children. Because of the way in which the mole is defined, for every element the number of grams in a mole is the same as the number of atomic mass units in the atomic mass of the element. For example, the mass of 1 mol of magnesium (atomic mass = 24.305 amu) is 2 ...
... greater than the total weight of 50 children. Because of the way in which the mole is defined, for every element the number of grams in a mole is the same as the number of atomic mass units in the atomic mass of the element. For example, the mass of 1 mol of magnesium (atomic mass = 24.305 amu) is 2 ...
Chapter 8
... Theoretical yield - The maximum amount of product that can be formed from the starting materials used in the reaction. Actual yield - The observed yield for a chemical reaction. Percent yield - The percent of the theoretical yield that is actually obtained. ...
... Theoretical yield - The maximum amount of product that can be formed from the starting materials used in the reaction. Actual yield - The observed yield for a chemical reaction. Percent yield - The percent of the theoretical yield that is actually obtained. ...
DEPARTMENT OF CHEMISTRY Requirements For Chemistry Major
... Courses For Chemistry Major All core Chemistry Courses for all the years must be taken by students intending to major in Chemistry. In addition, a useful selection of Elective Courses should be made in order to augment the depth of the subject expected of a Major. ...
... Courses For Chemistry Major All core Chemistry Courses for all the years must be taken by students intending to major in Chemistry. In addition, a useful selection of Elective Courses should be made in order to augment the depth of the subject expected of a Major. ...
Subject Area Standard Area Organizing Category Grade Level
... CHEM.B.1.4.1: Recognize and describe different types of models that can be used to illustrate the bonds that hold atoms together in a compound (e.g., computer models, ball‐ and‐ stick models, graphical models, solid‐ sphere models, structural formulas, skeletal formulas, Lewis dot structures). ...
... CHEM.B.1.4.1: Recognize and describe different types of models that can be used to illustrate the bonds that hold atoms together in a compound (e.g., computer models, ball‐ and‐ stick models, graphical models, solid‐ sphere models, structural formulas, skeletal formulas, Lewis dot structures). ...
Management of Peroxide Forming Chemicals
... Refrigeration and freezing can cause peroxides to precipitate. Blanketing peroxide formers with an inert gas reduces the opportunity for oxygen to reach the compound during storage, but must be done in accordance with MSDS requirements. Inorganic peroxides are generally stable, but some can be hazar ...
... Refrigeration and freezing can cause peroxides to precipitate. Blanketing peroxide formers with an inert gas reduces the opportunity for oxygen to reach the compound during storage, but must be done in accordance with MSDS requirements. Inorganic peroxides are generally stable, but some can be hazar ...
Ethylene Glycol
... Ethylene glycol can also be readily prepared in the laboratory by refluxing ethylene dichloride with a dilute solution of sodium carbonate [13]. One final interesting discover is that in the literature searches on the production of ethylene glycol, most companies used a plug flow reactor to react t ...
... Ethylene glycol can also be readily prepared in the laboratory by refluxing ethylene dichloride with a dilute solution of sodium carbonate [13]. One final interesting discover is that in the literature searches on the production of ethylene glycol, most companies used a plug flow reactor to react t ...
this page - Course Catalogs
... The Bachelor of Science degree is for those who wish to emphasize their study of chemistry. Students interested in chemical engineering can participate in the Engineering Dual-Degree Program described in the Department of Physics and Astronomy section of this catalog. A program of study emphasizing ...
... The Bachelor of Science degree is for those who wish to emphasize their study of chemistry. Students interested in chemical engineering can participate in the Engineering Dual-Degree Program described in the Department of Physics and Astronomy section of this catalog. A program of study emphasizing ...
Document
... E.Q.: What mathematical relationships can be determined from a balanced chemical equation? ...
... E.Q.: What mathematical relationships can be determined from a balanced chemical equation? ...
Stoichiometry and the Mole
... College of Education. The 100,000-square-foot building has enough office space to accommodate 86 full-time faculty members and 167 full-time staff. In a fit of monetary excess, the university administration offered to buy new furniture (desks and chairs) and computer workstations for all faculty and staff ...
... College of Education. The 100,000-square-foot building has enough office space to accommodate 86 full-time faculty members and 167 full-time staff. In a fit of monetary excess, the university administration offered to buy new furniture (desks and chairs) and computer workstations for all faculty and staff ...
Slide 1
... Changes in temperature • Raising the temperature of this system requires the addition of heat, which shifts the equilibrium to the left and reduces the concentration of hydrogen chloride. • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the ...
... Changes in temperature • Raising the temperature of this system requires the addition of heat, which shifts the equilibrium to the left and reduces the concentration of hydrogen chloride. • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the ...
Chemical plant
A chemical plant is an industrial process plant that manufactures (or otherwise processes) chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transformation and or separation of materials. Chemical plants use specialized equipment, units, and technology in the manufacturing process. Other kinds of plants, such as polymer, pharmaceutical, food, and some beverage production facilities, power plants, oil refineries or other refineries, natural gas processing and biochemical plants, water and wastewater treatment, and pollution control equipment use many technologies that have similarities to chemical plant technology such as fluid systems and chemical reactor systems. Some would consider an oil refinery or a pharmaceutical or polymer manufacturer to be effectively a chemical plant.Petrochemical plants (plants using chemicals from petroleum as a raw material or feedstock ) are usually located adjacent to an oil refinery to minimize transportation costs for the feedstocks produced by the refinery. Speciality chemical and fine chemical plants are usually much smaller and not as sensitive to location. Tools have been developed for converting a base project cost from one geographic location to another.