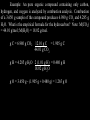* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 8
Multi-state modeling of biomolecules wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical industry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Safety data sheet wikipedia , lookup
Organic chemistry wikipedia , lookup
Drug discovery wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Biochemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Computational chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Water splitting wikipedia , lookup
Isotopic labeling wikipedia , lookup
History of molecular theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Electrochemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Thermometric titration wikipedia , lookup
Chemical reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Electrolysis of water wikipedia , lookup
Process chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Rate equation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Transition state theory wikipedia , lookup
CHAPTER 8 Chemical Reactions Dalton’s Atomic Theory We have previously discussed Dalton’s atomic theory. 1) Matter is composed of small indivisible particles called atoms. All atoms of a particular elements are identical. Atoms of different elements differ in their chemical and physical properties. (Chapter 2). 2) Compounds are composed of molecules. Each molecule contains the same number of atoms of each element making up the compound. (Chapter 5). 3) In a chemical reaction atoms are rearranged, but atoms are neither created, destroyed, or converted from atoms of one element to atoms of another element. We are now ready to discuss the last concept in Dalton’s theory, the rearrangement of atoms in a chemical reaction. Balanced Chemical Equation A balanced chemical equation indicates the starting substances (reactants) and ending substances (products) of a chemical reaction. The reactions may also indicate the state of the reactants and products, and/or the physical process used in the reaction. s = solid = liquid g = gas aq = aqueous = heat A balanced equation must satisfy conservation of mass (same number of atoms of each element on the reactant and product side) and conservation of electrical charge (same total charge on the reactant and product side). This means that chemical reactions must be consistent with Dalton’s theory. Examples of Balanced Chemical Equation Combustion of hydrogen (combustion is the reaction of a substance with molecular oxygen to form combustion products). 2 H2(g) + O2(g) 2 H2O() Nitration of toluene to form trinitrotoluene (TNT) C6H5CH3 + 3 HNO3 C6H2CH3(NO2)3 + 3 H2O Decomposition of calcium carbonate (decomposition is the breakdown of a single reactant into two or more products). CaCO3(s) CaO(s) + CO2(g) Dissolution of potassium sulfate, an ionic compound, in water. K2SO4(s) H2O 2 K+(aq) + SO42-(aq) Procedure For Balancing Chemical Equations 1) Begin with a complete set of reactants and products. These cannot be changed. 2) Find a set of numbers (stoichiometric coefficients) that satisfy mass balance. a) Change the coefficients of compounds before changing the coefficients of elements (save elements for last in balancing). b) Treat polyatomic ions that appear on both sides of the equation as a unit. c) Count atoms and polyatomic ions carefully, and track them every time you change a coefficient. Procedure For Balancing Chemical Equations 3) Convert the stoichiometric coefficients into the smallest set of whole numbers that correctly balance the chemical equation. 1/ 2 N2 + 3/2 H2 NH3 incorrect; fractional coefficient N2 + 3 H2 2 NH3 correct 2 N2 + 6 H2 4 NH3 incorrect; not smallest set of whole numbers This step can be saved for last. Remember that if a chemical equation is correctly balanced then multiplying all of the coefficients by the same number results in an equation that is also balanced. There are a few cases where we do sometimes use fractional coefficients to balance chemical equations because of how the reaction is defined, as will be discussed. Example - Combustion of Iso-octane Balance the following unbalanced chemical equation for the combustion of iso-octane, a hydrocarbon used to determine the octane rating of gasoline C8H18 + O2 CO2 + H2O (unbalanced) Example - Combustion of Iso-octane unbalanced C8H18 + O2 CO2 + H2O balance for C C8H18 + O2 8 CO2 + H2O balance for H C8H18 + O2 8 CO2 + 9 H2O balance for O C8H18 + 25/2 O2 8 CO2 + 9 H2O convert to whole numbers (multiply through by 2) 2 C8H18 + 25 O2 16 CO2 + 18 H2O Example - Precipitation of Lead (II) Chloride Precipitation reaction - A reaction where two soluble compounds are mixed and a solid product is formed. Pb(NO3)2(aq) + NaCl(aq) PbCl2(s) + NaNO3(aq) (unbalanced) Example - Precipitation of Lead (II) Chloride unbalanced Pb(NO3)2(aq) + NaCl(aq) PbCl2(s) + NaNO3(aq) balance for Cl Pb(NO3)2(aq) + 2 NaCl(aq) PbCl2(s) + NaNO3(aq) Balancing for Cl has now caused the above reaction to be unbalanced for Na. So we go back and rebalance for Na Pb(NO3)2(aq) + 2 NaCl(aq) PbCl2(s) + 2 NaNO3(aq) The above is now balanced. Remember that when you have a cation or anion group (such as NO3- above) it is often easier to balance equations treating this group as a unit. Types of Chemical Reactions There are literally thousands of different types of chemical reactions. Three common types are: Combination reaction - Two or more reactants form a single product. H2(g) + Cl2(g) 2 HCl(g) Decomposition reaction - A single reactant forms two or more products. CuSO4 • 5 H2O(s) CuSO4(s) + 5 H2O(g) Combustion reaction - Reaction of a single substance with molecular oxygen to form combustion products (CO2(g), H2O()). C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O() Combustion Analysis Combustion analysis is a method often used in the analysis of organic compounds. A sample of the compound is allowed to completely react with oxygen and the mass of carbon dioxide (CO2) and water (H2O) produced is measured. From this information the number of grams and the number of moles of carbon and hydrogen originally in the sample can be found. Combustion Analysis (Hydrocarbons) Combustion analysis can be carried out for hydrocarbons (compounds containing only carbon and hydrogen). This is because all of the carbon originally present in the compound is converted into CO2, and all of the hydrogen is converted into H2O. Example: C8H16 + 12 O2 8 CO2 + 8 H2O By knowing the mass of carbon dioxide and water formed by combustion, the mass and moles of carbon and hydrogen in the original compound can be found. From this, the empirical formula can be determined. Combustion analysis can also be carried out on organic compounds that contain C, H, and O, as the same two combustion products will form as with hydrocarbons. Example: 2 CH3CHO + 5 O2 4 CO2 + 4 H2O Example: A pure hydrocarbon is analyzed by combustion analysis. Combustion of a sample of the hydrocarbon produces 3.65 g CO2 and 1.99 g H2O. What is the empirical formula for the hydrocarbon? Note: M(CO2) = 44.01 g/mol; M(H2O) = 18.02 g/mol. Example: A pure hydrocarbon is analyzed by combustion analysis. Combustion of a sample of the hydrocarbon produces 3.65 g CO2 and 1.99 g H2O. What is the empirical formula for the hydrocarbon? Note: M(CO2) = 44.01 g/mol; M(H2O) = 18.02 g/mol. moles C = 3.65 g CO2 1 mol CO2 1 mol C = 0.0829 mol C 44.01 g CO2 1 mol CO2 moles H = 1.99 g H2O 1 mol H2O 2 mol H = 0.221 mol H 18.02 g H2O 1 mol H2O relative moles C = 0.0829 mol/0.0829 mol = 1.00 relative moles H = 0.221 mol/0.0829 mol = 2.67 To get values close to integers multiply by 3. Empirical formula = C3H8 (3.00) (8.01) Combustion Analysis (C, H, and O Present) For a compound containing carbon, hydrogen, and oxygen, combustion analysis is more complicated. That is because some of the oxygen in the combustion products comes from the compound, and some of the oxygen comes from the O2 used to carry out the combustion. Example: C6H12O6 + 6 O2 6 CO2 + 6 H2O While all of the carbon and hydrogen in the combustion products comes from the original compound, some of the oxygen comes from the molecular oxygen used to carry out the combustion reaction. Combustion Analysis (C, H, and O Present) To do combustion analysis for a compound in this case requires knowing the total mass of compound burned. Since mtotal = mC + mH + mO Then mO = mtotal – (mC + mH) The grams (and moles) of carbon and hydrogen present in the compound are found as before. We can then use the above equation to find the grams and moles of oxygen, and, from that, the empirical formula for the compound. Example: An pure organic compound containing only carbon, hydrogen, and oxygen is analyzed by combustion analysis. Combustion of a 3.650 g sample of the compound produces 6.980 g CO2 and 4.285 g H2O. What is the empirical formula for the hydrocarbon? Note: M(CO2) = 44.01 g/mol; M(H2O) = 18.02 g/mol. Example: An pure organic compound containing only carbon, hydrogen, and oxygen is analyzed by combustion analysis. Combustion of a 3.650 g sample of the compound produces 6.980 g CO2 and 4.285 g H2O. What is the empirical formula for the hydrocarbon? Note: M(CO2) = 44.01 g/mol; M(H2O) = 18.02 g/mol. g C = 6.980 g CO2 12.01 g C = 1.905 g C 44.01 g CO2 g H = 4.285 g H2O 2 (1.01 g H) = 0.480 g H 18.02 g H2O g O = 3.650 g - (1.905 g + 0.480 g) = 1.265 g O mol C 1.905 g C 1 mol C = 0.1586 mol C 12.01 g C mol H = 0.480 g H 1 mol H = 0.475 mol H 1.01 g H mol O = 1.265 g O 1 mol O = 0.07906 mol O 16.00 g O Relative moles C 0.1586 mol/0.07906 mol = 2.006 H 0.475 mol/0.07906 mol = 6.01 O 0.07906 mol/0.07906 mol = 1.000 So the empirical formula is C2H6O. Interpretation of Chemical Equations Consider the equation for the formation of water from hydrogen and oxygen (note that this is both a combustion reaction and a combination reaction). 2 H2(g) + O2(g) 2 H2O() We can interpret this equation in two ways: 1) Two molecules of hydrogen and one molecule of oxygen combine to form two molecules of water. 2) Two moles of hydrogen and one mole of oxygen combine to form two moles of water. Note that the second interpretation makes sense even if we have fractional stoichiometric coefficients. One of the most important uses of a balanced chemical equation is to provide the information needed to convert from the amount of one substance involved in a reaction and the amount of a second substance that is required for the reaction. Example: Consider the following balanced chemical equation 2 H2(g) + O2(g) 2 H2O() If we begin with 4.8 moles of H2, how many moles of O2 will be required for a complete reaction? What is the maximum number of moles of H2O that we will be able to produce? Example: Consider the following balanced chemical equation 2 H2(g) + O2(g) 2 H2O() If we begin with 4.8 moles of H2, how many moles of O2 will be required for a complete reaction? What is the maximum number of moles of H2O that we will be able to produce? mol O2 = 4.8 mol H2 1 mol O2 = 2.4 mol O2 2 mol H2 mol H2O = 4.8 mol H2 2 mol H2O = 4.8 mol H2O 2 mol H2 Example: reaction occurs. When sodium metal is placed in water a violent 2 Na(s) + 2 H2O() 2 NaOH(aq) + H2(g) If we begin with 4.0 moles of sodium metal, how many moles of water are required to completely react with the sodium metal? How many moles of hydrogen gas will form? 2 Na(s) + 2 H2O() 2 NaOH(aq) + H2(g) If we begin with 4.0 moles of sodium metal, how many moles of water are required to completely react with the sodium metal? How many moles of hydrogen gas will form? mol H2O = 4.0 mol Na 2 mol H2O = 4.0 mol H2O 2 mol Na mol H2 = 4.0 mol Na 1 mol H2 = 2.0 mol H2 2 mol Na Calculations Using Mass of Reactants and Products In the laboratory we work with masses of reactants and products. However, as we have seen, chemical equations give relationships among moles of reactants and products. We therefore need a method for going back and forth between moles of substance and grams of substance. We may do this conversion using M, the molecular mass or formula mass of a substance. For example, the molecular mass of ethane (C2H6) is M(C2H6) = 30.07 g/mol This is a conversion factor between mass of C2H6 and moles of C2H6. Strategy For Calculations Using Balanced Chemical Equations We may use the following strategy to find the mass of reactant or product in a chemical equation. 1) Convert from grams of the first substance into moles of the first substance using the molecular mass of the substance. 2) Use the balanced chemical equation to find the correct number of moles of the second substance. 3) Convert from number of moles of the second substance to mass of the second substance using the molecular mass of the substance. Example We can use a balanced chemical equation and our new concept of moles to do calculations involving chemical reactions. Example: Calcium chloride (CaCl2) can be produced by the reaction of hydrochloric acid (HCl) and calcium hydroxide (Ca(OH)2). 2 HCl + Ca(OH)2 CaCl2 + 2 H2O In a particular reaction we begin with 10.00 g HCl. How many grams of Ca(OH)2 are required to completely react with this amount of HCl? How many grams of calcium chloride can be produced by the reaction? calcium chloride 2 HCl + Ca(OH)2 CaCl2 + 2 H2O In a particular reaction we begin with 10.00 g HCl. How many grams of Ca(OH)2 are required to completely react with this amount of HCl? How many grams of calcium chloride can be produced by the reaction? M(HCl) = 36.46 g/mol M(CaCl2) = 110.98 g/mol M(Ca(OH)2) = 74.09 g/mol M(H2O) = 18.02 g/mol 1) Find the moles of HCl. n(HCl) = 10.00 g HCl 1 mol HCl 36.46 g HCl = 0.2743 mol HCl 2) Find the moles Ca(OH)2. n(Ca(OH)2) = 0.2743 mol HCl 1 mol Ca(OH)2 = 0.1371 mol Ca(OH)2 2 mol HCl 3) Find the grams of Ca(OH)2 m(Ca(OH)2) = 0.1371 mol Ca(OH)2 74.09 g Ca(OH)2 = 10.16 g 1 mol Ca(OH)2 We can also find the mass of calcium chloride that can be produced by the reaction m(CaCl2) = 0.2743 mol HCl 1 mol CaCl2 110.98 g CaCl2 2 mol HCl 1 mol CaCl2 = 15.22 g CaCl2 Yields For a Chemical Reaction There are three types of yields we can define for a chemical reaction. Theoretical yield - The maximum amount of product that can be formed from the starting materials used in the reaction. Actual yield - The observed yield for a chemical reaction. Percent yield - The percent of the theoretical yield that is actually obtained. . 100% % yield = actual yield theoretical yield For example, if the amount of CaCl2 actually obtained in the above reaction was 11.46 g, then the percent yield for the reaction would be % yield = 11.46 g . 100% = 75.3 % 15.22 g Limiting Reactant In most chemical reactions several reactants combine to form products. As soon as one of the reactants runs out, the reaction will stop, even if the other reactants are still present. We define the limiting reactant as the reactant the first runs out in a chemical reaction. Note that the theoretical yield of product is determined by the limiting reactant. Example: Consider making sandwiches out of cheese and bread. One sandwich consists of two slides of bread and one slice of cheese. If we begin with 10 slices of bread and 3 slices of cheese, what is the “limiting reactant” and the “theoretical yield” of cheese sandwiches? 2 slices bread + 1 slice cheese 1 cheese sandwich Start with 10 slices bread 3 slices cheese “limiting reactant” = cheese “theoretical yield” = 3 sandwiches “excess reactant” = bread Example: Methane can be formed by reacting carbon and hydrogen. If we begin with 3 carbon atoms and 10 hydrogen molecules, what is the limiting reactant, the excess reactant, and the theoretical yield of methane? The balanced equation is C + 2 H2 CH4 limiting reactant = carbon excess reactant = hydrogen theoretical yield = 3 methane molecules Note this works equally well if done in terms of moles. Finding the Limiting Reactant For a reaction involving two reactants (A and B) the limiting reactant can be found as follows. 1) Find the number of moles of A and B. 2) a) Find the number of moles of product that can be formed from reactant A, assuming it is the limiting reactant b) Find the number of moles of product that can be formed from reactant B, assuming it is the limiting reactant 3) Whichever number of moles of product is smallest corresponds to the limiting reactant. The other reactant is in excess. The smallest number of moles corresponds to the theoretical yield of product. The advantage of the above process is that it works no matter how many reactants there are in the reaction. Example: Consider the following chemical reaction (molecular masses are given below each reactant). C2H2 (26.04 g/mol) + 2 HCl (36.46 g/mol) C2H4Cl2 (98.96 g/mol) In a particular experiment we begin with 10.00 g C2H2 and 20.00 g HCl. What is the limiting reactant and the theoretical yield of C2H4Cl2? C2H2 (26.04 g/mol) + 2 HCl (36.46 g/mol) C2H4Cl2 (98.96 g/mol) In a particular experiment we begin with 10.00 g C2H2 and 20.00 g HCl. What is the limiting reactant and the theoretical yield of C2H4Cl2? moles C2H2 = 10.00 g 1 mol = 0.3840 mol C2H2 26.04 g moles HCl = 20.00 g 1 mol = 0.5485 mol HCl 36.46 g Moles C2H4Cl2 that could be produced if C2H2 is limiting moles C2H4Cl2 = 0.3840 mol C2H2 1 mol C2H4Cl2 = 0.3840 mol 1 mol C2H2 Moles C2H4Cl2 that could be produced in HCl is limiting moles C2H4Cl2 = 0.5485 mol HCl 1 mol C2H4Cl2 = 0.2743 mol 2 mol HCl The smallest number of moles corresponds to HCl. Therefore: HCl is the limiting reactant C2H2 is in excess The theoretical yield of C2H4Cl2 is 0.2743 mol We can now use the molecular mass of C2H4Cl2 to convert the theoretical yield into grams of product. theoretical yield (grams) = 0.2743 mol C2H4Cl2 98.96 g C2H4Cl2 = 27.14 g C2H4Cl2 1 mol C2H4Cl2 End of Chapter 8 “I have lived much of my life among molecules. They are good company.” - George Wald “The most exciting phrase to hear in science, the one that heralds new discoveries, is not Eureka! (I found it!) but rather, ‘hmm.... that's funny.…’” - Isaac Asimov “…it has become hard to find an important problem (in science) that is not already being worked on by crowds of people on several continents.” - Max Perutz “Not everyone learns from books.” - Sheila Rowbothum, Resistance and Revolution