* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download RXN-4-STUDENTS - Rothschild Science
Radical (chemistry) wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Water splitting wikipedia , lookup
Organic chemistry wikipedia , lookup
Process chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Isotopic labeling wikipedia , lookup
Atomic theory wikipedia , lookup
Asymmetric induction wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Hydroformylation wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Electrolysis of water wikipedia , lookup
Marcus theory wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Electrochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Rate equation wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Transition state theory wikipedia , lookup
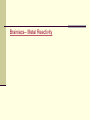
BALANCING EQUATIONS & CHEMICAL REACTIONS Evidence of Chemical Change Changes in Energy (E) Release of E as heat Release of E as light Production of sound Reduction or increase of temperature Absorption or release of electrical Energy Formation of new substances Formation of a gas Formation of a precipitate Change in color Change in odor Conservation of Mass Law of Conservation of Mass In any physical or chemical reaction, mass is neither created nor destroyed; it is conserved! Reactants Products Same number of atoms on both sides of the equation! Balancing Equations In every balanced equation each side of the equation has the same number of atoms of each element Vocab Terms Subscripts – tells how many atoms of each element you have NH3 (one nitrogen, three hydrogen)- DON’T mess with these!! Coefficients – small whole number that appears in front of a chemical formula in an equation – you get to “mess” with these. 2NH3 (Two molecules of ammonia or two moles of ammonia) the arrow means yields or reacts to produce REACTANTS PRODUCTS You have to get the same number of elements on each side of the reaction Balancing Rules 1. 2. 3. 4. Never touch subscripts when balancing equations, you will change the substance Include all sources of the element CH3CHOOH + NaOH Polyatomic ions that appear intact on both sides of the equation, can be balanced as a group (PO4) Coefficients in your balanced equation contain the lowest possible ratio. Balancing! Aluminum + Oxygen Aluminum Oxide Al + O2 Al2O3 Until you get good at this, it is helpful to write the number of atoms of each element on both sides of the equation. It helps you to keep track of the atoms. Balancing! Ethene + Oxygen Carbon Dioxide + water C2H4 + O2 CO2 + H2O Balancing! Hydrogen gas + nitrogen gas ammonia Remember your diatomic molecules!!! Try this one! NaNO3 + CrCl3 NaCl + Cr(NO3)3 Balance this equation… NaNO3 + CrCl3 NaCl + Cr(NO3)3 Write the word equation. Important stuff! Four abbreviations are used to indicate physical states of chemicals: shown as subscripts in the chemical equation (s) = solid (l) = liquid (g) = gas (aq)= aqueous solution (dissolved in water) Symbols over the arrow indicate the conditions of the reaction Heat Pressure Temperature Catalyst Reversible reaction Why does it happen? Use the file to make four hash marks perpendicular to the face of the penny. Carefully drop the penny into the 6M HCl solution. Check out the Pennys! Make some qualitative observations about the pennies. (Look at the dates- remember what you learned at the beginning of the year in the Penny Density Lab) As we proceed: You may want to write these down! 1. What kind of reaction 2. Write and balance the chemical equation. 3. Explain your observations. 4. What kind of quantitative observations would be helpful? Warm UpA sample contains a compound with 3.60 g of carbon and 0.61 g of hydrogen. The molar mass of the compound is 42.09g/mol. Determine the formula of the compound. Remind me to pass back your EMP Formula Packets. I only graded a few- answers are posted on line. Wonder Projects- HONORS ONLY Go to my website REACTIONS Combination Reaction or Synthesis Reaction Two or more simple substances react to form a more complicated one A + B AB Fe + S FeS Combination Reactions We can predict the products of ionic compounds by thinking about the charge! Ga (s) + O2 (g) Cu (s) + S(s) Are there 2 possible products to the 2nd reaction? How would we “say” these equations? Decomposition Reaction A decomposition reaction is the opposite of a composition reaction - a complex molecule breaks down to make simpler ones. AB A + B 2 H2O 2 H2 + O2 Decomposition Reactions Harder to predict the products…always remember your diatomics. Water is often a product! HgO(s) NH4NO3 CaCO3 (Carbonates usually decompose to CO2) Decomposition Reactions HgO(s) 2HgO(s) 2Hg(l) + O2 (g) NH4NO3 NH4NO3 N2O + 2H2O CaCO3 CaO + CO2 The only way to really know is to do the reaction!! Warm Up Predict the products of the following reactions. 1.H2O2 1.Mg + N Warm Up Predict the products of the following reactions. 1.H2O2 We heat the dihydrogen dioxide and the vapor turns out to be water… 2. Mg + N Mg3N2 How are you doing on the Word Equation WS? Single Replacement This is when one element trades places with another element in a compound. A + BC AC + B Mg + 2H2O Single Replacement Reactions Easy to predict the products. Look at the ions… remember that a cation has to bond to an anion!! Don’t forget about diatomics! Zn(s) + H2SO4(aq) Na(s) + H2O OOPS! Did we balance them? Single Replacement Reactions ZnSO4(aq) Zn(s) + Zn(s) + H2SO4(aq) H2SO4(aq) Na(s) + Na(s) + 2Na(s)+ H2O H+OH-(l) 2H+OH-(l) 2NaOH + H2(g) + H2(g) Single Replacement Reactions Reactivity of a metal makes a difference! If a metal is more reactive than the metal it is displacing a rxn will occur. If the metal is less reactive than the metal it is displacing, a rxn will not occur. Metal Reactivity Increases down a group Decreases across a period Brainiacs-- Metal Reactivity Reactivity in Single Displacement Cs Na KMnO4 CsMnO4 Will this occur?? + + + K KMnO4 NaMnO4 + K Will this occur? Double Replacement Reaction This is when the anions and cations of two different molecules switch places, forming two entirely different compounds AB + CD AD + CB Pb(NO3)2 + 2KI PbI2 + 2KNO3 Double Replacement Reactions Two ionic compounds in aqueous solution Generally 3 things happen: 1. A precipitate (solid) occurs Pb(NO3)2 + 2KI PbI2 + 2KNO3 Double Replacement Reactions 2. One product is a gas that bubbles out of the mixture 2NaCN(aq) + H2SO4 2HCN(g) + Na2 SO4(aq) Double Replacement Reactions 3. One product is a molecule while the other products remain ions Ca(OH)2(aq) + 2HCl(aq) Ca+2 + Cl- + 2 H2O(l) Warm Up- Determine the type of reaction and predict the products. 1.Al + Br2 2.FeO + Na 3.SnCl4 4.NaNO3 + CaCO3 Combustion Reactions A combustion reaction is when oxygen combines with another compound or element producing energy. When hydrocarbons (C?H?) combust, water, carbon dioxide and energy are produced. C10H8 + O2 CO2 + H2O + Energy Can you balance it? Combustion of Naphthalene Combustion Reactions Combustion of propane: C3H8 + O2 Combustion of methane: CH4 + O2 Combustion of butene (this one is tricky!) C4H6 + O2 Teacher Demo Extraordinaire! What is a flame video- Science Friday http://www.sciencefriday.com/video/06/08/2012/ what-is-a-flame.html Combustion Analysis Problems… Honors Only So, we can collect the water vapor and the carbon dioxide…. do some calculations…. and determine the empirical formula of the hydrocarbon. C?H? + O2 CO2 + 11.56g H2O 2.36g Start the Types of Reactions Lab 1. Spend about 5 minutes on the Background. 2. Read through the procedure at each station. 3. You need to get at least 3 of the 6 stations finished today. 4. Goggles must be worn at all times. 5. Listen carefully for safety concerns while I intro the labs. Predict the products, balance and classify the following reactions. Li + MgCl2 C6H12 + O2 Ca(NO3)2 + NaOH Warm Up- Friday Balance the following equations: C3H6 + O2 CO2 + H2O C7H14 + O2 CO2 + H2O Finish Types of Rxns Lab What do the equations mean? The coefficients indicate the number of moles of the reactants necessary… to form a certain mole of the product. Mole to Mole Ratio 3K + AlCl3 Al + 3KCl