
Clusters: Structure, Energetics, and Dynamics of Intermediate States
... Mixed clusters were soon studied, and extensive data are now available on the solvation of mixed molecular and protonated complexes, in addition to the aforementioned species.104,105,170 The structure of small gas-phase clusters is influenced by entropy as well as bonding. There are three major cont ...
... Mixed clusters were soon studied, and extensive data are now available on the solvation of mixed molecular and protonated complexes, in addition to the aforementioned species.104,105,170 The structure of small gas-phase clusters is influenced by entropy as well as bonding. There are three major cont ...
Answers to SelectedTextbook Questions
... Covalent forces hold the carbon and hydrogen atoms together within a methane molecule. Weak dispersion forces – a type of intermolecular or non‐bonding force – hold neighboring methane molecules together The CFCs do not have H atoms bonded to C. Such H atoms readily react with hydroxyl radicals. ...
... Covalent forces hold the carbon and hydrogen atoms together within a methane molecule. Weak dispersion forces – a type of intermolecular or non‐bonding force – hold neighboring methane molecules together The CFCs do not have H atoms bonded to C. Such H atoms readily react with hydroxyl radicals. ...
chapter 20 - Chemistry
... The species that can oxidize water to molecular oxygen must have an Ered more positive than 1.23 V. From Table 18.1 of the text we see that only Cl2(g) and MnO4 (aq ) in acid solution can oxidize water to oxygen. ...
... The species that can oxidize water to molecular oxygen must have an Ered more positive than 1.23 V. From Table 18.1 of the text we see that only Cl2(g) and MnO4 (aq ) in acid solution can oxidize water to oxygen. ...
HW 19
... ΔG° = −(1)(96500 J/V⋅mol)(0.37 V) = −36 kJ/mol Next, we can calculate K using Equation (19.5) of the text. ...
... ΔG° = −(1)(96500 J/V⋅mol)(0.37 V) = −36 kJ/mol Next, we can calculate K using Equation (19.5) of the text. ...
- Chemistry
... (a) The intermolecular force of attraction between water molecules and other water molecules or the molecules on the surface of the blade of grass is sufficient to allow water molecules to stick together on the blade of grass. (b) When a piece of paper burns, carbon-carbon and carbon-hydrogen bonds ...
... (a) The intermolecular force of attraction between water molecules and other water molecules or the molecules on the surface of the blade of grass is sufficient to allow water molecules to stick together on the blade of grass. (b) When a piece of paper burns, carbon-carbon and carbon-hydrogen bonds ...
Workshop materials for Class XII
... The materials have been developed by the teachers of the six participating regions: Bhubaneswar, Ranchi, Guwahati, Kolkata, Silchar and Tinsukia under the inspiring leadership of Ms Jayalakshmi Raju, Principal, K V Srikakulam (Bhubaneswar Region) and the dedicated guidance of the three Resource Pers ...
... The materials have been developed by the teachers of the six participating regions: Bhubaneswar, Ranchi, Guwahati, Kolkata, Silchar and Tinsukia under the inspiring leadership of Ms Jayalakshmi Raju, Principal, K V Srikakulam (Bhubaneswar Region) and the dedicated guidance of the three Resource Pers ...
The Reactions of Osmium(VIII) in Hydroxide
... Progress curves indicating the rate of change of the absorbance at 370 nm for various methanol concentrations. The progress curve depicting the reaction of osmium(VIII) with 0 mol/L methanol was superimposed onto the progress curves for those reactions involving varying methanol concentrations. ...
... Progress curves indicating the rate of change of the absorbance at 370 nm for various methanol concentrations. The progress curve depicting the reaction of osmium(VIII) with 0 mol/L methanol was superimposed onto the progress curves for those reactions involving varying methanol concentrations. ...
College Chemistry
... Dimensional calculations are greatly simplified if a consistent set of units is employed. The three major reference dimensions for mechanics are length, mass, and time, but length can be measured in units of inches, feet, centimeters, meters, etc. Which should be used? The scientific community has m ...
... Dimensional calculations are greatly simplified if a consistent set of units is employed. The three major reference dimensions for mechanics are length, mass, and time, but length can be measured in units of inches, feet, centimeters, meters, etc. Which should be used? The scientific community has m ...
Derivatization - Sigma
... determines the bonding and shape, type and strength of intermolecular forces, physical properties, and chemical reactivity of a molecule. Functional groups are less stable than the carbon backbone and are likely to participate in chemical reactions. For example, amine groups decrease compound volati ...
... determines the bonding and shape, type and strength of intermolecular forces, physical properties, and chemical reactivity of a molecule. Functional groups are less stable than the carbon backbone and are likely to participate in chemical reactions. For example, amine groups decrease compound volati ...
Copyright 2010 Scott R
... Na(H3BNMe2BH3), in tetrahydrofuran produces the new complex Th(H3BNMe2BH3)4. The thorium center forms bonds with fifteen hydrogen atoms; accordingly, this is the first example of a fifteen-coordinate atom of any kind. As determined by both single crystal X-ray and single crystal neutron diffraction ...
... Na(H3BNMe2BH3), in tetrahydrofuran produces the new complex Th(H3BNMe2BH3)4. The thorium center forms bonds with fifteen hydrogen atoms; accordingly, this is the first example of a fifteen-coordinate atom of any kind. As determined by both single crystal X-ray and single crystal neutron diffraction ...
© www.CHEMSHEETS.co.uk 17-Jul
... As with other cycles, the sum of the clockwise arrows equals the sum of the anticlockwise arrows. Be careful to ensure that arrow directions and number of moles are correct. ...
... As with other cycles, the sum of the clockwise arrows equals the sum of the anticlockwise arrows. Be careful to ensure that arrow directions and number of moles are correct. ...
Solutions - ChemConnections
... Ka for HF is less than one, while the other hydrogen halide acids have Ka > 1. In terms of ∆GE, HF must have a positive ∆G orxn value, while the other HX acids have ∆G°rxn < 0. The reason for the sign change in the Ka value, between HF versus HCl, HBr, and HI is entropy. ∆S for the dissociation of H ...
... Ka for HF is less than one, while the other hydrogen halide acids have Ka > 1. In terms of ∆GE, HF must have a positive ∆G orxn value, while the other HX acids have ∆G°rxn < 0. The reason for the sign change in the Ka value, between HF versus HCl, HBr, and HI is entropy. ∆S for the dissociation of H ...
Preparation and reactions of some lower tungsten halides and
... foam), resulted from the interference of the tungsten mineral in tin ores in the smelting of tin by slagging or eating up the tin. ...
... foam), resulted from the interference of the tungsten mineral in tin ores in the smelting of tin by slagging or eating up the tin. ...
UNIT 1. SOME BASIC CONCEPTS OF CHEMISTRY Concept
... Ans. Matter can neither be created nor destroyed in the course of a Physical or chemical process although it may change from one form to another. Q4. Which of the following statement about a compound is incorrect?(L2) (I) A molecule of a compound has atom of different elements. (II) A compound canno ...
... Ans. Matter can neither be created nor destroyed in the course of a Physical or chemical process although it may change from one form to another. Q4. Which of the following statement about a compound is incorrect?(L2) (I) A molecule of a compound has atom of different elements. (II) A compound canno ...
Question Bank (Class XI - Chemistry)
... Ans. Matter can neither be created nor destroyed in the course of a Physical or chemical process although it may change from one form to another. Q4. Which of the following statement about a compound is incorrect?(L2) (I) A molecule of a compound has atom of different elements. (II) A compound canno ...
... Ans. Matter can neither be created nor destroyed in the course of a Physical or chemical process although it may change from one form to another. Q4. Which of the following statement about a compound is incorrect?(L2) (I) A molecule of a compound has atom of different elements. (II) A compound canno ...
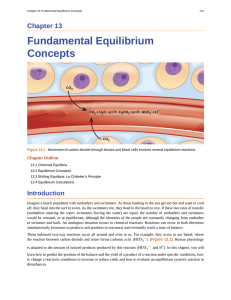
Fundamental Equilibrium Concepts
... The formation of NO2 from N2O4 is a reversible reaction, which is identified by the equilibrium arrow (⇌) . All reactions are reversible, but many reactions, for all practical purposes, proceed in one direction until the reactants are exhausted and will reverse only under certain conditions. Such re ...
... The formation of NO2 from N2O4 is a reversible reaction, which is identified by the equilibrium arrow (⇌) . All reactions are reversible, but many reactions, for all practical purposes, proceed in one direction until the reactants are exhausted and will reverse only under certain conditions. Such re ...
as a PDF
... hope that I have got (most of) them right) but I have tried to write the book with the needs of the teacher in mind, by providing plenty of bond lengths and also spectroscopic data (mainly vibrational, with a little NMR and ESR) that can be used as a teaching tool by hard-pressed lecturers or tutors ...
... hope that I have got (most of) them right) but I have tried to write the book with the needs of the teacher in mind, by providing plenty of bond lengths and also spectroscopic data (mainly vibrational, with a little NMR and ESR) that can be used as a teaching tool by hard-pressed lecturers or tutors ...
File
... molecules are also present. There is an apparent increase in ordering when these ions are placed in water as compared to the separated state. The hydrating water molecules must be in a highly ordered arrangement when surrounding these anions. G = RTlnK = H TS; HX(aq) ⇌ H+(aq) + X(aq) Ka re ...
... molecules are also present. There is an apparent increase in ordering when these ions are placed in water as compared to the separated state. The hydrating water molecules must be in a highly ordered arrangement when surrounding these anions. G = RTlnK = H TS; HX(aq) ⇌ H+(aq) + X(aq) Ka re ...
Chemistry Final Exam Review
... ____ 69. In a compound, the algebraic sum of the oxidation numbers of all atoms equals a. 0. ...
... ____ 69. In a compound, the algebraic sum of the oxidation numbers of all atoms equals a. 0. ...
CHAPTER SIXTEEN SPONTANEITY, ENTROPY, AND FREE
... molecules are also present. There is an apparent increase in ordering when these ions are placed in water as compared to the separated state. The hydrating water molecules must be in a highly ordered arrangement when surrounding these anions. G = RTlnK = H TS; HX(aq) ⇌ H+(aq) + X(aq) Ka re ...
... molecules are also present. There is an apparent increase in ordering when these ions are placed in water as compared to the separated state. The hydrating water molecules must be in a highly ordered arrangement when surrounding these anions. G = RTlnK = H TS; HX(aq) ⇌ H+(aq) + X(aq) Ka re ...
AP Chemistry-midterm review
... ____ 50. What mass of fluoristan, SnF2, would contain the same mass of tin as 306 grams of cassiterite, SnO 2? a. 295 g b. 318 g c. 278 g d. 367 g e. 335 g ____ 51. Heating MgSO4•7H2O at 150 C produces MgSO4•xH2O. If heating 24.4 g of pure MgSO4•7H2O at 150 C were to give 13.7 g of pure MgSO4•xH2O, ...
... ____ 50. What mass of fluoristan, SnF2, would contain the same mass of tin as 306 grams of cassiterite, SnO 2? a. 295 g b. 318 g c. 278 g d. 367 g e. 335 g ____ 51. Heating MgSO4•7H2O at 150 C produces MgSO4•xH2O. If heating 24.4 g of pure MgSO4•7H2O at 150 C were to give 13.7 g of pure MgSO4•xH2O, ...
EVS - RSC - Developments in Microwave Chemistry
... Initially, microwave chemistry was primarily used to carry out analytical processes such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic ...
... Initially, microwave chemistry was primarily used to carry out analytical processes such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic ...
master ap chemistry - NelnetSolutions.com
... ALL RIGHTS RESERVED. No part of this work covered by the copyright herein may be reproduced or used in any form or by any means—graphic, electronic, or mechanical, including photocopying, recording, taping, Web distribution, or information storage and retrieval systems—without the prior written perm ...
... ALL RIGHTS RESERVED. No part of this work covered by the copyright herein may be reproduced or used in any form or by any means—graphic, electronic, or mechanical, including photocopying, recording, taping, Web distribution, or information storage and retrieval systems—without the prior written perm ...
Joanna Kulesza
... Je tiens à exprimer ici la mémoire de mes anciennes amies: Anna Bresińska, Dominika Kozłowska, Anna Budzisz et Justyna Piotrowska. ...
... Je tiens à exprimer ici la mémoire de mes anciennes amies: Anna Bresińska, Dominika Kozłowska, Anna Budzisz et Justyna Piotrowska. ...
1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]
... The problem gives information about the amounts of both starting materials, so this is a limiting reactant situation. We must calculate the number of moles of each species, construct a table of amounts, and use the results to determine the final product mass. Start by determining the balanced net io ...
... The problem gives information about the amounts of both starting materials, so this is a limiting reactant situation. We must calculate the number of moles of each species, construct a table of amounts, and use the results to determine the final product mass. Start by determining the balanced net io ...
Photoredox catalysis
_Schematic.png?width=300)
Photoredox catalysis is a branch of catalysis that harnesses the energy of visible light to accelerate a chemical reaction via a single-electron transfer. This area is named as a combination of ""photo-"" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that otherwise would not react. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes and semiconductors. While each class of materials has advantages, soluble transition-metal complexes are used most often.Study of this branch of catalysis led to the development of new methods to accomplish known and new chemical transformations. One attraction to the area is that photoredox catalysts are often less toxic than other reagents often used to generate free radicals, such as organotin reagents. Furthermore, while photoredox catalysts generate potent redox agents while exposed to light, they are innocuous under ordinary conditions Thus transition-metal complex photoredox catalysts are in some ways more attractive than stoichiometric redox agents such as quinones. The properties of photoredox catalysts can be modified by changing ligands and the metal, reflecting the somewhat modular nature of the catalyst.While photoredox catalysis has most often been applied to generate known reactive intermediates in a novel way, the study of this mode of catalysis led to the discovery of new organic reactions, such as the first direct functionalization of the β-arylation of saturated aldehydes. Although the D3-symmetric transition-metal complexes used in many photoredox-catalyzed reactions are chiral, the use of enantioenriched photoredox catalysts led to low levels of enantioselectivity in a photoredox-catalyzed aryl-aryl coupling reaction, suggesting that the chiral nature of these catalysts is not yet a highly effective means of transmitting stereochemical information in photoredox reactions. However, while synthetically useful levels of enantioselectivity have not been achieved using chiral photoredox catalysts alone, optically-active products have been obtained through the synergistic combination of photoredox catalysis with chiral organocatalysts such as secondary amines and Brønsted acids.






















![1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]](http://s1.studyres.com/store/data/002731518_1-574ec10e88e667508364281b6325aeef-300x300.png)