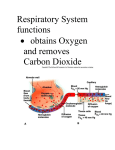
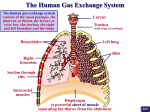
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Caveolae as potential macromolecule trafficking
Tissue engineering wikipedia , lookup
SNARE (protein) wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell growth wikipedia , lookup
Signal transduction wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell membrane wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Advanced Drug Delivery Reviews 49 (2001) 281–300 www.elsevier.com / locate / drugdeliv Caveolae as potential macromolecule trafficking compartments within alveolar epithelium Mark Gumbleton* Pharmaceutical Cell Biology, Welsh School of Pharmacy, Redwood Building, Cardiff University, Cardiff CF10 3 XF, UK Received 11 January 2001; accepted 3 April 2001 Abstract With inhalational delivery the alveolar epithelium appears to be the appropriate lung surface to target for the systemic delivery of macromolecules, such as therapeutic proteins. The existence of a high numerical density of smooth-coated or non-coated plasma membrane vesicles or invaginations within the alveolar epithelial type I cell has long been recognised. The putative function of these vesicles in macromolecule transport remains the focus of research in both pulmonary physiology and pharmaceutical science disciplines. These vesicles, or subpopulations thereof, have been shown to biochemically possess caveolin, a marker protein for caveolae. This review considers the morphometric and biochemical studies that have progressed the characterisation of the vesicle populations within alveolar type I epithelium. Parallel research findings from the endothelial literature have been considered to contrast the state of progress of caveolae research in alveolar epithelium. Speculation is made on a model of caveolae vesicle-mediated transport that may satisfy some of the pulmonary pharmacokinetic data that has been generated for macromolecule absorption. The putative transport function of caveolae within alveolar epithelium is reviewed with respect to in-situ tracer studies conducted within the alveolar airspace. Finally, the functional characterisation of in-vitro alveolar epithelial cell cultures is considered with respect to the role of caveolae in macromolecule transport. A potentially significant role for alveolar caveolae in mediating the alveolar airspace to blood transport of macromolecules cannot be dismissed. Considerable research is required, however, to address this issue in a quantitative manner. A better understanding of the membrane dynamics of caveolae in alveolar epithelium will help resolve the function of these vesicular compartments and may lead to the development of more specific drug targeting approaches for promoting pulmonary drug delivery. 2001 Elsevier Science B.V. All rights reserved. Keywords: Caveolin; Caveolae; Lung; Alveolar epithelium; Transport; Endocytosis and transcytosis Contents 1. Introduction ............................................................................................................................................................................ 2. Caveolae and the structural role of caveolins ............................................................................................................................. 3. Vesicular system in alveolar epithelium..................................................................................................................................... 3.1. Alveolar epithelial–pulmonary capillary barrier.................................................................................................................. 3.2. Membrane vesicles in alveolar epithelium .......................................................................................................................... 3.3. Caveolae and caveolin in alveolar epithelium ..................................................................................................................... *Corresponding author. Tel. / fax: 1 44-29-2087-5449. E-mail address: [email protected] (M. Gumbleton). 0169-409X / 01 / $ – see front matter 2001 Elsevier Science B.V. All rights reserved. PII: S0169-409X( 01 )00142-9 282 282 283 283 285 286 282 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 4. Transport role for caveolae in alveolar epithelium...................................................................................................................... 4.1. Endothelial paradigm ....................................................................................................................................................... 4.2. In-vivo kinetic considerations and vesicular transport ......................................................................................................... 4.3. In-situ tracer studies in alveolar airspace ............................................................................................................................ 4.4. Caveolae and receptor-mediated transport .......................................................................................................................... 5. Caveolae and caveolin in cultured alveolar epithelium ............................................................................................................... 6. Conclusion ............................................................................................................................................................................. References .................................................................................................................................................................................. 1. Introduction The permeability characteristics of the lung and recent advances in inhalational aerosol device technology have led to an increasing interest in exploiting the pulmonary route for the systemic delivery of macromolecule therapeutics, particularly recombinant proteins and polypeptides [1]. The lung deposition and absorption studies of Colthorpe et al. [2,3] elegantly demonstrated that the extents of systemic absorption of insulin or growth hormone following lung administration positively correlate with the depth of deposition of these administered proteins within the lung. Anatomical determinants [4] would also support the view that the lung periphery, and the alveolar epithelium in particular, is the appropriate lung surface to target when aiming to systemically deliver macromolecules. Some of these anatomical determinants would include: location of the alveolar surface beyond the clearance mechanisms of the mucociliary escalator; the large alveolar surface area potentially available for absorption; the high blood flow to the alveolar region, and the thin cellular barrier from airspace to capillary blood presented by the alveolar epithelial and pulmonary capillary cells. Further, in the transport of macromolecules across the pulmonary alveolar epithelial–capillary endothelial barrier, evidence indicates that it is the alveolar epithelium that possesses a more restrictive paracellular pathway than that provided by the capillary endothelium [5]. As a corollary the mechanisms of transport of macromolecules within alveolar epithelium are the subject of genuine interest [6], and in particular the nature and extent of any vesicular trafficking mechanism(s) such as that potentially provided by caveolae. 2. Caveolae and the structural role of caveolins At the electron-microscopic level caveolae are 287 288 289 292 295 295 297 297 most frequently observed as ‘‘smooth coated’’ or ‘‘non-coated’’ omega-shaped invaginations (diameter of 50–100 nm at the widest point) connected to the plasmalemma or plasma membrane by a neck-like structure which affords spatial continuity with the extracellular environment (Fig. 1a). At least in endothelial cells caveolae-like vesicles may also be observed as fused lines or clusters of vesicles at the plasmalemma [7]. The term ‘‘smooth coated’’ or ‘‘non-coated’’ vesicles has long been used to contrast them with the electron-dense cytoplasmic coat that can be seen associated with clathrin-coated pits when viewed under the electron microscope. However, it is probable that, both within a single cell and between cell types, that various subpopulations of smoothcoated or non-coated vesicles exist, and not all will be caveolae as defined by the presence of the marker protein caveolin (see later). Caveolae or caveolae-like structures are recognised as prominent morphological features in a variety of cell types, notably adipocytes, muscle cells (skeletal, cardiac and smooth), fibroblasts, capillary endothelium and type I alveolar epithelial cells, although to varying extents many other cell types may possess these morphological structures. A principal component constituting the striated coat of caveolae, and a critical structural and functional element of caveolae, is the cytoplasmically orientated integral membrane protein, caveolin [8]. As a biochemical marker caveolin has provided for an additional definition for caveolae beyond that morphological identification alone, i.e. caveolae as flattened caveolin-rich membrane microdomains morphologically indistinguishable from the plasmalemma itself (Fig. 1b). Caveolin comprises a family of proteins the most studied of which is caveolin-1. Caveolin-1 appears to be a critical, but not necessarily the sole, determining factor in caveolae formation in non-muscle cells [9–12], with the structural unit for the protein within the plasma membrane in the form of high molecular M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 283 Fig. 1. (a) Electron micrograph of a ‘flask-shaped’ caveolae invagination (CV) of diameter approximately 80 nm located within the apical plasmalemma (PL). The invagination lacks an electron dense cytoplasmic coat characteristic of a clathrin coated pit. At the neck of the caveolae can be seen a membraneous diaphragm (D) restricting the caveolae opening to 20–40 nm. (b) A schematic representation of putative caveolae dynamics. Flattened caveolin-rich domains (A) may exist in the plasma membrane that steadily increase in curvature (B ⇒ C) following appropriate physiological stimuli. The resultant invagination may remain attached to the plasma membrane or possibly completely close and detach to form a discrete intracellular vesicle. weight caveolin oligomers [13–15]. The relationship between caveolin-1 expression and caveolae biogenesis appears to require a threshold level of caveolin expression for the formation of caveolae [10,16], such that caveolin expression alone does not necessarily imply the presence of caveolae within a cell. 3. Vesicular system in alveolar epithelium 3.1. Alveolar epithelial–pulmonary capillary barrier The lower respiratory tract consists of the respiratory bronchioles, the alveolar ducts, and the alveoli themselves which represent the main location for gaseous exchange. Alveolar epithelium is predominantly comprised of two cell types, the terminally differentiated squamous alveolar epithelial type I (ATI) cell which constitutes approximately 93% of the alveolar epithelial surface area (33% of alveolar epithelial cells by number) and the surfactant producing cuboidal alveolar epithelial type II (ATII) cell comprising the remaining 7% by surface area and 67% by epithelial cell number [17]. The total alveolar epithelial surface area within an average adult human lung is estimated to be 100–120 m 2 , although potentially not all of this relatively large surface is likely to be concurrently available for the absorption of inhaled drug. The alveolar epithelial–pulmonary capillary bar- 284 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 rier comprises the alveolar epithelium and pulmonary capillary endothelium. In parts of the barrier the basal membranes of the epithelial and endothelial cells are directly in contact while in other parts they separated by interstitium (Fig. 2c). Morphometric reports [17] indicate that the human ATI cell has an abluminal or interstitial membrane surface area averaging 5098 m2 , with an average cell thickness of 0.36 m ranging from 2 to 3 m in the perinuclear region of the cell to approximately 0.2 m in the peripheral attenuated regions of the cell. Characteristically the ATI cell displays only sparse cellular organelles with the majority that are present, located in the cell’s perinuclear region. Some of these morphometric features of the ATI cell exemplify the ‘favourable’ anatomical determinants that have driven interest in the alveolar type I epithelium as a barrier across which to deliver systemically active proteins and peptides. In constrast, the cuboidal ATII cell is considerably smaller than the ATI cell (e.g. basal surface area averaging 183 m2 and a uniform cell thickness of about 5 m [17]) and is richly endowed with organelles and microvilli on it’s apical membrane. Current evidence would support a role for the ATII cell serving as the sole in-vivo progenitor for, and differentiating into, the terminally differentiated ATI cell (reviewed in [18]). The pulmonary capillary endothelial cell possesses a very similar thin attenuated squamous morphology to the ATI cell, although it’s cell surface area is reported to be up to three to four times smaller, e.g. luminal surface area averaging 1353 m2 [17]. 285 3.2. Membrane vesicles in alveolar epithelium The existence of a high numerical density of membrane vesicles within the alveolar epithelial type I cell has long been recognised. Morphometric data [19–22] obtained in the early 1980s provided important information on the vesicle populations within the in-vivo ATI cell. However, while the vesicle populations studied in these early investigations encompassed to a high extent smooth- or non-coated vesicle populations whose morphology is consistent with caveolae structures, ultrastructural appearance alone may not be sufficient to functionally define them as caveolae; the latter necessitating at least structural association of the vesicle membrane with caveolin protein. Therefore in the following description (below) of these early studies care has been taken to avoid defining the vesicles explicitly as caveolae. The studies of Gil and co-workers [19,20] described the number and distribution of plasmalemmal vesicles or invaginations within the ATI cell and pulmonary capillary endothelial cell of rabbit lung. These workers did not distinguish between different types of vesicles but described the presence of high numbers of non-coated vesicles or invaginations possessing an average diameter of 70 nm, and in many cases retaining a neck-like connection to the plasmalemma. The investigators did not count vesicles that appeared as free discrete entities within the cytoplasm of the cell with no apparent connection to either plasmalemmal surface. For the ATI cell they reported the presence of 150 Fig. 2. (a) A gallery of 19 optical images taken at steps of 0.46 m through a paraffin section of rat lung tissue using confocal laser scanning microscopy (CLSM). The rat lung tissue was immunostained with anti-caveolin-1 antibody and immunocolloidal gold. The colloidal gold was visually enhanced by silver development. The very reflective particles of dense gold / silver caveolin-1 immunostain are shown in black. The images are shown in the grid in order left to right, top to bottom, starting from the uppermost surface of the paraffin section and moving through to the bottom of the section. Caveolin-1 staining can be seen along the surfaces of the capillary endothelium and alveolar epithelium. (As – alveolar airspace; C – capillary lumen). (b) A red / green 3-dimensional reconstruction of the 19 optical sections shown in (2a) above. A transmission image of the bright-field view of the original section has been inverted and ghosted over the 3-dimensional reconstruction to provide a background of lung architecture. The nuclei of the cells are shown overlaid in light grey. The caveolin-1 immunostaining is shown as red / green pairs. The image when viewed through red / green stereo glasses shows the 3-dimensional structure of the original 10-m thick paraffin section with the profile of caveolin-1 stain. (c) and (d) Transmission electron micrographs of resin-embedded lung tissue (As – alveolar airspace; C – capillary lumen; S – surfactant). (c) Araldite thin section of tissue postfixed in osmium showing the alveolar–pulmonary capillary barrier in rat lung. Micrograph shows flask-shaped plasmalemmal invaginations or vesicles in both capillary endothelium (right-hand surface) and alveolar type I epithelium (left-hand surface); (d) LR White thin section (osmium omitted to retain antigenicity) immunolabelled for caveolin-1. An attenuated region similar to that in (c) shows anti-caveolin-1 colloidal-gold particles associated with plasmalemmal invaginations in both the alveolar epithelial and capillary endothelial cells. The epithelial surface is identified by the presence of surfactant (S). 286 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 vesicles per m2 of luminal or airspace membrane surface area, and the reproducible presence of a significantly higher vesicle load (230 vesicles per m2 cell membrane) at the basal or interstitial membrane surface. They also determined a vesicle density in the ATI cell of 145 per m3 cell volume and calculated that approximately 70% of the total plasmalemma surface area of the ATI cell was located within the membranes of the non-coated plasmalemmal invaginations. Between species the dimensions of the ATI cell appear similar [17,23], and based upon a conservative estimate of ATI cell surface area for the rabbit, the data of Gil et al. [19,20] would equate to approximately 600,000 plasmalemmal vesicles or invaginations on the luminal membrane of the ATI cell, and in excess of 900,000 on the abluminal membrane surface; statistics close to that calculated by the original investigator [20]. Morphometric studies in dogs by DeFouw and colleagues [21,22] have similarly reported high vesicle densities (vesicle diameters 48–60 nm) in the ATI cell with the number of vesicles per m3 cell volume reported to average 227–291. In the work of DeFouw [21], however, a significant differential distribution of vesicles between the luminal and interstitial membrane surfaces in the ATI cell was not observed. At first consideration the above statistics for the ATI cell may appear extreme. Nevertheless, high numbers of non-coated vesicles or invaginations morphologically conforming to caveolae are a consistent feature within endothelial microvascular cells. Reports from a number of laboratories [22,24–26] each analysing different tissue beds, indicates the numerical density of such vesicles in capillary endothelial cells to range from 150 to 600 per m3 cell volume. Indeed in the above lung morphometric studies of Gil and coworkers [19,20], data for the rabbit pulmonary capillary endothelial cell was also presented, with vesicle numbers reported at 131 per m3 endothelial cell volume, and vesicle loads upon each membrane of 196 per m2 for the luminal surface and 181 per m2 for the abluminal surface. For the smaller endothelial cell the statistics of Gil and coworkers [19,20] would equate to approximately 200,000 plasmalemmal vesicles or invaginations on each of the luminal and abluminal pulmonary capillary endothelial cell membranes. Clathrin-coated membrane pits or invaginations are generally readily characterised at the morphologi- cal level. In none of the early lung alveolar morphometric papers described above were micrographs presented, or data discussed, pertaining to the presence of electron-dense-coated pits within the in-vivo ATI cell. Atwal and coworkers in their electron tracer studies [27] in goat lung alluded to the lack of clathrin-coated pits in alveolar type I epithelium. Further, in our own experience of the electron-microscopic analysis of rat and human alveolar type I epithelium we have failed to identify membrane structures within the ATI cell conforming to the morphological characteristics of clathrin-coated pits. However, an isolated immunoelectron microscopy report in 1989 [28] localised clathrin light and heavy chains to the smooth coated vesicles seen in the type I alveolar epithelial cell. These workers did not report the morphological presence of clathrin coated pits in these cells but hypothesised that some components that participate in clathrin-coated pits may also be involved in the formation of other vesicle types. This work has not been further substantiated. The alveolar type I epithelium therefore appears to parallel the endothelial microvasculature in that the majority (but not exclusively all) of the vesicles present in the cell are the smaller non-coated or smooth-coated vesicle populations that morphologically are recognised as caveolae. 3.3. Caveolae and caveolin in alveolar epithelium With the discovery of caveolin protein, ultrastructural appearance alone is no longer sufficient to functionally define smooth-coated or non-coated plasmalemmal vesicles as caveolae. In 1994 Lisanti et al. [29] described an association between caveolin protein expression and the lung. They isolated caveolin rich domains from whole lung homogenates by the use of sucrose density gradients. Based upon ultrastructural evidence and theoretical calculations they concluded that 80% of the caveolin signal generated by Western blot analysis is contributed by the ATI cell. However, given the range of cell types that display caveolae in lung periphery (e.g. capillary endothelial, fibroblast and alveolar type I cells) coupled with the architectural complexity and structural diversity of the lung tissue, the calculation is tenuous. The work of Kasper et al. in 1998 [30] was the M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 first full publication to spatially localise caveolin to alveolar type I epithelium. Using double immunofluorescence with both frozen and paraffin wax sections these workers reported the localisation of caveolin-1 in alveolar type I epithelium of rats and mini-pigs. In these studies the type II alveolar epithelial cells were found to be devoid of caveolin-1 staining. Following X-ray irradiation of these animals to induce lung injury, with subsequent initiation of lung fibrogenesis, Kasper and colleagues noted a dramatic loss of caveolin-1 expression in the alveolar epithelium but increases in expression of caveolin-1 in the pulmonary capillary endothelium. They proposed that caveolin-1 may serve as an early indicator of subcellular alteration during the initial stages of lung fibrosis. In 1999 Newman et al. [31] undertook an immunocytochemical study for caveolin in the alveolar epithelial–pulmonary capillary barrier of rat lung. At the light microscopic level they used a combination of bright-field and confocal laser scanning microscopy spatially localise in a 3-dimensional manner caveolin-1 immunomarker to alveolar epithelial and pulmonary capillary surfaces of lung tissue (Fig. 2a and b). At the electron microscopic level they reported observing a greater number of caveolae-like structures in the capillary endothelium compared to that seen in the ATI epithelium (Fig. 2c), although no quantitative morphometric analyses were undertaken. These workers also noted the absence of caveolae-like structures in the ATII cell. At the immunoelectron microscopic level, however, specific low-level labelling of the ATII cell for caveolin-1 was observed. Both the ATI epithelium and pulmonary capillary endothelium were specifically labelled with anti-caveolin-1 immunogold particles, with immunogold particle frequency generally greater in the endothelium than epithelium. In both cell types plasmalemmal invaginations could be observed decorated with immunogold label, although not all invaginations were labelled in such a manner (Fig. 2d). This labelling of some, but not all plasmalemmal vesicles (despite their morphological similarity) was considered to reflect either a true biochemical heterogeneity in the smooth-coated vesicle populations within the ATI cell, or an antigen threshold requirement for caveolin-1 coupled with a variability in the level of this protein (or of epitope access) between caveolae structures. 287 The biochemical confirmation that the plasmalemmal invaginations or vesicles in the in vivo ATI cell are caveolae has a number of implications. From a pharmaceutical perspective it provides a biological framework for studies addressing the mechanistic role caveolae vesicles may fulfil in the trafficking of therapeutic macromolecules across the alveolar– capillary barrier, and indeed the development of targeting strategies that could exploit caveolae membrane domains for receptor mediated transcytosis (see article by Jan Schnitzer in this series). From a more fundamental basis it should provide insights into the potential regulation of endogenous solute trafficking and cell signal regulation within the alveolar region. The work of Newman et al. [31] and Kasper et al. [30] has provided some initial characterisation of ATI cell plasmalemma vesicles upon which their transport role can be further studied with reference to established functions of caveolae in other cell types. 4. Transport role for caveolae in alveolar epithelium While the morphometric data for the ATI cell vesicle populations is intriguing in terms of their putative function as endocytic or transcytotic compartments, direct evidence for their role in transport within alveolar epithelium is at present extremely limited. Certainly, the functional characterisation of ATI vesicle populations lags considerably behind the progress made in determining a transport role for caveolae in endothelium. However, until comparatively recently the role of endothelial caveolae in vesicular trafficking events was itself much debated, due in part to the lack of recognised inhibitors specific for caveolae mediated pathways, the lack of ligands specifically targeting caveolae membranes, and also a paucity in knowledge of the underlying mechanisms modulating caveolae membrane vesicle dynamics. A fuller discussion on the controversy relating to the role of caveolae in endothelial transport is provided in Jan Schnitzer’s article within this series. However, to constrast the status of caveolae transport research in alveolar epithelium to progress that has been made in the endothelial literature, a brief overview of the latter is provided below. 288 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 4.1. Endothelial paradigm Ultrastructural studies by Bungaard et al. [32,33] and Frøkjaer-Jensen et al. [34,35] undertaking ultrathin serial sectioning with reconstruction revealed endothelial caveolae to be organised as fused lines or clusters of vesicles continuous with either the luminal or abluminal plasmalemma. They found a paucity of free discrete vesicles in the cytoplasm, and argued as a result that caveolae are merely static invaginations of the cell surface and not dynamic structures capable of mediating endocytosis or transcytosis. This work raised the level of debate about the trafficking function of endothelial caveolae, but did not provide absolute evidence against such a role, either via the vectorial shuttling of single discrete vesicles carrying solute from one plasma membrane surface to another, or with solute transfer mediated via transient interconnections or fusions occurring between lines or clusters of adjacent vesicles as described by Charles Michel [7,36]. During recent years a growing body of experimental data has led to the general consensus that the endothelial vesicle system can mediate the transendothelial transport of macromolecules. Of particular note in this context is the work from George Palade’s laboratory [37–39] and that from the laboratory of Jan Schnitzer [40–45]. The use of electron dense tracer probes to localise macromolecules to plasma membrane vesicles such as caveolae, and deduce from this a functional transport role for the vesicles is impaired by a number of factors. For example, artifacts may be introduced during processing of the tissue for microscopic analysis, or by failing to account for the three-dimensional structure of a cell’s tubulo-vesicular system. Work by Predescu and Palade during the 1990s [37–39], however, exploited a combination of labelled tracer probes, quantitative permeability investigations, and application of vesicular transport inhibitors to identify that caveolae could serve as the structural equivalents of both small and large pores in continuous microvascular endothelium. For intact continuous microvascular endothelium theoretical pore models generally predict the presence of a small pore population (diameter 10 nm; | 18 units / mm 2 ) and a large pore population (diameter # 50 nm) of much lower numerical density. The above series of in-situ studies by Palade and coworkers demonstrated the transcytosis of labelled albumin and orosomucoid (tracers that qualify as large and small pore probes, respectively) via endothelial caveolae. They hypothesised that caveolae could act as large pores when fully opened, and as small pores when the neck of the plasma membrane invagination is constricted to less than 10 nm in diameter. Conversely, caveolae could fulfil such structural equivalents through the presence of functionally distinct caveolae subpopulations with a cell. Using both cultured endothelial monolayers and an in-situ model of rat lung pulmonary endothelial microvasculature, Schnitzer and co-workers [40–42] demonstrated a role for caveolae-mediated transport in the endothelial permeability to albumin. A specific binding protein (originally known as gp-60 and renamed albondin) has been identified on endothelial surfaces, and appears to mediate the transcytosis of native albumin, while other endothelial surface binding proteins (gp-18 and gp-30) appear to mediate the endocytosis of modified albumins [42]. Schnitzer and coworkers [40] used the sterol binding agent, filipin, to disassemble caveolae invaginations leading to a reduction in their surface density to less than 15% of control, but without effect on the structural integrity of coated pits. Correspondingly, filipin inhibited, in a concentration-dependent manner up to 60% of the transendothelial transport of native albumin across both in-vitro cultured endothelial monolayers and in-situ rat lung capillaries. Fillipin treatment exhibited no effect upon paracellular transport pathways as indicated by a lack of effect upon inulin permeability, or indeed upon the transport of a 2 macroglobulin, a substrate internalised via clathrincoated pits. Further, these workers showed [41] Nethylmaleimide (NEM), an inhibitor of NEM-sensitive factor (NSF)1 to reduce the endothelial transport 1 NSF is a key component in a group of proteins collectively termed the SNARE complex [46] involved in the subcellular trafficking and membrane fusion of vesicles. Certain components of the SNARE machinery, namely SNAP-25 and syntaxin, reside on the target membrane whilst other components reside in the membrane of the free cytoplasmic vesicles (vesicle associated membrane protein-VAMP). Other components, NSF and soluble NSF attachment protein (a-SNAP) represent two soluble cytosolic proteins that mediate the docking of free vesicles to target membranes. M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 of albumin species, and therefore demonstrated an association between the SNARE trafficking complex and a transport role for caveolae in endothelial cells. Similarly, they examined the direct involvement of certain SNARE components in the intracellular endothelial trafficking of caveolae by selectively inhibiting the internalisation of cholera toxin subunit B (a putative caveolar transport marker) through the functional disruption of vesicle associated membrane protein-2 (VAMP-2) [44]. Extending these studies Schnitzer and co-workers [43] isolated caveolae membranes from pulmonary microvascular endothelium, and revealed that the caveolae membrane preparations contained several key components of the SNARE complex as well as members of the annexin family (II and IV) and heterotrimeric GTPbinding proteins, which are both believed to influence plasma membrane dynamics. More recently the same workers [45] have co-localised dynamin, a member of a multigene family of large GTPases, to the neck of endothelial caveolae and shown its functional involvement in severing the caveolae invagination from the plasma membrane to form transport vesicles. As mentioned above, the SNARE protein, VAMP2, has been functionally localised to caveolar membranes of rat lung microvascular endothelium [44]. The same paper reported on the level of VAMP-2 specific colloidal gold label associated with the alveolar type I epithelial cells within intact lung tissue. It was noted that VAMP-2 expression was evident in the ATI cell although the immunostaining in the alveolar cell was significantly lower than that in the capillary endothelial cell, even when differences in caveolae density between the two cell types are taken into consideration. This data would not exclude caveolae within the ATI cell from being dynamic entities able to detach from a plasma membrane location, as the caveolae (or subpopulations thereof) within alveolar type I epithelium (if they utilise the SNARE complex) may have a reduced requirement for VAMP-2, or functionally utilise other distinct VAMP related molecules. The functional characterisation of endothelial caveolae has provided a framework of knowledge and study design to allow for the rational characterisation of caveolae function within alveolar epithelium. Fundamental to an improved understanding 289 of this function will be our ability to address questions such as: ‘‘Do caveolae in alveolar epithelium detach from the plasmalemma?’’ ‘‘What role does the SNARE trafficking complex, or other vesicular membrane components, fulfil in the movement of alveolar epithelial caveolae?’’, ‘‘What contribution do microtubule-dependent versus microtubule-independent mechanisms play in caveolae detachment and trafficking in the thin attenuated regions of the alveolar type I cell?’’ ‘‘What regulates the polarised trafficking of caveolae versus their recycling to the original plasma membrane?’’. These are but a few of the questions that need to be experimentally addressed. However, it should be acknowledged that the in-vivo and in-vitro experimental models exploited in studies of alveolar epithelium are less amenable to investigation than endothelial models. For example, this is exemplified by the difficulty in animal models of achieving reproducible and quantifiable solute access to the luminal alveolar epithelial surface, and by the lack of success so far in being able to isolate and culture alveolar type I epithelial cells (see later). 4.2. In-vivo kinetic considerations and vesicular transport Tracer experiments that attempt to spatially localise solute to morphologically defined structures are frequently considered in studies addressing the role that vesicular transport may serve in the permeability of alveolar epithelium. An implicit need in much of the research upon alveolar epithelial permeability is, however, the required resolution of mechanistic information with quantitative transport data, and vice versa. The recognition of an inverse correlation between solute molecular size and the rate of transport or absorption across lung epithelium is substantiated for a range of molecule classes (including peptides and proteins), and by a number of different laboratories (reviewed in [6]). In many studies, however, the results are not unequivocally derived from permeability data for alveolar epithelium alone, or do not involve investigation of solutes across a particularly wide range of molecular weight (MW). Nevertheless, evidence for an inverse molecular weight dependency in systemic absorption from the 290 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 lung is substantial, and the contributions in the physiological literature from Lewis Shanker [47], and Taylor and Garr [48] are perhaps the most widely known. Theodore et al. [49] reported the transalveolar transport of sucrose (MW 342 Da), inulin (5 kDa) and dextran (MW 60–90 kDa) following direct administration (via catheter) to the terminal airways of dogs. These workers observed alveolar permeability to decrease with increasing solute molecular weight, and the kinetics of absorption to be consistent with a first-order process. Effros and Mason [50] compiled data from a number of published in-vivo pulmonary permeability investigations which utilised a range of animal species, involved various hydrophillic solutes of MW up to 500 kDa, and which were performed using different solute administration techniques into the lung. Despite these experimental variations the analysis by Effros and Mason clearly demonstrated solute clearance from the lung to decrease with increasing molecular weight. Within the pharmaceutical sciences Taylor and Farr and co-workers [51,2,3] have generated data from studies aerosolising into rabbit lung polypeptides and proteins of pharmaceutical interest including, oxytocin (MW 1007 Da), insulin (MW 5.7 kDa), human growth hormone (MW 22 kDa). Their data showed an inverse relationship between MW and the rate of pulmonary absorption, where the latter was determined not from ‘time to maximal plasma concentration’ (Tmax), but more appropriately from pharmacokinetic calculation of apparent absorption rate constants (ka), which were 6.16, 0.678, and 0.12 h – 1 , respectively. Indeed an important feature in the comparison of pulmonary kinetic data between solutes and between different studies, is that such comparison is based upon permeability coefficients or absorption rate constants such that differences in dosing rates or solute clearances are accounted for. The simplistic interpretation of the kinetic data from the above studies would support that the major mechanism for protein or macromolecule transport across pulmonary epithelium involves a first-order diffusional process. Upon initial consideration, this type of kinetics may not appear to conform to a mechanism of macromolecule transport via a transcytotic pathway mediated by ATI caveolae-like vesicles. Clearly, even if this latter interpretation were correct then it would represent a generalisation only, and not exclude the possibility of a significant role for the receptor-mediated transcytotic transport of certain individual macromolecules. In the following discussion some consideration has been given to the question ‘‘Can the kinetic data for pulmonary drug absorption be resolved with a vesicle-mediated pathway of transport ? ’’ In addition to specific receptor-mediated transcytosis, macromolecules may also be captured within membrane vesicles via a non-specific adsorptive membrane binding process, or via simple internalisation as part of vesicle-mediated fluid uptake. In the case of both adsorptive and fluid-phase vesicular capture, the vectorial shuttling of individual discrete caveolae vesicles transcytosing solute from one plasma membrane surface to the opposing membrane would not appear to fulfil the above observed molecular weight dependence in solute permeability of alveolar epithelium. This can be argued on the basis that fluid-phase uptake while able to display concentration-dependent kinetics, would not show discrimination between solutes based upon molecular size. Similarly, under the condition that non-specific adsorptive solute binding to vesicle membrane does not display saturation, then an adsorptive process would likewise display a concentration-dependence in solute transport but no molecular weight dependence. Although in the case of the latter process it could be envisaged if saturable binding conditions prevailed, and where the binding capacity of the caveolae membranes display differential molar binding capacities between solutes of low and high molecular size, that some molecular size discrimination may well be evident. However, under this condition the transport kinetics would approximate to a concentration-independent, zero-order, process. If the aim were to resolve the kinetics described above with a vesicular mechanism of pulmonary transport then an alternative form of vesicle-mediated transfer needs to be considered beyond that involving the vectorial shuttling of discrete membrane compartments from one plasma membrane surface to another. Once again the discussion is considered with respect to adsorptive or fluid-phase capture of solute. The numerical density of the caveolae-like vesicles in the ATI cell is high, and in the thin attenuated M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 regions of the cell the luminal and abluminal membrane surfaces are closely opposed, separated by 100–300 nm of cytoplasm. Under these circumstances frequent interactions between vesicles may be expected (driven potentially through the forces of Brownian Motion [52,53]) leading to transient interconnections or fusions between adjacent vesicles. Without giving rise to a complete or continuous transepithelial channel or pore, which would allow for convective transfer and be detrimental to alveolar fluid homeostasis, such transient vesicle interconnections or fusions would provide for a series of discontinuous fluid pathways allowing the diffusion of solute (Fig. 3). This fluid pathway would confer an approximate first-order process on solute transfer, would display MW dependence, and be mediated via a vesicular mechanism, albeit one that does not rely on the vectorial shuttling of individual discrete vesicles from luminal to abluminal plasmalemmal surfaces. An additional condition that would need to be satisfied is that the frequency of interaction and fusion between vesicles forming the ‘diffusion pathway’ is not rate-limiting in terms of solute transfer, i.e. the rate of transcellular passage of solutes 291 remains proportional to their relative diffusion coefficients. It is evident, however, that certain kinetic features of pulmonary absorption may not readily be explained by any vesicular model. In particular, the inhalational volume-induced increases in the rate of pulmonary solute absorption reported in humans, not only for small molecules such as DTPA [64] and nedocromil [65], but also for insulin [66]. These volume-induced changes in solute absorption do not have to be the result of extreme ventilation conditions such that epithelial membrane damage occurs, e.g. in the insulin report [66] the high volume inspiratory manoeuvres were limited by the lung vital capacity (averaged 4.1 L) and the low volume manoeuvre controlled by the inhaler device at 2.2 L. However, the exact mechanism underlying this volume-induced phenomenon is still to be resolved but one hypothesis [66] suggests that increased alveolar expansion leads to changes in paracellular permeability as a result of epithelial stretching and transient disruption of the tight-junctional complexes between alveolar cells. Only at very high lung distending volumes, as used in animal experimenta- Fig. 3. A speculative scheme showing how a vesicular transport system may operate in the alveolar epithelium which may afford the pulmonary absorption kinetics for macromolecules to display both a molecular weight dependence and concentration-dependence absorption rate. Frequent interactions between vesicles leading to transient interconnections or fusions between adjacent vesicles could provide for a series of discontinuous fluid pathways allowing the diffusion of solute. Solute deposited in alveolar airspace is shown as closed black circles. See text for more details. 292 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 tion [67–69], does the alveolar epithelium show expansion-mediated permeability to large proteins. It must also be reemphasised that a common mechanistic interpretation of the kinetics of pulmonary absorption across a broad range of solute physicochemical characteristics may obscure the underlying presence of multiple transport pathways, e.g. for insulin the paracellular pathway may predominate, whereas a molecular-weight dependence in pulmonary transport across a higher range of molecular weight, e.g. FITC-dextrans of 10–150 kDa [70], may involve a vesicular mechanism. Finally, some evidence exists [71] indicating that caveolae fulfil a role in the rapid transduction of mechanical signals (flowinduced) in vascular endothelium. How caveolae vesicles within alveolar epithelial cells respond to a mechanically-induced stretch stimuli remains to be examined. Given the morphometric information on the densities of caveolae-like vesicles in the alveolar epithelial type I cell it is interesting to consider some calculations on the potential capacity of this vesicular system for fluid capture from the alveolar airspace: a single vesicle represented by a sphere of diameter 70 nm would occupy a volume of 1.8 3 10 24 m3 or 1.79 3 10 213 ml. Morphometric data indicates a density of approximately 150 vesicles per m2 of luminal ATI surface area, or a total of 375 3 10 13 vesicles for an alveolar luminal surface area of 25 m 2 — a perhaps not unrealistic alveolar epithelial drug deposition area that could be achieved following drug delivery with advanced inhaler technology. If it is assumed that for any unit time 5% of the vesicles (19 3 10 13 vesicles) have detached from the luminal membranes of ATI epithelium, then the calculated fluid capture for a 25 m 2 area would approximate to 35 ml per unit time. To put this into perspective a reasonable estimate of the steady-state alveolar fluid volume for a 25 m 2 area would be 2–5 ml [72]. The issue of speculating upon the frequency of vesicle detachment from the ATI luminal membrane (i.e. quantifying ‘unit time’ in the above calculation) is, more complicated. Nevertheless, of interest in this context are predictions [52–55] based upon combined morphometric and transport data, or those derived from theoretical modelling, that have generated average times (ranging from , 10 s to 5 min) for a caveolae vesicle to traverse a capillary endothelial cell. If the membrane vesicle system is maintained at steady-state (i.e. area of vesicle membrane detaching from a plasmalemmal surface is balanced by the area of vesicle membrane joining) then these transit times would also provide a timeframe for the potential frequency of plasma membrane vesicle detachment, at least in endothelium. Clearly the above calculations are based on assumptions not addressed by experimental data within the alveolar epithelium itself, not least that the ATI vesicles in the in-vivo cell are able to detach from the luminal plasmalemma. A considerable research effort is required to provide a clear understanding of the role of caveolae in pulmonary macromolecule transport. This will involve well designed pulmonary pharmacokinetic studies as well as combined cell biology and permeability investigations in appropriate in-vitro alveolar epithelial cell models. 4.3. In-situ tracer studies in alveolar airspace Despite potential problems with the interpretation of tracer experiments useful information on putative transport pathways can be provided, information that has allowed for the further refinement of hypotheses regarding macromolecule transport pathways in alveolar lung. The classical studies of Schneeberger and Karnovsky [56,57] helped define the alveolar epithelium as the limiting restrictive membrane in the alveolar– pulmonary capillary barrier. Using ultrastructural cytochemical techniques these workers studied the permeability of alveolar epithelium to protein tracers such as horseradish peroxidase (HRP) (MW 40 kDa). They showed that within 90 s of an intravenous injection of HRP into adult mice, the HRP had passed through the pulmonary endothelial intercellular junctions into the underlying basement membrane, but was prevented from gaining access to the alveolar space by the tight-junctional complexes of the alveolar epithelium. Both the luminal and abluminal endothelial cell vesicles, (morphologically consistent with caveolae) appeared to contain HRP reaction product. HRP product was also observed to be intracellularly located within endothelial vesicles that appeared as discrete structures in the cytoplasm not attached to the plasma membrane. Only rarely were plasma membrane invaginations in the alveolar M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 type I cell seen to contain HRP, and these were usually connected to the abluminal or interstitial surface of the alveolar epithelial cell. No HRP was seen to access the alveolar airspace even by 60 min post-injection. Similar observations were seen for injected ferritin. By means of intranasally instilled peroxidase, Schneeberger and Karnovsky attempted to establish the manner of protein absorption from the alveolar airspace. Following this route of administration into the respiratory tract, limited access of HRP to the alveolar membrane was observed, with labelled pinocytic vesicles within the ATI cells only rarely demonstrated. By 6 h post instillation some alveolar type I cells were seen to contain large vacuoles of HRP reaction product, but no HRP was observed discharged into the interstial regions of the alveolar–capillary barrier. A range of cationic probes of varying pI values have been used as probes to decorate membrane surfaces. In lung studies cationic ferritin has been one of the most commonly used tracers. Simionescu and Simionescu [58] studied the differential distribution of cell surface charge in alveolar epithelium of mouse lung following airway perfusion with cationic ferritin. Their results showed that while the luminal surface of the ATII cells showed heavy decoration with the cationic probe, the luminal surface of the ATI cell displayed only very light labelling. This was interpreted as a difference in the density of anionic sites on the respective cell membranes, with a relative paucity of anionic charge on the ATI cell surface. A not unrelated series of investigations by these and other workers [59] describes in-situ studies addressing the surface charge and chemistry of endothelial plasmalemmal vesicles. In a variety of endothelial vascular beds it appears that anionic sites of low pKa occur at high density over the entire luminal membrane surface, but are characteristically absent over the large majority of the membranes of the non-coated plasmalemmal vesicles. If the membranes of the ATI plasmalemmal invaginations possess a less anionic nature this may have implications for electrostatic interactions of these domains with proteins in the alveolar airspace. In the rat lung instillation tracer studies of Mary Williams [60], cationic ferritin was observed to be internalised most rapidly, and in the greatest 293 amounts, by the ATII cells. At the earliest timepoint of study (10 min after instillation into the lung) clusters of cationic ferritin were observed adhered to the ATII plasmalemma with tracer present within vesicle compartments proximal to the ATII luminal cell membrane. Within 30–60 min post-instillation the tracer was trafficked to multi-vesicular bodies and lamellar bodies of the ATII cell. At 2 h some tracer was observed in the interstitial space below the basal membrane of the ATII cell. Although the surfaces of the ATI cell had adhered cationic ferritin particles the numerous vesicles present in the ATI cell appeared to be largely devoid of tracer. In the same body of work, it was observed that while ATII cells were also seen to internalise instilled dextran (70 kDa), the uptake of this probe by the ATI cell was minimal; neither the ATII or the ATI cell appeared to internalise neutral ferritin. Another study examining cationic ferritin uptake and intracellular transport by intact alveolar epithelium of the rat [61], also concluded that type I alveolar epithelium internalised only very limited amounts of tracer, with the majority that appeared to be transported to the interstitial space following tracheal instillation, doing so via transport across the type II cell. In studies examining the uptake of cationised ferritin by alveolar type I cells from goat lung, Atwal et al. [27] made observations that appeared contrary to the cationic ferritin reports described above. Atwal and co-workers instilled cationised ferritin into the right lung via a bronchoscope and observed cationic ferritin decorating the surfaces of both the ATI and ATII cells, although the temporal pattern of staining indicated that the membranes of the non-coated plasmalemmal invaginations or caveolae of the ATI cell were preferentially stained, indicating at these sites the presence of highly charged anionic domains. Within 2 min of instillation the cationic ferritin was found to be ultrastructurally associated with ATI vesicles, and within 5 min these vesicles were heavily decorated with tracer. They reported evidence of discharge of tracer on the abluminal or interstitial surface of the ATI cell indicative of trancystosis. The authors hypothesised that their divergent findings to that of other studies with cationic ferritin, reflected a change in the cell surface charge of the ATI cell membranes in goat lung following exposure of the alveolar epithelial surface 294 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 to ruminant gases as part of the natural physiological cycling of gas from goat rumen to lung. They postulated that the induced change in surface charge may occur as a mechanism to facilitate the transport out of the lung of ruminal fluids and solutes which may also enter the goat lung. Intriguingly, Gordon et al. [62] observed a 5-fold increase in the numerical density of non-coated vesicles within the ATI cells of hamster lung following exposure to NO 2 , suggesting a change had occurred in the steady-state regulation of the cell’s membrane trafficking. These workers also reported that with NO 2 exposure the affinity of anionic surface probes, cationic ferritin and ruthenium red, for the plasmalemmal surface was found to increase [63], suggesting a modification in cell surface characteristics. By means of antibodies labelled with HRP, Bignon and co-workers [73] were the first to identify under physiological conditions the presence of endogenous serum proteins, mainly albumin and immunoglobulin G (IgG), within the fluid lining the alveolar epithelial surface. Their immunocytochemical investigations in rat lung showed these proteins to be present also in the non-coated vesicles or invaginations of the ATI cell; at the electron-microscopic level they noted an absence of caveolae-like vesicles in the ATII cell. Hastings and co-workers [74,75] explored the clearance pathway of native proteins following their exogenous instillation into the lungs of rabbits. Their tracer studies [74] showed that alveolar macrophages rapidly (within 2 h) internalise both soluble albumin and colloidal-gold labelled albumin. By 6 h postinstillation they demonstrated the association, or apparent internalisation, of soluble albumin within the caveolae-like vesicles of the ATI cell, and within the vesicle system of the ATII cell. Neither of the alveolar epithelial cell types appeared to internalise the colloidal gold conjugated albumin probe, highlighting the differing physico-chemical properties and biological interactions of the soluble and insoluble albumin tracers. In 1994 Hastings and co-workers [75] combined morphological tracer techniques with the use of vesicular transport inhibitors and the examination of alveolar protein clearances [75]. Among their experiments, they studied the effects of the microtubule disrupting agent, nocodazole, and the endosomal acidification inhibitor, monensin, upon the alveolar clearance of soluble albumin from rabbit lung. Within 2 h of albumin instillation they showed an increased albumin immunoreactivity in both ATI and ATII cells, by this time they also observed that both inhibitors co-instilled into the airways could reduce this albumin staining to background levels. Nocodazole significantly increased the numerical density of the ATI vesicles by approximately 100% without effects upon the size of the vesicles (average diameters 70 nm), indicating a disruption in steadystate membrane trafficking by this microtubule inhibitor; similar effects of nocodazole upon vesicle density were seen in the pulmonary capillary endothelium. While at the immunocytochemical level the inhibitors appeared to have an effect upon albumin association with the alveolar epithelial cells, at neither 2 nor 8 h post albumin instillation did the endocytic inhibitors have an effect upon the alveolar clearance of albumin ( 131 I-labelled albumin), where clearance was determined by sampling of lung lavage fluid or of lung tissue homogenate post experiment. The authors concluded that while their data would not exclude an endocytic route contributing significantly to the removal of trace protein from the alveolar region, this mechanism is probably insufficient to clear large quantities of serum protein that may enter the alveolar airspace, as may occur in hydrostatic pulmonary oedema. In combination, the studies described above using HRP, ferritin, dextran and albumin probes would suggest that the plasmalemmal vesicle system in the ATI cell does not fulfil a significant role in macromolecule trafficking. However, the interpretation of electron microscopic tracer experiments can be imprecise, especially with the realisation that dynamic membrane events such as vesicle internalisation and trafficking will continue for some time after the initiation of tissue fixation. Further, using this technique alone to interpret vesicular labelling in terms of quantitative vesicular transport is not possible. Patton [6] raised several reasons why the inhibitor studies of Hastings et al. should be interpreted with caution, including the possibility that vesicle trafficking in the thin squamous ATI cell might occur independently of the microtubule network, and the potential lack of the sustained presence, and hence effectiveness, of the endocytic inhibitors in the alveolar region over an 8 h period. The role of vesicular-mediated trafficking in the transport of proteins from alveolar airspace to capillary blood M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 remains to be established but as yet cannot be dismissed. Given what is known about caveolae transport in other tissues and cell types, it would be surprising if caveolae in alveolar epithelial type I cells did not fulfil a trans-alveolar macromolecule trafficking function. 4.4. Caveolae and receptor-mediated transport An established role for caveolae in the transport of potential inhaled therapeutic macromolecules across the alveolar epithelium remains to be determined. However, inferences relating to caveolae functioning in receptor-mediated trancytosis may be gained from the study of caveolae in cell types of non-alveolar lineage. Insulin is perhaps the most studied protein therapeutic in regard to pulmonary absorption. The insulin receptor has been localised to caveolae in endothelial [76] and adipocyte [77] cell types, although the insulin receptor undergoes dynamic transfer between different membrane domains, including clathrincoated pits. Schnitzer and colleagues [40] have shown receptor-mediated endothelial transcytosis of insulin to be undertaken, at least in part, by caveolae. In the in-vitro study of Roberts and Sandra [76], comparison was made of the relative contribution of a clathrin-coated pit pathway and a non-coated vesicle (morphologically identifiable as caveolae) pathway in the transcytosis of insulin across cultured bovine pulmonary artery endothelial cells. Using semi-quantitative immunocytochemical analyses they reported that both vesicular populations were associated with insulin, although a greater (approximately 70% of the total) amount of the gold-labelled insulin probe was associated with caveolae structures. However, morphometric evaluation determined the surface density of clathrin-coated pits in their cultured cell type to approximate only 5% of that for caveolae, leading the authors to interpret that, when normalised for vesicle density, insulin shows a preferential interaction with clathrin-coated pits. The lung delivery of recombinant human growth hormone (rhGH) has been investigated, with the studies [78,79] describing a relatively rapid, and dose-dependent [79], systemic absorption for rhGH. In his review in 1996, Patton [6] alluded to unpublished work using immunocytochemical tech- 295 niques that localised instilled rhGH to caveolae in rat alveolar epithelium, although Patten and co-workers indicated that no evidence of hGH receptor expression could be found on the luminal surface of rat lung alveolar epithelium. In Chinese hamster ovary (CHO) cells bearing recombinant rhGH-receptor, a component in the internalisation of rhGH has been shown to be mediated via caveolae [80]. The kinetics of rhGH internalisation in the recombinant CHO cells displayed a bi-phasic response which consisted of a relatively more rapid initial period of internalisation (5–15 min), followed by a slower uptake phase. The inhibitor for caveolae formation, the sterol binding agent fillipin, reduced internalisation during the slower component of uptake only. The mechanisms by which chemokines penetrate the alveolar barrier to stimulate systemic granulocytes is of interest to both pharmaceutical and biomedical science disciplines. The studies of Middleton et al. [81] addressed the sub-cellular fate of gold labelled IL-8 in venular endothelial cells following intradermal injection in rabbits. These workers reported that 30 min after administration the IL-8 gold-conjugate had become bound to the abluminal endothelial surface and incorporated into omegashaped plasma membrane invaginations that proved to be reactive for caveolin. Following internalisation the IL-8 was observed to be transcytosed via caveolae to the luminal side of the cell into capillary blood. Maybe caveolae within the alveolar type I cell can fulfil a similar role. The Duffy antigen, a broad spectrum scavenging receptor for chemokines, including IL-8, has been reported to be localised to the caveolae within alveolar epithelium [82]. 5. Caveolae and caveolin in cultured alveolar epithelium Due to the complex nature of the lung architecture, the alveolar epithelium is not a readily accessible absorption surface to study. Therefore the use of cultures of alveolar epithelium cells as an in-vitro experimental model for the prediction of the extent, rate and mechanism of alveolar absorption of pharmaceuticals has gained acceptance amongst investigators [83]. Consistent with the in-vivo hypothesis of the ATII cell transdifferentiating into the in-vivo ATI cell 296 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 [18], isolated ATII cells in primary culture grown over a 5–6 day period on a substratum of tissue culture plastic loose their characteristic ATII phenotype and acquire with time the morphology, and expression of certain biochemical markers, characteristic of an ATI-‘like’ phenotype [84,85]. When grown on semi-permeable membranes these ATI‘like’ monolayers generate a restrictive paracellular permeability pathway. Such cultures derived most commonly from rat have been extensively used in the pharmaceutical sciences to investigate the alveolar transport properties of select macromolecules (reviewed in [83] and [86]). A study by Matsukawa et al. [87] evaluated the flux of radiolabelled albumin across cultured rat ATI-‘like’ monolayers. The transport rate was much faster than that predicted by simple passive diffusion alone, and found to be asymmetric. For example the apparent permeability of 14 C-bovine serum albumin was 0.768 ( 3 10 27 cm sec 21 ) and 0.39 ( 3 10 27 cm sec 21 ) in the apical to basolateral, and basolateral to apical directions, respectively. This led the authors to speculate that the likely route of transport for albumin was via a receptor-mediated transcytotic pathway. Kim and co-workers [88] identified on the apical membrane of rat ATI-‘like’ cultures the presence of an albumin binding protein which was antigenically similar to the albumin receptor, gp60, which appears to mediate the transcytosis of native unmodified albumin across capillary endothelium [42]. The same laboratory [89] examined the permeability of the ATI-‘like’ cultures to probes of fluid-phase vesicular transport. They found the permeability coefficients for HRP in both the A → B and B → A directions to be symmetrical, with permeability coefficients calculated at | 7.0 ( 3 10 29 cm sec 21 ). At 48C the transport of HRP was decreased by 70% suggesting it’s translocation across the alveolar cell model did not take place via a paracellular route, but rather by a vesicle-mediated pathway. In the report of Cheek et al. [90] the appearance was noted of a vesicle-like structure attached to the plasma membrane of rat ATI-‘like’ monolayer. However, no further characterisation was undertaken. Recently the expression of caveolin-1 and caveolae biogenesis within in-vitro rat primary alveolar epithelial monolayer cultures has been reported, and shown to occur as a function of transdifferentiation of the cultured cell from the ATII to an ATI-‘like’ phenotype [16]. This work showed in freshly isolated rat ATII cells and cells grown to 2 days post-seeding a lack of caveolae-like structures at the electronmicroscopic level, and a lack or low expression of caveolin-1 protein. As the ATII cells acquired an ATI-‘like’ phenotype with continued primary culture over a number of days, the expression of caveolin-1 increased, with caveolin-1 signal at day 8 postseeding up to 50-fold greater than at day 2. In parallel with the increase in caveolin-1 expression, plasmalemmal invaginations characteristic of caveolae (determined morphologically and using caveolin-1 immunolabelling) became evident in the ATI-‘like’ cultures between day 6 and 8 post-seeding. In contrast, when the differentiated ATII phenotype was maintained with time by culturing the freshly isolated ATII cells upon a collagen matrix with an apical interface of air [38], the temporal increase in caveolin-1 expression was not observed, with only very faint signals evident even at day 8 post-seeding, and no generation of caveolae. Although parallels between in-vitro and in-vivo ATII transdifferentiation remain to be fully defined, the in vitro caveolae and caveolin studies described above correspond to observations in intact lung tissue showing the presence of caveolae in the in-vivo ATI cell and an absence in the in-vivo ATII cell [31]. Work from the same laboratory (unpublished) has recently identified components of the SNARE complex to be expressed in the cultured ATI-‘like’ monolayers and for albumin endocytosis by these cells to be modulated by the caveolae inhibitor, filipin, without effects upon the internalisation of transferrin (a probe for clathrin-mediated internalisation). The ability to isolate primary ATII cells and to culture them to form a polarised monolayer which acquires the characteristics of the in vivo ATI cell has allowed various research groups to study putative alveolar vectorial electrolyte and drug transport processes. With further characterisation these ATI‘like’ monolayers should provide a suitable in-vitro model system to examine the potential trafficking mechanisms regulating the pulmonary absorption of therapeutic macromolecules, mechanisms that to date are poorly understood. M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 6. Conclusion There is a genuine clinical and commercial interest in exploiting the pulmonary route for the systemic delivery of macromolecules, particularly recombinant proteins and polypeptides. The alveolar epithelium appears to be the appropriate lung surface to target when aiming to systemically deliver macromolecules. The existence of a high numerical density of smooth-coated or non-coated plasma membrane vesicles or invaginations within the alveolar epithelial type I cell has long been recognised. These vesicles, or subpopulations thereof, have been shown to biochemically possess caveolin, a marker protein for caveolae. From what is known about caveolae function in other cell types a role within alveolar type I epithelium for the endocytic and / or transcytotic trafficking of solute by caveolae may be expected. Pulmonary pharmacokinetic data and tracer experiments within the alveolar airspace have tended to support the view that alveolar vesicle mediated trafficking will in general function as a minor pathway in protein absorption. A model, not inconsistent with some of the pulmonary pharmacokinetic data, of how alveolar epithelial vesicles may serve as a pathway for macromolecule transport can be envisaged, and a potentially significant role for alveolar caveolae in mediating the alveolar airspace to blood transport of macromolecules cannot be dismissed. A better understanding of the membrane dynamics of caveolae in alveolar epithelium will help resolve the function of these vesicular compartments and may lead to the development of more specific drug targeting approaches for promoting pulmonary drug delivery. References [1] J.S. Patton, Breathing life into protein drugs, Nat. Biotechnol. 16 (1998) 141–143. [2] P. Colthorpe, S.J. Farr, G. Taylor, I.J. Smith, D. Wyatt, The pharmacokinetics of pulmonary delivered insulin: a comparison of intra-tracheal and aerosol administration to the rabbit, Pharm. Res. 9 (1992) 765–768. [3] P. Colthorpe, S.J. Farr, I.J. Smith, D. Wyatt, G. Taylor, The influence of regional deposition on the pharmacokinetics of pulmonary-delivered human growth hormone in rabbits, Pharm. Res. 12 (1995) 356–359. 297 [4] E.R. Weibel, Morphological basis of alveolar–capillary gas exchange, Physiol. Rev. 53 (1973) 419–495. [5] E.E. Schneeberger, The integrity of the air–blood barrier, in: J.D. Brain, D.F. Proctor, L.M. Reid (Eds.), Respiratory Defense Mechanisms, Dekker, New York, 1977, pp. 687– 708. [6] J.S. Patton, Mechanisms of macromolecule absorption by the lungs, Adv. Drug Deliv. Rev. 19 (1996) 3–36. [7] G. Clough, C.C. Michel, The role of vesicles in the transport of ferritin through frog endothelium, J. Physiol. 315 (1981) 127–142. [8] K.G. Rothberg, J.E. Heuser, W.C. Donzell, Y. Ying, J.R. Glenny, R.G.W. Anderson, Caveolin, a protein component of caveolae membrane coats, Cell 68 (1992) 673–682. [9] S. Li, F. Galbiati, D. Volonte, M. Sargiacomo, J.A. Engelman, K. Das, P.E. Scherer, M.P. Lisanti, Mutational analysis of caveolin-induced vesicle formation, FEBS Lett. 434 (1998) 127–134. [10] A.M. Fra, E. Williamson, K. Simons, R.G. Parton, De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin, Cell Biol. 92 (1995) 8655–8659. [11] S. Li, K.S. Song, S.S. Koh, A. Kikuchi, M.P. Lisanti, Baculovirus-based expression of mammalian caveolin in Sf21 insect cells, J. Biol. Chem. 271 (1996) 28647–28654. [12] U. Vogel, K. Sandvig, B. van Deurs, Expression of caveolin1 and polarised formation of invaginated caveolae in Caco-2 and MDCK II cells, J. Cell Sci. 111 (1998) 825–832. [13] S. Monier, R.G. Parton, F. Vogel, J. Behlke, A. Henske, T.V. Kurzchalia, VIP21-caveolin, a membrane protein constituent of caveolae coat, oligomerizes in-vivo and in-vitro, Mol. Biol. Cell 6 (1995) 911–927. [14] M. Sargiacomo, P.E. Scherer, Z.-L. Tang, E. Kubler, K.S. Song, M.C. Sanders, M.P. Lisanti, Oligomeric structure of caveolin: implications for caveolae membrane organisation, Proc. Natl. Acad. Sci. U.S.A. 92 (1995) 9407–9411. [15] K.S. Song, Z.-L. Tang, S. Li, M.P. Lisanti, Mutational analysis of the properties of caveolin-1, J. Biol. Chem. 272 (1997) 4398–4403. [16] L. Campbell, A.J. Hollins, A. Al-Eid, G.R. Newman, C. von Ruhland, M. Gumbleton, Caveolin-1 expresion and caveolae biogenesis during cell transdifferentiation in lung alveolar epithelial primary cultures, Biochem. Biophys. Res. Commun. 262 (1999) 744–751. [17] J.D. Crapo, B.E. Barry, P. Gehr, M. Bachofen, E.R. Weibel, Cell number and cell characteristics of normal human lung, Am. Rev. Respir. Dis. 125 (1982) 332–337. [18] B.D. Uhal, Cell cycle kinetics in the alveolar epithelium, Am. J. Physiol. 272 (1997) L1031–L1045. [19] J. Gil, D.A. Silage, J.M. McNiff, Distribution of vesicles in cells of the air–blood barrier in the rabbit, J. Appl. Physiol. 50 (1981) 334–340. [20] J. Gil, Number and distribution of plasmalemma vesicles in the lung, Fed. Proc. 42 (1983) 2414–2418. [21] D.O. DeFouw, Ultrastructural features of alveolar epithelial transport, in: E.D. Crandall (Eds.) Fluid balance across alveolar epithelium, Am. Rev. Res. Dis. Supplement 127 (1983) S9–S13. 298 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 [22] D.O. DeFouw, F.P. Chinard, Numerical denisties of cellular vesicles and cellular attentuation in the pulmonary alveolar septa of edematous dog lungs, Microvasc. Res. 23 (1982) 248. [23] D.M. Haies, J. Gil, E.R. Weibel, Morphometric study of rat lung cells: numerical and dimensional characteristics of parenchymal cell populations, Am. Rev. Respir. Dis. 123 (1981) 533–541. [24] J. Gil, D.A. Silage, Morphometry of pinocytic vesicles in the capillary endothelium of rabbit lungs using automated equipment, Circ. Res. 47 (1980) 384–391. [25] R.W. Mazzone, S.M. Kornblau, Pinocytotic vesicles in the endothelium of rapidly frozen rabbit lung, Microvasc. Res. 21 (1981) 193–211. [26] M. Simionescu, N. Simionescu, G.E. Palade, Morphological data on the endothelium of blood capillaries, J. Cell Biol. 60 (1974) 128–152. [27] O.S. Atwal, L. Viel, K.J. Minhas, An uptake of cationized ferritin by alveolar type I cells in airway-instilled goat lung: distribution of anionic sites on the epithelial surface, J. Submicrosc. Cytol. Pathol. 22 (1990) 425–432. [28] R.E. Gordon, S. Puszkin, Endocytic vesicles of type I pneumocytes. Immunocytochemical colocalisation of calmodulin with clathrin molecules, J. Histotechnol. 12 (1989) 185–191. [29] M.P. Lisanti, P.E. Scherer, J. Vidugiriene, Z.L. Tang, A. Hermanowski-Vosatka, Y. Tu, R.F. Cook, M. Sargiacomo, Characterisation of caveolin-rich domains isolated from an endothelial rich source: implications for human disease, J. Cell Biol. 126 (1994) 111–126. [30] M. Kasper, T. Reimann, U. Hempel, K.W. Wenzel, A. Bierhaus, D. Schuhl, V. Dimmer, G. Haroske, M. Muller, Loss of caveolin-1 expression in type I pneumocytes as an indicator of subcellular alterations during lung fibrogenesis, Histochem. Cell Biol. 109 (1998) 41–48. [31] G.R. Newman, L. Campbell, C. von Ruhland, B. Jasani, M. Gumbleton, Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium: implications for alveolar type I cell function, Cell Tissue Res. 295 (1999) 111–120. [32] M. Bungaard, J. Frøkjaer-Jensen, C. Crone, Endothelial plasmalemmal vesciles as elements in a system of branching invaginations from the cell surface, Proc. Natl. Acad. Sci. U.S.A. 76 (1979) 6439–6442. [33] M. Bungaard, P. Hagman, C. Crone, The three–dimensional organisation of plasmalemmal vesicular profiles in the endothelium of rat heart capillaries, Microvasc. Res. 25 (1983) 358–368. [34] J. Frøkjaer-Jensen, Three dimensional organisation of plasmalemmal vesicles in endothelial cells: an analysis by serial sectioning of frog mesenteric capillaries, J. Ultrastruct. Res. 73 (1980) 9–20. [35] J. Frøkjaer-Jensen, The endothelial vesicle systems in cryofixed frog mesenteric capillaries analysed by ultrathin serial sectioning, J. Electron Microsc. Tech. 19 (1991) 291– 304. [36] C.C. Michel, Transport of macromolecules through microvascular walls, Cardiovasc. Res. 32 (1996) 644–653. [37] D. Predescu, R. Horvat, S. Predescu, G. Palade, Transcytosis in the continuous endothelium of the myocardial microvasculature is inhibited by N-ethylmaleimide, Proc. Natl. Acad. Sci. U.S.A. 91 (1994) 3014–3018. [38] D. Predescu, G. Palade, Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium, Am. J. Physiol. 265 (1993) H725–H733. [39] S. Predescu, D. Predescu, G. Palade, Plasmalemmal vesicles function as transcytotic carriers for small proteins in the continuous endothelium, Am. J. Physiol. 272 (1997) H937– H949. [40] J.E. Schnitzer, P. Oh, E. Pinney, J. Allard, Fillipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis and capillary permeability of select macromolecules, J. Cell Biol. 127 (1994) 1217–1232. [41] J.E. Schnitzer, J. Allard, P. Oh, NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia, Am. J. Physiol. 268 (1995) H48–H55. [42] J.E. Schnitzer, P. Oh, Albondin-mediated capillary permeability to albumin: differentiation role for receptors in endothelial transcytosis and endocytosis of native and modified albumins, J. Cell Biol. 269 (1994) 6072–6082. [43] J.E. Schnitzer, J. Liu, P. Oh, Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases, J. Biol. Chem. 24 (1995) 14399–14404. [44] D.P. McIntosh, J.E. Schnitzer, Caveolae require intact VAMP for targeted transport in vascular endothelium, Am. J. Physiol. 277 (1999) H2222–H2232. [45] P. Oh, D.P. McIntosh, J.E. Schnitzer, Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium, J. Cell Biol. 141 (1998) 101–114. [46] M. Linial, SNARE proteins — why so many, why so few?, J. Neurochem. 69 (1997) 1781–1792. [47] L. Shanker, J.A. Hemberger, Relation between molecular weight and pulmonary absorption rate of lipid-insoluble compounds in neonatal and adult rats, Biochem. Pharmacol. 32 (1983) 2599–2601. [48] A.E. Taylor, K.A. Gaar, Estimation of equivalent pore radii of pulmonary capillary and alveolar membranes, Am. J. Physiol. 218 (1970) 1133–1140. [49] J. Theodore, E.D. Robin, R. Gaudio, J. Acevedo, Transalveolar transport of large polar solutes (sucrose, inulin and dextran), Am. J. Physiol. 229 (1975) 989–996. [50] R.M. Effros, G.M. Mason, Measurements of pulmonary epithelial permeability in–vivo, Am. Rev. Respir. Dis. 127 (1983) S59–S65. [51] G. Taylor, P. Colthorpe, S.J. Farr, Pulmonary absorption of proteins influence of deposition site and competitive elimination processes, Resp. Drug Deliv. IV (1994) 25–30. [52] S.M. Shea, M.J. Karnovsky, Vesicular transport across endothelium: simulation of a diffusion model, J. Theor. Biol. 24 (1969) 30–42. [53] H.S. Green, J.R. Casley-Smith, Calculations on the passage of small vesicles across endothelial cells by brownian motion, J. Theor. Biol. 35 (1972) 103–111. M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 [54] E.M. Renkin, Transport of large molecules across capillary walls, Physiologist 7 (1964) 13–17. [55] R.R. Bruns, G.E. Palade, Studies on blood capillaries: transport of ferritin molecules across the wall of muscle capillaries, J. Cell Biol. 37 (1968) 277–299. [56] E.E. Schneeberger-Keeley, M.J. Karnovsky, The ultrastructural basis of alveolar–capillary membrane permeability to peroxidase used as a tracer, J. Cell Biol. 37 (1968) 781–793. [57] E.E. Schneeberger, M.J. Karnovsky, The influence of intravascular fluid volume on the permeability of newborn and adult mouse lungs to ultrastructural protein tracers, J. Cell Biol. 49 (1971) 319–334. [58] D. Simionescu, M. Simionescu, Differential distribution of the cell surface charge on the alveolar capillary unit. Characteristic paucity of anionic sites on the air–blood barrier, Microvasc. Res. 25 (1983) 85–100. [59] D. Simionescu, M. Simionescu, Endothelial transport of macromolecules: transcytosis and endocytosis, Cell Biol. Rev. 25 (1991) 1–80. [60] M.C. Williams, Endocytosis in alveolar type II cells: effect of charge and size of tracers, Proc. Natl. Acad. Sci. U.S.A. 81 (1984) 6054–6058. [61] T. Ito, H. Kitamura, Y. Inayama, A. Nozawa, M. Kanisawa, Uptake and intracellular transport of cationic ferritin in the bronchiolar and alveolar epithelia of the rat, Cell Tiss. Res. 268 (1992) 335–340. [62] R.E. Gordon, B.W. Case, J. Kleinerman, Acute NO 2 effects on penetration and transport of horseradish peroxidase in hamster respiratory epithelium, Am. Rev. Respir. Dis. 128 (1983) 528–533. [63] R.E. Gordon, The effects of NO 2 on ionic surface charge on type I pneumocytes of hamster lungs, Am. J. Pathol. 121 (128) (1985) 291–297. [64] A.M. Lorino, M. Meignan, P. Bouissou, G. Atlan, Effects of sustained exercise on pulmonary clearance of aerosolized 99 m Tc-DTPA, J. Appl. Physiol. 67 (1989) 2055–2059. [65] S.K. Ghosh, M.G. Neale, K.R. Patel, The effect of physiological manoeuvres on the absorption of inhaled nedocromil sodium, Br. J. Clin. Pharmacol. 37 (1994) 305–308. [66] S.J. Farr, I. Gonda, V. Licko, Physico-chemical and physiological factors influencing the effectiveness of inhaled insulin, in: P.R. Byron (Ed.), Respiratory Drug Delivery VI, CRC Press, Boca Raton, FL, 1998, pp. 25–33. [67] E.A. Egan, R.M. Nelson, R.E. Olver, Lung inflation and alveolar permeability to non-elecrolytes in the adult sheep in vivo, J. Physiol. 260 (1976) 409–424. [68] E.A. Egan, Lung inflation, lung solute permeability, and alveolar edema, J. Appl. Physiol. 53 (1982) 121–125. [69] E.A. Egan, Response of alveolar epithelial solute permeability to changes in lung inflation, J. Appl. Physiol. 49 (1980) 1032–1036. [70] T. Ohtani, M. Murakami, A. Yamamoto, K. Takada, S. Muranishi, Effect of absorption enhancers on pulmonary absorption of fluorescein isothiocyanate dextrans with various molecular weights, Int. J. Pharm. 77 (1991) 141–150. [71] V. Rizzo, A. Sung, P. Oh, J.E. Schnitzer, Rapid mechanotransduction in situ at the luminal cell surface of vascular [72] [73] [74] [75] [76] [77] [78] [79] [80] [81] [82] [83] [84] [85] [86] 299 endothelium and its caveolae, J. Biol. Chem. 273 (1998) 26323–26329. J. Bastacky, C.Y. Lee, J. Goerke, H. Koushafar, D. Yager, L. Kenaga, T.P. Speed, Y. Chen, J.A. Clements, Alveolar lining layer is thin and continuous: low temperature scanning electron microscopy of rat lung, J. Appl. Physiol. 79 (1995) 1615–1628. J. Bignon, M.C. Jaurand, M.C. Pinchon, C. Sapin, J.M. Warnet, Immunoelectron microscopic and immunochemical demonstration of serum proteins in the alveolar lining material of the rat lung, Am. Rev. Resp. Dis. 113 (1976) 109–120. R.H. Hastings, H.G. Folkesson, V. Petersen, R. Ciriales, M.A. Matthay, Cellular uptake of albumin from lungs of anesthetised rabbits, Am. J. Physiol. 269 (1995) L453–L462. R.H. Hastings, J.R. Wright, K.H. Albertine, R. Ciriales, M.A. Matthay, Effect of endocytosis inhibitors on alveolar clearance of albumin, immunoglobulin G and SP-A in rabbits, Am. J. Physiol. 266 (1994) L544–L552. R.L. Roberts, A. Sandra, Receptor-mediated endocytosis of insulin by cultured endothelial cells, Tissue Cell 24 (1992) 603–611. R.I. Goldberg, R.M. Smith, L. Jarrett, Insulin and a 2 -macroglobulin-methylamine undergo endocytosis by different mechanisms in rat adipocytes: comparison of cell surface events, J. Cell. Physiol. 133 (1987) 203–212. J.S. Patton, J.G. McGabe, S.E. Hansen, A.L. Daugherty, Absorption of human growth hormone from the rat lung, Biotechnol. Ther. 1 (1990) 213–228. H.G. Folkesson, L. Hedin, B.R. Westrom, Lung to blood passage of human growth hormone (hGH) after intra-tracheal instillation: stimulation of growth in hypophysectomised rats, J. Endocrinol. 134 (1992) 197–203. P.E. Lobie, R. Sadir, R. Graichen, H.C. Mertani, M. Morel, Caveolar internalisation of growth hormone, Exp. Cell Res. 246 (1999) 47–55. J. Middleton, S. Neil, J. Wintle, I. Clarke-Lewis, H. Moore, C. Lam, M. Auer, E. Hub, A. Rot, Transcytosis and surface presentation of IL-8 by venular endothelial cells, Cell 91 (1997) 385–395. A. Chaudhuri, S. Nielsen, M.-L. Elkjaer, V. Zbrzezna, F. Fang, A.O. Pogo, Detection of duffy antigen in the plasma membranes and caveolae of vascular endothelial and epithelial cells of nonerythroid organs, Blood 89 (1997) 701– 712. N.R. Mathias, F. Yamashita, V.H.L. Lee, Respiratory epithelial cell culture models for evaluation of ion and drug transport, Adv. Drug Deliv. Rev. 22 (1996) 215–249. L.G. Dobbs, M.C. Williams, R. Gonzalez, Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells, Biochim. Biophys. Acta 970 (1988) 146–156. S.I. Danto, S.M. Zabski, E.D. Crandall, Reactivity of alveolar epithelial cells in primary culture with type I cell monoclonal antibodies, Am. J. Respir. Cell Mol. Biol. 6 (1992) 296–306. ¨ H.G. Folkesson, M.A. Matthay, B.R. Westrom, K.J. Kim, 300 M. Gumbleton / Advanced Drug Delivery Reviews 49 (2001) 281 – 300 B.W. Karlsson, R.H. Hastings, Alveolar epithelial clearance of protein, J. Appl. Physiol. 80 (1996) 1431–1445. [87] Y. Matsukawa, H. Yamahara, F. Yamashita, V.H.L. Lee, E.D. Crandall, K.J. Kim, Rates of protein transport across rat alveolar epithelial cell monolayers, J. Drug Target. 7 (2000) 335–342. [88] K.J. Kim, V. Rattan, P. Oh, J.E. Schnitzer, V.K. Kalra, E.D. Crandall, Specific albumin-binding protein in alveolar epithelial cell monolayers (abstract), Am. J. Respir. Crit. Care Med. 151 (1995) A190. [89] Y. Matsukawa, H. Yamahara, V.H.L. Lee, E.D. Crandall, K.J. Kim, Horseradish peroxidase transport across rat laveolar epithelial cell monolayers, Pharm. Res. 13 (1996) 1331– 1336. [90] J.M. Cheek, M.J. Evans, E.D. Crandall, Type 1 cell-like morphology in tight alveolar epithelial monolayers, Exp. Cell Res. 184 (1989) 375–387.