* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lectures 15, 16 and 17
Homoaromaticity wikipedia , lookup
Kinetic resolution wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Elias James Corey wikipedia , lookup
Metal carbonyl wikipedia , lookup
Ene reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Wolff rearrangement wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Stille reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
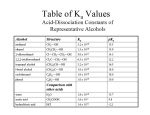
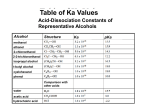
19.13) Which of the following bases are strong enough to deprotonate CH3COOH? a) F- pka of CH3COOH is 4.8. b) (CH3)3CO- b) has a pka of 18 c) has a pka of 50 c) CH3- d) has a pka of 38 d) NH2e) Cl- pka of conjugate acid must be greater than that of the carboxylic acid being deprotonated. 1 19.14) Rank the labeled protons in order of increasing acidity. Ha H OHb OHc O Ha<Hb<Hc The more stable the conjugate base the more acidic the proton. 2 19.15) Match each pka value with each carboxylic acid.(3.2, 4.9 and 0.2) a) CH3CH2COOH 4.9 b) CF3COOH 0.2 c) ICH2COOH 3.2 Electron withdrawing groups make acids more acidic. 3 19.16) Why is formic acid more acidic than acetic acid? H OH O OH O The methyl group is electron donating and stabilizes the acid while destabilizing the conjugate base thus making it less acidic. 4 19.17) Rank the compounds within each group in order of decreasing acidity. a) CH3COOH, HSCH2COOH, HOCH2COOH 3 2 1 b) ICH2COOH, I2CHCOOH, ICH2CH2COOh 2 1 3 5 19.18) Rank each group of compounds in order of decreasing acidity. a) CO2H CO2H CO2H Cl 2 1 3 CO2H b) CO2H CO2H O H3CO 2 1 3 6 19.19) Is the following compound more or less acidic than phenol? OH HO R The more electron donating groups present, the less acidic a compound is. This compound has an additional hydroxy and alkyl group, both electron donating. So it is less acidic. 7 19.22) Comparing CF3SO3H and CH3SO3H, which has the weaker conjugate base? Which conjugate base is the better leaving group? Which of these acids has the higher pka? CF3SO3H is the weaker conjugate base. CF3SO3H is the better leaving group because it is the weaker conjugate base. CH3SO3H, with the electron donating methyl group, has the higher pka and is thus a weaker acid. 8 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation and Reduction Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that contain an electronegative atom bonded to the carbonyl 9 • The presence or absence of a leaving group on the carbonyl determines the type of reactions the carbonyl compound will undergo. • Carbonyl carbons are sp2 hybridized, trigonal planar, and have bond angles that are ~1200. In these ways, the carbonyl group resembles the trigonal planar sp2 hybridized carbons of a C=C. 10 • In one important way, the C=O and C=C are very different. • The electronegative oxygen atom in the carbonyl group means that the bond is polarized, making the carbonyl carbon electron deficient. • Using a resonance description, the carbonyl group is represented by two resonance structures. 11 General Reactions of Carbonyl Compounds Carbonyls react with nucleophiles. 12 Aldehydes and ketones react with nucleophiles to form addition products by a two-step process: nucleophilic attack followed by protonation. 13 • The net result is that the bond is broken, two new bonds are formed, and the elements of H and Nu are added across the bond. • Aldehydes are more reactive than ketones towards nucleophilic attack for both steric and electronic reasons. 14 Carbonyl compounds with leaving groups react with nucleophiles to form substitution products by a two-step process: nucleophilic attack, followed by loss of the leaving group. The net result is that Nu replaces Z, a nucleophilic substitution reaction. This reaction is often called nucleophilic acyl substitution. 15 16 • Nucleophilic addition and nucleophilic acyl substitution involve the same first step—nucleophilic attack on the electrophilic carbonyl carbon to form a tetrahedral intermediate. • The difference between the two reactions is what then happens to the intermediate. • Aldehydes and ketones cannot undergo substitution because they do not have a good leaving group bonded to the newly formed sp3 hybridized carbon. 17 Preview of Oxidation and Reduction • Carbonyl compounds are either reactants or products in oxidation-reduction reactions. 18 The three most useful oxidation and reduction reactions of carbonyl starting materials can be summarized as follows: 19 Reduction of Aldehydes and Ketones • The most useful reagents for reducing aldehydes and ketones are the metal hydride reagents. • Treating an aldehyde or ketone with NaBH4 or LiAlH4, followed by H2O or some other proton source affords an alcohol. 20 • The net result of adding H:¯ (from NaBH4 or LiAlH4) and H+ (from H2O) is the addition of the elements of H2 to the carbonyl bond. 21 • Catalytic hydrogenation also reduces aldehydes and ketones to 1° and 2° alcohols respectively, using H2 and a catalyst. • When a compound contains both a carbonyl group and a carbon—carbon double bond, selective reduction of one functional group can be achieved by proper choice of the reagent. A C=C is reduced faster than a C=O with H2 (Pd-C). A C=O is readily reduced with NaBH4 and LiAlH4, but a C=C is inert. 22 • Thus, 2-cyclohexenone, which contains both a C=C and a C=O, can be reduced to three different compounds depending upon the reagent used. 23 The Stereochemistry of Carbonyl Reduction • Hydride converts a planar sp2 hybridized carbonyl carbon to a tetrahedral sp3 hybridized carbon. 24 Enantioselective Carbonyl Reductions • Selective formation of one enantiomer over another can occur if a chiral reducing agent is used. • A reduction that forms one enantiomer predominantly or exclusively is an enantioselective or asymmetric reduction. • An example of chiral reducing agents are the enantiomeric CBS reagents. 25 • CBS refers to Corey, Bakshi and Shibata, the chemists who developed these versatile reagents. • One B—H bond serves as the source of hydride in this reduction. • The (S)-CBS reagent delivers H:- from the front side of the C=O. This generally affords the R alcohol as the major product. • The (R)-CBS reagent delivers H:- from the back side of the C=O. This generally affords the S alcohol as the major product. 26 • These reagents are highly enantioselective. For example, treatment of propiophenone with the (S)-CBS reagent forms the R alcohol in 97% ee. 27 Reduction of Carboxylic Acids and Their Derivatives • LiAlH4 is a strong reducing agent that reacts with all carboxylic acid derivatives. • Diisobutylaluminum hydride ([(CH3)2CHCH2]2AlH, abbreviated DIBAL-H, has two bulky isobutyl groups which makes this reagent less reactive than LiAlH4. • Lithium tri-tert-butoxyaluminum hydride, LiAlH[OC(CH3)3]3, has three electronegative O atoms bonded to aluminum, which makes this reagent less nucleophilic than LiAlH4. 28 • Acid chlorides and esters can be reduced to either aldehydes or 1° alcohols depending on the reagent. 29 • In the reduction of an acid chloride, Cl¯ comes off as the leaving group. • In the reduction of the ester, CH3O¯ comes off as the leaving group, which is then protonated by H2O to form CH3OH. 30 • The mechanism illustrates why two different products are possible. 31 • Carboxylic acids are reduced to 1° alcohols with LiAlH4. • LiAlH4 is too strong a reducing agent to stop the reaction at the aldehyde stage, but milder reagents are not strong enough to initiate the reaction in the first place. 32 • Unlike the LiAlH4 reduction of all other carboxylic acid derivatives, which affords 1° alcohols, the LiAlH4 reduction of amides forms amines. • Since ¯NH2 is a very poor leaving group, it is never lost during the reduction, and therefore an amine is formed. 33 34 35 Oxidation of Aldehydes • A variety of oxidizing agents can be used, including CrO3, Na2Cr2O7, K2Cr2O7, and KMnO4. • Aldehydes can also be oxidized selectively in the presence of other functional groups using silver(I) oxide in aqueous ammonium hydroxide (Tollen’s reagent). Since ketones have no H on the carbonyl carbon, they do not undergo this oxidation reaction. 36 For Wednesday, 20.1-20.16. 37 20.1) What type of orbitals make up the indicated bonds? And in what orbitals do the lone pairs on the oxygen lie? O a b c a. sp3-sp2 b. sp2-sp2, p-p c. sp3-sp2 The lone pairs lie in sp2 hybridized orbitals. 38 20.2) Which compounds undergo nucleophilic addition and which substitution? O a) O b) H3CH2CH2C addition Cl substitution O c) O d) H H3C OCH3 substitution addition 39 20.3) Which compound in each pair is more reactive toward nucleuphilic attack? a) H3CH2CH2C H H3C(H3C)HC CH2CH3 H3CH2C O O O c) H3CH2C H O O H3CH2C d) H3CH2CH2C H3CH2CH2C O b) O O O Cl O OCH3 Cl O O OCH3 H3CH2C NHCH3 OCH3 40 20.4) What alcohol is formed when each compound is treated with NaBH4 in MeOH? a) O OH NaBH4 H3CH2CH2C MeOH H H3CH2CH2C H H b) OH O NaBH4 MeOH c) NaBH4 MeOH O OH 41 20.5) What aldehyde or ketone is needed to synthesize each alcohol by metal hydride reduction? a) b) OH OH O O c) OH O 42 20.6) Why can’t 1-methylcyclohexanol be prepared from a carbonyl by reduction? OH Tertiary alcohols can not be made by reduction of a carbonyl because there are no hydrogens on the carbon with the -OH. 43 20.7) Draw the products of the following reactions? a) O OH LiAl4 H2O O b) OH NaBH4 MeOH c) O O H2 (1 equiv.) Pd-C d) O OH H2 (excess) Pd-C 44 e) O OH NaBH4 (excess) MeOH f) O NaBD4 D OH MeOH 45 20.8) Draw the products when the following compounds are treated with NaBH4 in MeOH. a) O HO NaBH4 H H MeOH b) + NaBH4 O OH OH MeOH c) (H3C)3C O NaBH4 MeOH (H3C)3C OH (H3C)3C + OH 46 20.9) What reagent is needed to carry out the reaction below? O HO Cl H Cl Two reagents are needed to carry out this reaction. First, the (S)-CBS reagent to produce the R-enantiomer. Followed by H2O to protonate the alcohol. 47 20.10) Draw a stepwise mechanism for the following reaction. O LiAlH4 OH H2O Cl O O O Cl Cl H H H3Al H + Cl + AlH3 O O H OH OH H H H H H3Al H H + OH + AlH3 48 20.11) Draw an acid chloride and an ester that can be used to produce each product. a) O O Cl OCH3 CH2OH Cl OCH3 b) O OH O O c) OH H3CO H3CO Cl O H3CO OCH3 49 20.12) Draw the products of LiAlH4 reduction of each compound. O a) OH OH O b) NH2 NH2 c) O N(CH3)2 N(CH3)2 50 d) O NH NH 51 20.13) What amide will form each of the following amines when treated with LiAlH4? O a) NH2 NH2 O b) N c) N O N H N H 52 20.14) Predict the products of these compounds when treated with the following reagents. O O OH a) LiAlH4 OCH3 H2O OH OH O NaBH4 MeOH b) O OCH3 O LiAlH4 H3CO OH H2O NaBH4 MeOH HO OH No reaction 53 c) H3CO H3CO O OH LiAlH4 H2O NaBH4 H3CO OH MeOH 54 20.15) Predict the products in the following reactions. a) OH Ag2O No Reaction NH4OH O Na2Cr2O7 H2SO4, H2O b) OH O OH OH O Ag2O NH4OH OH Na2Cr2O7 O O H2SO4, H2O OH 55 20.16) Predict the products of the compound below when OH reacted with each reagent. O HO a) OH NaBH4 OH MeOH HO b) LiAlH4 OH H2O OH HO 56 OH O HO c) O PCC O O OH d) O Ag2O OH NH4OH HO O e) O CrO3 OH H2SO4, H2O HO O 57 Organometallic Reagents • Other metals in organometallic reagents are Sn, Si, Tl, Al, Ti, and Hg. General structures of the three common organometallic reagents are shown: 58 • Since both Li and Mg are very electropositive metals, organolithium (RLi) and organomagnesium (RMgX) reagents contain very polar carbon—metal bonds and are therefore very reactive reagents. • Organomagnesium reagents are called Grignard reagents. • Organocopper reagents (R2CuLi), also called organocuprates, have a less polar carbon—metal bond and are therefore less reactive. Although they contain two R groups bonded to Cu, only one R group is utilized in the reaction. • In organometallic reagents, carbon bears a - charge. 59 • Organolithium and Grignard reagents are typically prepared by reaction of an alkyl halide with the corresponding metal. • With lithium, the halogen and metal exchange to form the organolithium reagent. With Mg, the metal inserts in the carbon—halogen bond, forming the Grignard reagent. 60 • Grignard reagents are usually prepared in diethyl ether (CH3CH2OCH2CH3) as solvent. • It is thought that two ether O atoms complex with the Mg atom, stabilizing the reagent. 61 • Organocuprates are prepared from organolithium reagents by reaction with a Cu+ salt, often CuI. 62 • Acetylide ions are another example of organometallic reagents. • Acetylide ions can be thought of as “organosodium reagents”. • Since sodium is even more electropositive than lithium, the C—Na bond of these organosodium compounds is best described as ionic, rather than polar covalent. 63 • An acid-base reaction can also be used to prepare sp hybridized organolithium compounds. • Treatment of a terminal alkyne with CH3Li affords a lithium acetylide. • The equilibrium favors the products because the sp hybridized C—H bond of the terminal alkyne is more acidic than the sp3 hybridized conjugate acid, CH4, that is formed. 64 • Organometallic reagents are strong bases that readily abstract a proton from water to form hydrocarbons. • Similar reactions occur with the O—H proton of alcohols and carboxylic acids, and the N—H protons of amines. 65 • Since organolithium and Grignard reagents are themselves prepared from alkyl halides, a two-step method converts an alkyl halide into an alkane (or other hydrocarbon). • Organometallic reagents are also strong nucleophiles that react with electrophilic carbon atoms to form new carbon—carbon bonds. • These reactions are very valuable in forming the carbon skeletons of complex organic molecules. 66 Examples of functional organometallic reagents: group transformations involving [1] Reaction of R—M with aldehydes and ketones to afford alcohols [2] Reaction of R—M with carboxylic acid derivatives 67 [3] Reaction of R—M with other electrophilic functional groups 68 Reaction of Organometallic Reagents with Aldehydes and Ketones. • Treatment of an aldehyde or ketone with either an organolithium or Grignard reagent followed by water forms an alcohol with a new carbon—carbon bond. • This reaction is an addition because the elements of R’’ and H are added across the bond. 69 • This reaction follows the general mechanism for nucleophilic addition—that is, nucleophilic attack by a carbanion followed by protonation. • Mechanism 20.6 is shown using R’’MgX, but the same steps occur with RLi reagents and acetylide anions. 70 Note that these reactions must be carried out under anhydrous conditions to prevent traces of water from reacting with the organometallic reagent. 71 • This reaction is used to prepare 1°, 2°, and 3° alcohols. 72 Retrosynthetic Analysis of Grignard Products • To determine what carbonyl and Grignard components are needed to prepare a given compound, follow these two steps: 73 • Let us conduct a retrosynthetic analysis of 3-pentanol. 74 • Writing the reaction in the synthetic direction—that is, from starting material to product—shows whether the synthesis is feasible and the analysis is correct. • Note that there is often more than one way to synthesize a 20 alcohol by Grignard addition. 75 Protecting Groups • Addition of organometallic reagents cannot be used with molecules that contain both a carbonyl group and N—H or O—H bonds. • Carbonyl compounds that also contain N—H or O—H bonds undergo an acid-base reaction with organometallic reagents, not nucleophilic addition. 76 Solving this problem requires a three-step strategy: [1] Convert the OH group into another functional group that does not interfere with the desired reaction. This new blocking group is called a protecting group, and the reaction that creates it is called “protection.” [2] Carry out the desired reaction. [3] Remove the protecting group. This reaction is called “deprotection.” A common OH protecting group is a silyl ether. 77 tert-Butyldimethylsilyl ethers are prepared from alcohols by reaction with tert-butyldimethylsilyl chloride and an amine base, usually imidazole. The silyl ether is typically removed with a fluoride salt such as tetrabutylammonium fluoride (CH3CH2CH2CH2)4N+F¯. 78 The use of tert-butyldimethylsilyl ether as a protecting group makes possible the synthesis of 4-methyl-1,4pentanediol by a three-step sequence. 79 Figure 20.7 General strategy for using a protecting group 80 For Friday, 20.17-20.25 81 20.17) Write out the rea tions needed to convert CH3CH2Br to each of the following reagents. a) H3CH2C H3CH2C b) Li H3CH2C H3CH2C c) + 2 Li Br Li H3CH2C MgBr + Li Br MgBr + Mg Br H3CH2C CuLi H3CH2C 2 H3CH2C H3CH2C Br Li + 2 Li H3CH2C Li Li Cu + CuI H3CH2C + Li Br + Li CH2CH3 I 82 20.18) 1-octyne reacts readily with NaH, forming a gas that bubbles out of the reaction mixture. 1-octyne also reacts with CH3MgBr and a different gas is produced. Write out balanced equations for each reaction. HC CCH2CH2CH2CH2CH2CH3 + NaH NaC HC CCH2CH2CH2CH2CH2CH3 CCH2CH2CH2CH2CH2CH3 + H2 + CH3MgBr BrMgC CCH2CH2CH2CH2CH2CH3 + CH4 83 20.19) Draw the product of the following reactions. a) Li + H2O + LiOH b) MgBr c) + H2O MgBr + H2O + HOMgBr + HOMgBr 84 d) LiC CCH2CH3 + H2O HC CCH2CH3 + LiOH 85 20.20) Draw the product formed when each compound is treated with C6H5MgBr followed by H2O. a) H O OH H b) H H O CH2CH3 OH H3CH2C CH2CH3 CH2CH3 O c) CH2CH3 H3CH2C OH H H 86 d) OH O 87 20.21)Draw the products of each reaction. a) H3CH2CH2C Li + LiOH H2O O HO b) CH2CH2CH3 Li H O + LiOH HO H H2O H H OH c) C6H5Li O + LiOH H2 O 88 d) CNa H2C H2 C O C H2O OH + NaOH 89 20.22) Draw the products (including stereochemistry) of the following reactions. a) O H H3CH2C H3C b) H OH H MgBr OH + H2O CH2CH3 H3CH2C O Li OH H2O + CH2CH3 OH 90 20.23) What Grignard and carbonyl are needed to prepare each alcohol? a) O OH + H3C MgBr H MgBr O OH b) + H H O OH c) + H3CH2C MgBr or O MgBr |+ 91 d) OH O H3C + Br or O MgBr + 92 20.24) Tertiary alcohols with three different R groups on the carbon attached to the OH can be prepared in three different ways using the Grignard reagent. Show them. a) OH O H3C CH2CH3 CH2CH3 CH2CH2CH3 + H3C MgBr + H CH C 3 2 MgBr H3CH2CH2C O H3C CH2CH2CH3 O + H CH CH C 3 2 2 H3C MgBr CH2CH3 93 b) OH O + H3C MgBr MgBr O + MgBr O + 94 c) OH O MgBr + MgBr O + + H3CH2C MgBr O 95 20.25) Show the steps for the following reaction. CH2CH2CH2CH3 HO O HO OH TBDMS-Cl HO O TBDMSO H N O N BrMg CH2CH2CH2CH3 H2O CH2CH2CH2CH3 FN(CH2CH2CH2CH3)4 HO OH CH2CH2CH2CH3 TBDMSO H2O OH 96 Reaction of Organometallic Reagents with Carboxylic Acid Derivatives. • Both esters and acid chlorides form 3° alcohols when treated with two equivalents of either Grignard or organolithium reagents. 97 98 • To form a ketone from a carboxylic acid derivative, a less reactive organometallic reagent—namely an organocuprate—is needed. • Acid chlorides, which have the best leaving group (Cl¯) of the carboxylic acid derivatives, react with R’2CuLi to give a ketone as the product. • Esters, which contain a poorer leaving group (¯OR), do not react with R’2CuLi. 99 Reaction of Compounds Organometallic Reagents with Other • Grignards react with CO2 to give carboxylic acids after protonation with aqueous acid. • This reaction is called carboxylation. • The carboxylic acid formed has one more carbon atom than the Grignard reagent from which it was prepared. 100 • The mechanism resembles earlier reactions of nucleophilic Grignard reagents with carbonyl groups. 101 • Like other strong nucleophiles, organometallic reagents—RLi, RMgX, and R2CuLi—open epoxide rings to form alcohols. 102 • The reaction follows the same two-step process as opening of epoxide rings with other negatively charged nucleophiles—that is, nucleophilic attack from the back side of the epoxide, followed by protonation of the resulting alkoxide. • In unsymmetrical epoxides, nucleophilic attack occurs at the less substituted carbon atom. 103 ,-Unsaturated Carbonyl Compounds • ,-Unsaturated carbonyl compounds are conjugated molecules containing a carbonyl group and a C=C separated by a single bond. • Resonance shows that the carbonyl carbon and the carbon bear a partial positive charge. 104 • This means that ,-unsaturated carbonyl compounds can react with nucleophiles at two different sites. 105 • The steps for the mechanism of 1,2-addition are exactly the same as those for the nucleophilic addition of an aldehyde or a ketone—that is, nucleophilic attack, followed by protonation. 106 107 • Consider the conversion of a general enol A to the carbonyl compound B. A and B are tautomers: A is the enol form and B is the keto form of the tautomer. • Equilibrium favors the keto form largely because the C=O is much stronger than a C=C. Tautomerization, the process of converting one tautomer into another, is catalyzed by both acid and base. 108 109 110 111 Summary Reagents of the Reactions of Organometallic [1] Organometallic reagents (R—M) attack electrophilic atoms, especially the carbonyl carbon. 112 [2] After an organometallic reagent adds to the carbonyl group, the fate of the intermediate depends on the presence or absence of a leaving group. [3] The polarity of the R—M bond determines the reactivity of the reagents: —RLi and RMgX are very reactive reagents. —R2CuLi is much less reactive. 113 Synthesis Figure 20.8 Conversion of 2–hexanol into other compounds 114 Reactions of Alcohols—Dehydration • Dehydration, like dehydrohalogenation, is a elimination reaction in which the elements of OH and H are removed from the and carbon atoms respectively. • Dehydration is typically carried out using H2SO4 and other strong acids, or phosphorus oxychloride (POCl3) in the presence of an amine base. 115 • Typical acids used for alcohol dehydration are H2SO4 or ptoluenesulfonic acid (TsOH). • More substituted alcohols dehydrate more easily, giving rise to the following order of reactivity. 116 • When an alcohol has two or three carbons, dehydration is regioselective and follows the Zaitsev rule. • The more substituted alkene is the major product when a mixture of constitutional isomers is possible. 117 • Secondary and 3° alcohols react by an E1 mechanism, whereas 1° alcohols react by an E2 mechanism. 118 • Since 1° carbocations are highly unstable, their dehydration cannot occur by an E1 mechanism involving a carbocation intermediate. Therefore, 1° alcohols undergo dehydration following an E2 mechanism. 119 Dehydration of Alcohols Using POCl3 and Pyridine • Some organic compounds decompose in the presence of strong acid, so other methods have been developed to convert alcohols to alkenes. • A common method uses phosphorus oxychloride (POCl3) and pyridine (an amine base) in place of H2SO4 or TsOH. • POCl3 serves much the same role as a strong acid does in acid-catalyzed dehydration. It converts a poor leaving group (¯OH) into a good leaving group. • Dehydration then proceeds by an E2 mechanism. 120 121 Conversion of Alcohols to Alkyl Halides with HX • Substitution reactions do not occur with alcohols unless ¯OH is converted into a good leaving group. • The reaction of alcohols with HX (X = Cl, Br, I) is a general method to prepare 1°, 2°, and 3° alkyl halides. 122 • More substituted alcohols usually react more rapidly with HX: • This order of reactivity can be rationalized by considering the reaction mechanisms involved. The mechanism depends on the structure of the R group. 123 124 125 • The reactivity of hydrogen halides increases with increasing acidity. • Because Cl¯ is a poorer nucleophile than Br¯ or I¯, the reaction of 10 alcohols with HCl occurs only when an additional Lewis acid catalyst, usually ZnCl2, is added. Complexation of ZnCl2 with the O atom of the alcohol makes a very good leaving group that facilitates the SN2 reaction. 126 Conversion of Alcohols to Alkyl Halides with SOCl2 and PBr3 • Primary and 2° alcohols can be converted to alkyl halides using SOCl2 and PBr3. • SOCl2 (thionyl chloride) converts alcohols into alkyl chlorides. • PBr3 (phosphorus tribromide) converts alcohols into alkyl bromides. • Both reagents convert ¯OH into a good leaving group in situ—that is, directly in the reaction mixture—as well as provide the nucleophile, either Cl¯ or Br¯, to displace the leaving group. 127 • When a 1° or 2° alcohol is treated with SOCl2 and pyridine, an alkyl chloride is formed, with HCl and SO2 as byproducts. • The mechanism of this reaction consists of two parts: conversion of the OH group into a better leaving group, and nucleophilic cleavage by Cl¯ via an SN2 reaction. 128 129 • Treatment of a 10 or 20 alcohol with PBr3 forms an alkyl halide. • The mechanism of this reaction also consists of two parts: conversion of the OH group into a better leaving group, and nucleophilic cleavage by Br¯ via an SN2 reaction. 130 131 132 Tosylate—Another Good Leaving Group • Alcohols can be converted into alkyl tosylates. • An alkyl tosylate is composed of two parts: the alkyl group R, derived from an alcohol; and the tosylate (short for ptoluenesulfonate), which is a good leaving group. • A tosyl group, CH3C6H4SO2¯, is abbreviated Ts, so an alkyl tosylate becomes ROTs. 133 • Alcohols are converted to tosylates by treatment with ptoluenesulfonyl chloride (TsCl) in the presence of pyridine. • This process converts a poor leaving group (¯OH) into a good one (¯OTs). • Tosylate is a good leaving group because its conjugate acid, p-toluenesulfonic acid (CH3C6H4SO3H, TsOH) is a strong acid (pKa = -7). 134 • (S)-2-Butanol is converted to its tosylate with retention of configuration at the stereogenic center. Thus, the C—O bond of the alcohol is not broken when tosylate is formed. 135 • Because alkyl tosylates have good leaving groups, they undergo both nucleophilic substitution and elimination, exactly as alkyl halides do. • Generally, alkyl tosylates are treated with strong nucleophiles and bases, so the mechanism of substitution is SN2, and the mechanism of elimination is E2. 136 • Because substitution occurs via an SN2 mechanism, inversion of configuration results when the leaving group is bonded to a stereogenic center. • We now have another two-step method to convert an alcohol to a substitution product: reaction of an alcohol with TsCl and pyridine to form a tosylate (step 1), followed by nucleophilic attack on the tosylate (step 2). 137 • Step 1, formation of the tosylate, proceeds with retention of configuration at a stereogenic center. • Step 2 is an SN2 reaction, so it proceeds with inversion of configuration because the nucleophile attacks from the backside. • Overall there is a net inversion of configuration at a stereogenic center. Example: 138 Figure 9.8 Summary: Nucleophilic substitution and β elimination reactions of alcohols 139 For Monday, 20.26-20.36 140