* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lehninger Principles of Biochemistry 5/e
Basal metabolic rate wikipedia , lookup
Catalytic triad wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Photosynthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
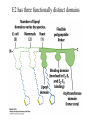
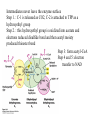
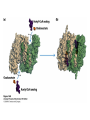
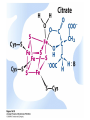
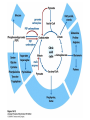
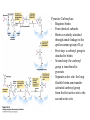
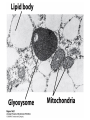
David L. Nelson and Michael M. Cox LEHNINGER PRINCIPLES OF BIOCHEMISTRY Fifth Edition CHAPTER 16 The Citric Acid Cycle © 2008 W. H. Freeman and Company Cellular Respiration occurs in three stage - Organic fuel molecules are oxidized to yield twocarbon fragemnts in the form of acetyla –coA - The acetyl group is oxidized into carbon dioxide in the citric acid cycle; energy released is conserved in the NADH and FADH2 - This reduced coenzyme transferred electron to oxygen through ETS and oxidative phosphorylation Pyruvate is oxidized to acetyl-coA and CO2 Oxidative decarboxylation by PDH complex The PDH complex requires five coenzyme TPP, FAD, NAD, CoA, lipoate Reactive thiol, acyl carrier, thioester ( high acyl group transfer poteintial and donate their acyl group to acceptor molecule) Two thiol groups, ser both as an electron carrier and an acyl carrier 1. PHD complex contains three enzyme - pyruvate DH (E1) - dihydrolipoyl transacetylase (E2) - dihydrolipoyl DH (E3) 2. E2 is the point of connection for the lipoate, attached to Lys. 3. The active site of E1 has bound TPP 4. E3 has bound FAD E2 has three functionally distinct domains Intermediates never leave the enzyme surface Step 1. : C-1 is released as CO2, C-2 is attached to TPP as a hydroxyethyl group Step 2. : this hydroxyethyl group is oxidized into acetate and electrons reduced disulfide bond and then acetyl moiety produced thioester bond Step 3: form acetyl-CoA Step 4 and 5: electron transfer to NAD 1. Central to the mechanism are the winging lipoyllysyl arms of E2, which accept from E1 the two electrons and the acetyl group, passing them to E3 2. Substrate Channeling: allowing the intermediates to react quickly without diffusing away from the surface of enzyme complex Thus, local concentration of substrate is kept high 1. In each turn of the cycle, on acetyl froup enters as acetyl-CoA and two Co2 leave; 1 OAA used and 1 OAA generated; NADH and FADH2, GTP or ATP 2. Four or five carbon intermediate serve as precursor of biomolecule 3. In eucaryotes, cycle takes place in mitochondria - the site of most energyyielding oxidative reactions and of the coupled synthesis of ATP 3. In procaryotes. cycle are in the cytosol, plasma memb. plays a role analogues to that of inner mitrochondrial memb. in ATP synthesis 1. - Formation of citrate: the condensation of acetyl-CoA with oxaloacetate. Methyl carbon of acetyl group is joined to the carbonyl group (C-2) of OAA The hydrolysis of high-energy thioester makes the forward reaction Citrate sythase : homodimeric enzyme; induce conformational change by OAA binding, creating binding site for acetyl-CoA; when citroylCoA has formed , another conformational change brings about thioester hysorlysis; ordered bisubstrate mechanism 2. Formation of isocitrate via cis-aconitate - The aconitase catalyzes the reversible transformation of citrate to isocitrate - Because isocitrate is rapidly consumed in the next step of cycle, the reaction is pulled to the right - Iron sulfur center: binding site of the substrate, catalytic addition or removal of water 3. Oxidation of isocitrate to a-ketoglutarate and carbon dioxide - Isocitrate dehydrogenase catalyzed oxidative decarboxylation - Mn2+ in active site: stabilized intermediates 4. Oxidation of a-ketoglutarate to succinyl-CoA and carbon dioxide - a-ketoglutarate dehydrogenase complex: NAD serve as an electron aceeptor and CoA as the carrier of the succinyl group. - Resemble to pyruvate dehydrogenase complex:E1, E2, E3 complex. TPP. Lipate, FAD, NAD, CoA - Energy of oxidation is conserved in the formation of thioester bond 5. Conversion of succinyl-CoA to succinate - Succinyl-CoA sythetase - Thioester bond phophoanhydride bond of GTP or ATP 6. Oxidation of succinat to fumarate - Succinate dehydrogenase: flavoprotein, bind to the mitochondrial inner membrane, three different iron-sulfur cluster and FAD - Electorn pass from succinate through the FAD and iron-sulfur center before entering ETS Malonate is a competitive inhibitor of succinate dehydrogenase and block the citric acid cycle 6. Hydration of fumarate to malate 6. Oxidation of Malate to Oxalocetate - The equilibrium of this reaction less far to the left under standard condition, but in intact cells oxaloacetate is continually remove by the highly exergonic citrate synthase reaction 1. The energy of oxidation in the cycle is efficiently conserved 3 NADH, 1FADH2, 1 GTP or ATP 2. Any compound that give rise to four- or five carbon intermediates of the citric acid cycle can be oxidized by the cycle glutamate a-ketoglutarate aspartate oxloactate 3. The citric acid cycle is amphibolic, serving in both catabolism and an anabolism; cycle intermediates can be drawn off and used as the starting material for variety of biosynthetic products Anaplerotic reactions replenish citric acid cycle intermediates - As intermediates of the citric acid cycle are removed to serve as biosynthetic precursors, they are replenished by anaplerotic reactions - The most important anaplerotic reaction in mammalian liver and kideny is the reversible carboxylation of pyruvate by CO2 to form oxaloacetate. Pyruvate Carboxylase - Requires biotin - Four identical subunits - Biotin covalently attached through amide linkage to the epsilon-amino group of Lys - First step: a carboxyl group is attached to biotin - Second step: the carboxyl group is transferred to pyruvate - Separate active site: the long flexible biotin arm transfer activated carboxyl group from the first active site to the second active site Long flexible arms in biochemical reaction Pyruvate dehydrogenase complex 1. Allosteric regulation - Inhibited by ATP, acetyl-coA, NADH, long-chain fatty acid - Activated by AMP, CoA, and NAD+ 2. Covalent protein modification - Inhibited by phosphorylation of E1 - Kinase allosteically activated by ATP - Thus [ATP] high inhibit pyruvate dehydrogenase complex Regulation of citric acid cycle 1. Three factors govern the rate of the cycle - Substrate availability, inhibition by products, allosteric feedback inhibition - Substrate availability: acetyl-CoA and oxaloacetate limits the rate of citrate formation - Inhibition by product: citrate synthase by citrate, aketoglutarate dehydrogenase by succinyl-CoA - allosteric feedback inhibition: ATP inhibits citrate synthase and isocitrate dehydrogenase 2. In muscle, Ca2+, the signal for increase in demand for ATP, acitvate isocitrate DH, a-ketoglutarate DH as well as PDH complex Regulation of citric acid cycle Glyoxylate Cycle 1. Vertebrate cannot convert fatty acid, or the acetate derived from them, to carbohydrate - PEP to PA and PA to AcetylCoA are so exergonic as to be essentially irreversible - In many organism, other than vertebrates, the glyoxylate cycle serve as a mechanism for converting acetate to carbohydrate - Two enzymes mediates glyoxylate cycle: isocitrate lyase and malate synthase - Vetebrates do not have these enzymes - Overall, 2 acetyl-CoA produces1 succinate - - - - In plant, glyoxysomes contain all the enzymes needed for the degradation of the fatty acid Acetyl-CoA from fatty acid is converted to succinate and then succinate is exported to mitochondria Succinate is converted into malate in citric acid cycle malate is exported to cytosol and converted into glucose by gluconeogenesis Glyoxylate Cycle and citric acid cycle are coordinately regulated - - - Isocitrate dehydrogenase is regulated by covalent modification; phosphoylation inactivated isocitrate enter to glyoxylate cycle glucose Phosphatae remove phosphate group from isocitrate dehydrogenase and reactive this enzyme Regulatory protein kinase and phosphatase in single polypeptide The same intermediates of glycolysis and the citric acid cycle activate isocitrate dehydrogenase wherase inhibits isocitrate lyase