* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Samantha Landolfa Amy Ryan Section 10 Experiment 9 – Alkenes
Woodward–Hoffmann rules wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
George S. Hammond wikipedia , lookup
Petasis reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
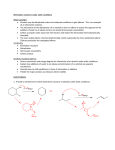
Samantha Landolfa Amy Ryan Section 10 Experiment 9 – Alkenes from Alcohols: Analysis of a Mixture by Gas Chromatography H2SO4 OH 2-methyl-2-butanol H2O + 2-methyl-1-butene 2-methyl-2-butene Introduction In this experiment, 2-methyl-2-butanol is dehydrated to produce a mixture of two isomers that can be analyzed by gas chromatography. The dehydration of the alcohol is accomplished via an E1 elimination reaction. The rates of reactivity are tertiary > secondary > primary. An E1 reaction is favored when a tertiary alcohol is reacted in the presence of an acid. A carbocation intermediate forms and the products follow Zaitsev’s Rule, which states that the major product will be the most substituted alkene because it is the most stable. In this experiment, the most stable alkene is 2-methly-2-butene, which is tri-substituted about the double bond. The minor product is 2-methyl-1-butene, a di-substituted alkene. The hydroxyl group is a poor leaving group, but protonation by concentrated sulfuric acid forms water, which acts as a good leaving group. This step is fast. Sulfuric acid is useful in E1 reactions as both a catalyst and a dehydrating agent. When the water is eliminated, a carbocation forms, from which a proton is easily abstracted by a weak base such as water or HSO4-. This is the slow, rate-determining step. A double bond forms when the proton on an adjacent carbon is abstracted. The formation of 2methyl-1-butene requires the loss of a proton from an adjacent primary carbon, while 2-methyl-2butene requires the loss of a proton from an adjacent secondary carbon. An E2 reaction differs from an E1 reaction in that the elimination occurs in one step rather than two, and there is no carbocation formation. E1 reaction rates are first order and depend solely on the concentration of the substrate, while E2 are second order, dependent on the concentration of both the substrate and the base. Rearrangements are common in E1 reactions, but impossible for E2 reactions because there is no intermediate. The strength of the base or nucleophile is the most important factor for determining the order of a reaction. If 2-methyl-2-butanol were reacted in the presence of a strong base, the reaction would proceed via an E2 mechanism instead of E1 because the strong base forces second-order kinetics. In gas chromatography, a sample is separated based on temperature, polarity, and rate of gas flow. The mobile phase is a gas such as nitrogen or helium and the stationary phase is a nonvolatile liquid with a high boiling point. The sample is vaporized and then passed through a column where the separation occurs. Mechanism: + O H2SO4 C slow slow H + H2O - OH + fast + HO S O OH2 H O H O H2SO4 H slow - OH + fast OH2 slow + + HO S O + H2O C O H Possible side products (in addition to H2O, OH-, H3O+, and HSO4-): H HO + - :H shift + CH C H + HO H - S O - :H shift + + C H S + O O - CH O HO O OH O H + H + C OH CH2 - :H shift - + HO OH O S O O H + H CH2 - :H shift + C - HO + S O O HO - + C :CH3 shift + - + H2C HO O HO S O - + C :CH3 shift + H2C + O S - OH O O O O Experimental 1. Add 1.5 mL distilled water to a 10 mL conical vial. 2. Add 1.0 mL concentrated sulfuric acid dropwise while mixing. 3. Cool solution in an ice bath to 0oC. 4. Add 1.0 mL 2-methyl-2-butanol and mix thoroughly. 5. Add a boiling stone and perform a simple distillation at 30-45oC. 6. Cool receiving vial and add calcium chloride pellets to dry the product. 7. Place the cap on the vial to prevent evaporation and weigh product. 8. Determine the percent yield of the mixture of butenes. 9. Inject 1.0 μL of mixture into gas chromatograph. 10. Record conditions and run gas chromatography to get printout. 11. Interpret the results. Results Percent yield = actual/theoretical x 100 = (0.945 g/70.13 g/mol)/[2(0.80 g/88.146 g/mol)] x 100 = 0.0135 mol/0.0181 mol x 100 = 74.58% Retention time Percentage A 1.662 min 10.15315% B 2.092 min 89.84685% Conclusion According to the gas chromatography results, the mixture of alkenes contained approximately 10% of alkene A and 90% of alkene B. The retention time of alkene A was 1.662 minutes and for alkene B it was 2.092 minutes. This indicates that alkene A is more volatile, and therefore less stable, than alkene B. from the structure of each alkene, it can be concluded that alkene A is 2-methyl-1-butene and alkene B is 2-methyl-2-butene. This is evident because 2-methyl-1-butene is only di-substituted, while 2methyl-2-butene is tri-substituted. Dehydration reactions follow Zaitsev’s rule, which states that the major product of a reaction will be the alkene with the most substituents about the double bond. The reason behind this is that the more substituted an alkene is, the more stable it becomes. Therefore, the major product is 2-methyl-2butene.