* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
Kinetic resolution wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Bottromycin wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Discodermolide wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ene reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Elias James Corey wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Aldol reaction wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
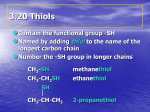
Organosulfur compounds wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
CH 420, Spring 2015 CH 18 practice problems Name ___________________________ 1) Draw the products of the following ester reduction. Consider the mechanism of the reaction and relative reactivity of carbonyl compounds towards nucleophiles. 2) Tert-butyloxycarbonyl (Boc) is a common protecting group for amines. Removal of this group is accomplished by treatment with acid. Outline the mechanism of the initial steps of Boc cleavage, noting that this reaction works only for tert-butyl carbamate – not methyl, ethyl, propyl, etc. 3) Determine the product obtained by treatment of the following chiral alcohol with base. Clearly indicate stereochemistry. What is the mechanism in the above reaction? 4) Indicate the product with its appropriate regio- and stereochemistry. Note the reaction conditions. 5) Thiols can be converted to disulfides by treatment with iodine. In this transformation, iodine is a(n): a. b. c. d. e. f. Acid Base Nucleophile Electrophile Oxidizing agent Reducing agent 6) Complete the following scheme. The pKa of thiophenol is ca. 7. Are thiols more or less nucleophilic than their corresponding alcohols? Are sulfides (thioethers) more or less prone to oxidation than the corresponding ether? 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ________________ > Most acidic ________________ > ________________ > ________________ Least acidic