* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia
Neurogenomics wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Development of the nervous system wikipedia , lookup
Environmental enrichment wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Nervous system network models wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Synaptic gating wikipedia , lookup
Spike-and-wave wikipedia , lookup
Neuroanatomy wikipedia , lookup
Circumventricular organs wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Hypothalamus wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Optogenetics wikipedia , lookup
Endocannabinoid system wikipedia , lookup
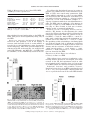
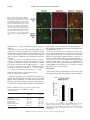
Am J Physiol Regul Integr Comp Physiol 284: R1454–R1465, 2003. First published January 30, 2003; 10.1152/ajpregu.00286.2002. special topic Peptides that Regulate Food Intake Orexin gene expression is increased during states of hypertriglyceridemia Katherine E. Wortley,* Guo-Qing Chang,* Zoya Davydova, and Sarah F. Leibowitz The Rockefeller University, New York, New York 10021 Submitted 20 May 2002; accepted in final form 17 January 2003 Wortley, Katherine E., Guo-Qing Chang, Zoya Davydova, and Sarah F. Leibowitz. Orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol 284: R1454–R1465, 2003. First published January 30, 2003; 10.1152/ajpregu.00286. 2002.—Previous reports implicate the orexins in eating and body weight regulation. This study investigated possible functional relationships between hypothalamic orexins and circulating hormones or metabolites. In situ hybridization and quantitative PCR were used to examine orexin expression in the perifornical hypothalamus (PF) of rats and mice on diets varying in fat content and with differential propensity toward obesity. The results showed that orexin gene expression was stimulated by a high-fat diet in close association with elevated triglyceride levels, suggesting a functional relationship between these measures. Results obtained in obesity-prone rats and mice revealed a similar increase in orexin in close relation to triglycerides. A direct test of this orexin-triglyceride link was performed with Intralipid, which increased PF orexin expression along with circulating triglycerides. Whereas PF galanin is similarly stimulated by dietary fat, double-labeling immunofluorescence studies showed that orexin and galanin neurons are anatomically distinct. This evidence suggests that the orexins, like galanin, are “fat-responsive” peptides that respond to circulating lipids. (hypocretin) peptides A and B derive from a common precursor protein, prepro-orexin, which is expressed specifically in the lateral hypothalamus (LH), most densely within the perifornical region (PF) (15, 16). In addition to their role in the control of arousal and sleep-wake cycle (34), there is evidence that the orexins are involved in body weight regulation. Food deprivation stimulates orexin gene expression (11, 48), and injection of orexin A causes a small enhancement of food intake in rats (32, 48, 52). Conversely, administration of an orexin receptor 1 antagonist (30, 31) or anti-orexin antibody (61) inhibits feeding behavior. That these pharmacological results reflect a physiological function is supported by a recent study showing that genetic ablation of orexin neurons in mice causes hypophagia (29). However, these mice also develop late-onset obesity, indicating that the orexins may have additional effects beyond the stimulation of food intake. Indeed, other studies indicate that orexin peptides can increase metabolic rate and sympathetic nervous system (SNS) activity (3, 19, 49, 51) and have variable effects on lipid utilization depending on time of day (38). Orexin neurons express leptin receptor immunoreactivity (27), and leptin administration downregulates orexin A levels in this area (6, 37). Whereas these findings suggest an inhibitory influence of leptin on the orexins, other studies show that orexin gene expression is downregulated in ob/ob mice and fa/fa rats, both of which have disturbed leptin signaling (7, 20, 51, 62). There are few studies that have actually tested the relationship between leptin and orexin peptides under physiological conditions. Two reports that exist fail to reveal a change in orexin expression in feeding paradigms associated with elevated leptin (11, 53), suggesting that this hormone may not be a key physiological regulator of the orexins. Other evidence implicates glucose and insulin in the control of the orexins. Because the LH contains neurons that are stimulated by a decline in glucose levels (44), it is not surprising that a subset of orexin neurons concentrated in this area is also affected by this change in glucose (41). In addition, orexin expression increases in response to insulin-induced hypoglycemia and also to food deprivation, which reduces glucose as well as insulin (11). Further studies are needed to characterize a possible relationship between the orexins and this metabolite or hormone under physiological conditions. Circulating lipids may also influence peptide gene expression in the hypothalamus. This has been demonstrated by studies with galanin (36) and dynorphin (58), which are stimulated by a high-fat diet. Circulating lipid levels rise considerably with dietary obesity (4, 17), and there is evidence that circulating fatty acids derived from triglycerides can cross the bloodbrain barrier and gain access to the brain (35, 57). Although not yet directly tested, the possibility that lipids modulate the orexin system is suggested by evi- * K. E. Wortley and G.-Q. Chang contributed equally to this work. Address for reprint requests and other correspondence: S. F. Leibowitz, The Rockefeller Univ., 1230 York Ave., New York, NY 10021 (E-mail: [email protected]). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. perifornical lateral hypothalamus; triglycerides; high-fat diet; obesity THE OREXIN R1454 0363-6119/03 $5.00 Copyright © 2003 the American Physiological Society http://www.ajpregu.org OREXINS AND CIRCULATING TRIGLYCERIDES dence that orexin-synthesizing neurons may coexpress dynorphin (5, 60) and galanin (27). To further understand the various signals that modulate the orexin system, this study used different animal models of dietary obesity to measure orexin gene expression in relation to shifts in circulating levels of leptin, insulin, triglycerides, and glucose. METHODS Animals Adult male Sprague-Dawley rats (275–285 g; Charles River Breeding Labs, Kingston, NY) or 8-wk-old male inbred C57L/J and AKR/J mice (Jackson Laboratories, Bar Harbor, ME) were used. They were individually housed in a fully accredited American Association for the Accreditation of Laboratory Animal Care facility (22°C, with lights off at 3:30 PM for 12 h), according to institutionally approved protocols as specified in the NIH Guide to the Use and Care of Animals and also with the approval of the Rockefeller University Animal Care Committee. All animals were given 1 wk to acclimate to lab conditions, and all protocols fully conformed to the “Guiding Principles for Research Involving Animals and Human Beings” of the American Physiological Society (2). Diets All diets were supplemented with vitamins and minerals, as described previously (50, 55). In experiments 1 and 4, rats were maintained on a single diet varying in fat composition. Diet composition, calculated as percentage of total kilocalories, ranged from a low-fat diet (LFD: 10% fat, 65% carbohydrate, 25% protein) with 3.75 kcal/g, to a moderate-fat diet (MFD: 25% fat, 50% carbohydrate, 25% protein) with 3.98 kcal/g, to a high-fat diet (HFD: 50% fat, 25% carbohydrate, 25% protein) with 4.7 kcal/g. In experiments 2 and 5, rats were maintained on an HFD. In experiment 3, inbred mice were allowed to choose from separate sources of protein (casein, 3.7 kcal/g), carbohydrate (dextrin, sucrose, cornstarch, 3.7 kcal/g), and fat (lard, 7.7 kcal/g) (55). In experiment 6, rats were maintained on laboratory chow. Test Procedures All rats were maintained ad libitum on food and water for the duration of the experiment. At the end of the experiment, food was removed 1 h before death (except in experiment 6), and the animals were killed by rapid decapitation 1–2 h before dark onset. Trunk blood was collected, and body fat pads from four regions (inguinal, retroperitoneal, gonadal, and mesenteric) were collected and weighed. In experiments 1–4, the rats or mice were maintained on their respective diets for 3 wk, and food intake was measured three times per week and body weight one time per week for the duration of the experiment. In experiment 2, the rats were killed after 3 wk on an HFD and were designated lean or obese based on the combined weight of their four fat pads. The rats were ranked, with the lightest 33% designated lean and the heaviest 33% designated obese, as described before (36). Rats with a combined fat pad weight that fell in the middle range were not used in this study. In experiment 5, rats were killed after 5 days on an HFD and were designated obesity resistant or obesity prone based on their weight gain during this 5-day period. Preliminary studies from this lab, consistent with the work of Hill and colleagues (13), show this weight gain measure on a HFD to R1455 be a strong and reliable correlate of long-term body fat accrual, permitting one to characterize obesity-prone rats during the very early stages in their development of obesity. The identification of obesity-prone rats with this measure can occur even before a significant rise in fat pad weights, with the elevated 5-day weight gain presumably reflecting increased weight of nonadipose tissues, extra food in the gut, and greater water retention. Based on this, a daily weight gain score across the 5 days was recorded and averaged for each rat. The subjects were then rank ordered and designated obesity resistant (lowest 33%) or obesity prone (highest 33%), with the middle range eliminated from the study. In experiment 6, rats in the absence of food were given an intraperitoneal injection of saline or 20% Intralipid (5 ml/rat, Clintech Nutrition). This mode of administration was employed to minimize trauma to the rat and avoid the anesthesia required for intravenous injections, which can interfere with triglyceride metabolism (8, 40). The rats were killed 4 h after injection, which in pilot studies revealed the largest increase in circulating triglycerides. Hormone and Metabolite Assays Trunk blood was assayed for insulin, leptin, glucose, and triglycerides. Insulin (RIR-1685) and leptin (RLR-1143) were assayed with radioimmunoassay kits from Linco Research. Serum levels of glucose and triglycerides were analyzed using a commercially available serum chemistry analyzer at Amgen. In Situ Hybridization In situ hybridization with digoxigenin-labeled probes was used to quantify orexin gene expression. This technique measures specifically the density of neurons expressing the orexin gene above threshold levels, rather than the level of mRNA expressed per cell. This technique was chosen because it is sensitive, due in part to our procedure of using freefloating sections, and it has a high signal-to-noise ratio, with almost no background, making quantification possible (12, 36). It is also time efficient, allowing analyses of large groups of animals, and it maintains high-quality morphology, permitting examination and quantification of cells in specific hypothalamic nuclei. Particularly important, it also avoids problems of isotope contamination and decay. Results obtained with this technique are generally consistent with those obtained, in this and other labs, using such techniques as immunocytochemistry, radioimmunoassay, RNase protection assay, and isotopic in situ hybridization. In the present study, they are further validated by measurements of mRNA using quantitative PCR, as described below. After rapid decapitation, brains were removed, fixed, and stored, as described previously (36). On the day of use, brains were cut into 30-m-thick sections. An anti-sense cRNA probe (final concentration, 5 g/ml), labeled with digoxigenin, was prepared by in vitro transcription. Briefly, a pBC SK plasmid, containing a 565-bp rat prepro-orexin cDNA (a generous gift of Dr. L. de Lecea, The Scripps Research Institute), was linearized with EcoRI and NotI and was transcribed in vitro with T3 or T7 RNA polymerase for the anti-sense and sense probes, respectively. Free-floating coronal sections were processed, as described previously (36). They were incubated with the cRNA probe at 55°C for 18 h, and, based on pilot studies, a 6-h period was chosen for the orexin color reaction. The sense probe control was performed in the same tissue, and no signal was found. AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org R1456 OREXINS AND CIRCULATING TRIGLYCERIDES Quantification of In Situ Hybridization A digital imaging system, with the help of a rat brain atlas (45), was used for quantification of cell density (density of cells/mm2). The hypothalamic areas examined were the PF and the LH (bregma ⫺2.8 to ⫺3.6 mm). To obtain a total of six separate readings per subject at a given level, the density of cells in three to four sections at the same level was routinely analyzed, with a fourth section read if an area was unreadable due to tissue damage. A Leitz microscope was used with a ⫻4 illumination objective, and a Leitz volts monitor (model 050260) kept the light voltage at 12 V to maintain light intensity. The digital image was collected by a video camera connected to an IBM computer with WScan Array Software (Galai Lat, Migdal Haemek, Israel). The area of interest was outlined using the Drawing Tool available in the software. The defined area was always at the same level on all sections chosen for control and experimental animals. To count the number of black pixels above background level within the areas of interest, a threshold value was first established, as described in the instruction manual. Briefly, using 10 randomly selected sections from the experiment, this threshold was set by matching the number of objects counted by the software in a defined area of each section with the number of objects counted manually in that same area. When these numbers of objects were found to match in all 10 sections, this threshold value was kept constant and used for all sections counted within a given experiment. By setting the threshold, the software then permitted the experimenter to count the objects above background level. Double-Labeling Studies Sprague-Dawley rats (350–450 g) were injected intracerebroventricularly with colchicine (120 g in 20 l NaCl; Sigma, St. Louis, MO) and killed 48 h later by rapid decapitation. The brains were removed and treated, as described before (36). Thirty-micrometer free-floating cryostat sections were blocked in 1% normal serum with 0.1% Triton X-100PBS for 30 min, followed by incubation in rabbit anti-orexin serum (1:200, supplied by Dr. L. de Lecea, The Scripps Research Institute) and guinea pig anti-GAL serum (1:100, Peninsula Laboratories) overnight. After three washes in PBS, 5 min each, the tissue was incubated in FITC-conjugated donkey anti-rabbit (1:50, JacksonImmuno Research, PA) and Cy3-conjugated donkey anti-guinea pig serum (1: 100, JacksonImmuno Research, PA) for 30 min. Finally, the tissue was washed in PBS, mounted onto glass slides, and covered with Vectashield mounting medium (Vector Laboratories). Sections were then examined, and double-labeling images were scanned using a Zeiss 510 laser confocal microscope. RNA Extraction and cDNA Synthesis The whole hypothalamus or PF, using the fornix and third ventricle as landmarks, was rapidly microdissected. Total RNA from individual and pooled samples was extracted with TRIzol reagent (Invitrogen) and filtered with Qiagen RNeasy, according to the manufacturer’s instructions. One microgram of RNA was reverse transcribed into cDNA using the Reverse Transcription System (Invitrogen) with oligo dT primer and SuperScript Reverse Transcriptase. Before RT, the RNA was treated with RNase-free DNase I (Invitrogen) to remove any contaminating genomic DNA. The RT reaction was carried out at 42°C for 50 min and terminated by heating at 70°C for 15 min. Samples in which RT was omitted were also forwarded for PCR as negative control samples, to confirm that no genomic DNA contamination occurred. Quantitative PCR For quantitative PCR, the SYBR Green PCR core reagents kit (Applied Biosystems) was used, and the real-time PCR was performed in MicroAmp Optic 96-well reaction plates (Applied Biosystems) on an ABI PRISM 7700 Sequence Detection system (Applied Biosystems). The reaction mixture (20 l) was composed of 1⫻ SYBR Green buffer, 3.5 mM MgCl2, 200 M dNTP mixed with dUTP, 0.625 U AmpliTaq Gold, 0.25 U AmpErase UNG, 400 nM -actin primers, 800 nM orexin primers, 0.5 l RT product, and RNase-, DNasefree water. The primers for rat -actin were 5⬘-GGCCAACCGTGAAAAGATGA-3⬘ (forward) and 5⬘-CACAGCCTGGATGGCTACGT-3⬘ (reverse). The primers for rat orexin were 5⬘-AGATACCATCTCTCCGGATTGC-3⬘ (forward) and 5⬘-ACCAGGGAACCTTTGTAGAAGG-3⬘ (reverse). These primers were designed with ABI Primer Express v.1.5a software. The reaction conditions were an initial 2 min at 50°C, followed by 10 min at 95°C, then 40 cycles of 15 s at 95°C and 1 min at 60°C. The whole study consisted of four independent runs of RT-PCR in triplicate. In addition to samples, each run included a standard curve, a nontemplate control, and a negative RT control. The levels of orexin mRNA expression were quantified relative to the level of the housekeeping gene -actin, using a standard curve method. Data Analysis Caloric intake, presented as kilocalories per 24 h, was averaged from values taken in the last week of a given experiment, unless otherwise stated. Body weight, body weight gain, and fat pad weights are calculated for the final week of measurements. Hypotheses regarding diet groups, brain areas, and endocrine and behavioral measures were tested using either a one-way or two-way ANOVA, followed by a Bonferroni post hoc test for multiple comparisons between groups, when appropriate, or using unpaired or paired t-tests. For quantitative PCR, the ratio of the relative concentration of orexin to -actin in the test vs. control samples was analyzed by a paired t-test. Within-group measures of fat pad weights, hormones, metabolites, and orexin cell density were related using a Pearson’s product-moment correlation. The criterion for the use of the term “significant” in the text is that the probability value (P) for a given test is ⬍0.05. RESULTS Experiment 1: Impact of an HFD on Orexin Expression in Rats This experiment tested the relationship between the orexin system and dietary fat. Orexin mRNA was examined in three groups of rats (n ⫽ 7/group) maintained for 3 wk on either an LFD, MFD, or HFD. Behavioral, hormonal, and metabolite measures. Rats maintained on an HFD compared with an LFD had greater body weight and fat pad weights (Table 1). This increase in body weight was accompanied by, and presumably attributed to, hyperphagia seen during the first week on the HFD (87 ⫾ 2.9 kcal) vs. LFD (73 ⫾ 2.0 kcal, P ⬍ 0.05), but not during week 2 (HFD 109 ⫾ 1.9 vs. LFD 104 ⫾ 2.3) or week 3 (Table 1). The HFD rats had higher levels of leptin but lower insulin levels compared with the LFD rats (Table 1). In addition, AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org OREXINS AND CIRCULATING TRIGLYCERIDES Table 1. Measurements in rats fed an LFD, MFD, or HFD for 3 wk in experiment 1 Final body weight, g Body fat, g Caloric intake, kcal/day Leptin, ng/ml Insulin, ng/ml Triglycerides, mg/dl Glucose, mg/dl LFD MFD HFD 476 ⫾ 5 16 ⫾ 1.2 96 ⫾ 9.4 15 ⫾ 2.5 2.8 ⫾ 0.20 87 ⫾ 15 143 ⫾ 15 483 ⫾ 13 17 ⫾ 0.5 101 ⫾ 10.1 16 ⫾ 0.9 1.6 ⫾ 0.36† 189 ⫾ 49† 138 ⫾ 6 519 ⫾ 13†§ 23 ⫾ 0.2†§ 85 ⫾ 3.1 21 ⫾ 1.0†§ 1.5 ⫾ 0.32† 207 ⫾ 32† 177 ⫾ 17*‡ Data are means ⫾ SE; n ⫽ 7/group. LFD, low-fat diet; MFD, moderate-fat diet; HFD, high-fat diet. * P ⬍ 0.05, † P ⬍ 0.01 vs. LFD; ‡ P ⬍ 0.05, § P ⬍ 0.01 vs. MFD. they exhibited elevated triglycerides on the MFD and HFD, with glucose levels significantly rising only on the HFD. Orexin gene expression. An increase in dietary fat from 10 to 50% produced a rise in the density of neurons with detectable levels of orexin mRNA, as revealed by in situ hybridization using digoxigeninlabeled riboprobes. This effect reached statistical significance in the PF, as shown in Fig. 1, A and B, but not in the LH (LFD 490 ⫾ 105; MFD 715 ⫾ 72; HFD 739 ⫾ 65 cells/mm2), an area immediately lateral to the PF. R1457 To validate this diet-induced increase in orexin expression, two additional sets of rats, maintained on an HFD vs. LFD, were tested using quantitative PCR. Orexin mRNA, expressed as a ratio to -actin mRNA, was measured in the PF dissection of the first set of rats with pooled tissue samples (n ⫽ 4/group) and the second set of rats with individual samples (n ⫽ 7/group). Consistent with the in situ hybridization results, the HFD subjects exhibited a clear increase in the ratio of orexin to -actin mRNA in the PF dissection (Fig. 2). This occurred in both sets of tissues, whether pooled or examined individually. Relation of orexins to circulating hormones and metabolites. The density of cells expressing the orexin gene in the PF was strongly positively correlated with circulating triglycerides. This was evident in rats on the MFD (r ⫽ ⫹0.67, P ⬍ 0.05) or HFD (r ⫽ ⫹0.81, P ⬍ 0.05), with a similar but statistically insignificant correlation seen in the LFD rats (r ⫽ ⫹0.57, P ⬎ 0.10). The specificity of this relationship to circulating lipids was indicated by the lack of significant correlations obtained on an HFD, for example, between orexin cell density and the measures of kilocalorie intake (r ⫽ ⫹0.29), fat pad weight (r ⫽ ⫺0.31), leptin (r ⫽ ⫺0.26), insulin (r ⫽ ⫺0.04), and glucose (r ⫽ ⫹0.30), measures that are altered by the HFD. Experiment 2: Orexin Expression in Relation to Obesity in Rats on a HFD With evidence from experiment 1 indicating a relationship between dietary fat, blood lipids, and orexin neurons in the PF, this experiment tested an additional animal model, comparing obese vs. lean groups on an HFD, which also involves elevated triglycerides. Behavioral, hormonal, and metabolite measures. Rats that became obese on an HFD accumulated a total of 28 ⫾ 1.1 g of fat pad weight, compared with only 18 ⫾ 1.2 g for the lean rats on the same HFD. Similar Fig. 1. Density of orexin-expressing cells in the perifornical hypothalamus (PF) of rats maintained on a low-fat (LFD), moderate-fat (MFD), or high-fat diet (HFD) in experiment 1 (n ⫽ 7/group), as measured by in situ hybridization. A: bar graph shows an increase in orexin cell density in the PF as dietary fat content increases from 10 to 50%. Data are means ⫾ SE. * P ⬍ 0.05 vs. LFD. B: photomicrographs illustrate the increase in orexin cell density in the dorsal region of the PF of rats fed HFD vs. LFD. F, fornix. Fig. 2. Orexin mRNA in the PF of rats maintained on LFD or HFD in experiment 1, as measured by quantitative PCR. Data (means ⫾ SE) show a significant increase in orexin mRNA levels, expressed as a ratio to -actin mRNA, in both pooled (n ⫽ 4/group) and individual (n ⫽ 7/group) PF samples from HFD rats (* P ⬍ 0.05 vs. LFD rats). AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org R1458 OREXINS AND CIRCULATING TRIGLYCERIDES to the group differences in experiment 1 (Table 1), the obese (vs. lean) rats (Table 2) had greater body weight and exhibited higher levels of triglycerides as well as leptin, with glucose levels tending to be elevated. These two animal models differed, however, in their measures of insulin and total caloric intake. Both measures were elevated in the obese (vs. lean) rats of this experiment (Table 2), while insulin levels were lower and caloric intake unchanged in the final week in the HFD (vs. LFD) rats of experiment 1. Orexin gene expression. Despite these differences, the density of orexin-expressing cells was increased in the PF of obese compared with lean rats on the HFD (Fig. 3A). No significant change in orexin cell density was seen in the LH. To validate this obesity-induced increase in orexin expression, two additional sets of obese vs. lean rats on an HFD were examined using quantitative PCR. Orexin mRNA, as a ratio to -actin mRNA, was measured in the whole hypothalamus of rats with pooled tissue (n ⫽ 4/group) or individual samples (n ⫽ 8/group). The obese rats of these groups showed, respectively, a 35 and 45% increase in the ratio of orexin to -actin mRNA in the hypothalamus compared with lean rats (Fig. 3B), confirming the increase seen with in situ hybridization. Relation of orexins to circulating hormones and metabolites. The density of cells with detectable orexin mRNA in the PF, as revealed by in situ hybridization, was positively correlated with triglyceride levels across individual subjects within the whole group (r ⫽ ⫹0.70, P ⬍ 0.05). Orexin cell density was additionally related to fat pad weight (r ⫽ ⫹0.69, P ⬍ 0.05) and to strong correlates of body fat, namely, leptin (r ⫽ ⫹0.65, P ⬍ 0.05) and insulin (r ⫽ ⫹0.70, P ⬍ 0.05). It showed little relation, however, to glucose levels (r ⫽ ⫺0.27, P ⬎ 0.10) or to total kilocalorie intake (r ⫽ ⫹0.48, P ⬎ 0.10). Experiment 3: Orexin in Relation to a Propensity Toward Obesity in Inbred Mice To cross-validate these findings observed in rats, we examined two strains of inbred mice. Orexin expression in AKR/J mice, which are susceptible to obesity on a HFD, was compared with that of C57L/J mice, which are less responsive to the obesity-promoting effects of a HFD (59). All mice were maintained ad libitum for 3 wk with a choice of three macronutrient diets: fat, carbohydrate, and protein (see METHODS). Table 2. Measurements in lean and obese rats on HFD in experiment 2 Final body weight, g Caloric intake, kcal/day Leptin, ng/ml Insulin, ng/ml Triglycerides, mg/dl Glucose, mg/dl Fig. 3. Density of orexin-expressing cells in the hypothalamus of obese vs. lean rats on an HFD in experiment 2. A: data (means ⫾ SE) reveal an increase in orexin cell density in the perifornical hypothalamus (PF) but not the lateral hypothalamus (LH) of obese rats, as measured by in situ hybridization (n ⫽ 6/group). B: orexin mRNA (ratio to -actin mRNA) in the hypothalamus of lean and obese rats in experiment 2, as measured by quantitative PCR, shows a significant increase in orexin expression in both pooled (n ⫽ 4/group) and individual samples (n ⫽ 8/group) from obese rats. (* P ⬍ 0.05 vs. lean rats). Behavioral, hormonal, and metabolite measures. The lean C57L/J mice on these macronutrient diets exhibited a strong preference for dietary carbohydrate over fat, consuming 62% of their daily kilocalorie intake in the form of carbohydrate and only 13% as fat (Table 3). The obese AKR/J mice, in contrast, consumed 33% of their daily caloric intake in the form of fat. They also Table 3. Measurements in lean C57L/J and obese AKR/J mouse strains given 3 macronutrient diets in experiment 3 C57L/J Lean Obese 463 ⫾ 28 99 ⫾ 2.3 12 ⫾ 0.8 0.7 ⫾ 0.06 86 ⫾ 9 104 ⫾ 7.4 525 ⫾ 5* 107 ⫾ 2.7* 22 ⫾ 0.7† 1.0 ⫾ 0.04† 127 ⫾ 21* 117 ⫾ 5.3 Data are means ⫾ SE; n ⫽ 6/group. * P ⬍ 0.05, † P ⬍ 0.01 vs. lean. Final body weight, g Fat intake, %caloric intake Carbohydrate intake, %caloric intake Protein intake, %caloric intake Body fat, g Leptin, ng/ml Triglycerides, mg/dl AJP-Regul Integr Comp Physiol • VOL AKR/J 24 ⫾ 0.49 32 ⫾ 0.81* 13 ⫾ 2.2 33 ⫾ 4* 62 ⫾ 1.7 42 ⫾ 3.7* 17 ⫾ 1.9 1.0 ⫾ 0.18 2.4 ⫾ 0.8 66 ⫾ 9.0 26 ⫾ 2.2* 1.9 ⫾ 0.17* 8.1 ⫾ 1.1* 101 ⫾ 6.6* Data are means ⫾ SE; n ⫽ 5/group. * P ⬍ 0.01 vs. C57L/J. 284 • JUNE 2003 • www.ajpregu.org OREXINS AND CIRCULATING TRIGLYCERIDES R1459 had significantly greater fat pad weights, in addition to higher levels of triglycerides and leptin compared with the C57L/J mice (Table 3). Levels of insulin and glucose were not measured, due to the low volume of blood collected from these mice. Orexin gene expression. Together with elevated triglycerides and body weight, the obese AKR/J mice exhibited a greater density of cells expressing the orexin gene in the PF compared with the lean C57L/J mice (Fig. 4). These results are very similar to those obtained in the obese rats of experiment 2. Experiment 4: Relationship of the Orexins to Galanin in the PF This experiment performed two tests to determine whether galanin-expressing cells in the PF are responsive to dietary fat and, if so, whether the orexin and galanin peptides actually colocalize in neurons of the PF, as suggested by a preliminary report (27). In two sets of rats maintained on either an LFD or HFD, this experiment examined diet-induced changes in the density of PF galanin-expressing cells in one set (n ⫽ 5/group) and analyzed peptide immunoreactivity (peptide-ir) for orexin and galanin together in the second set (n ⫽ 3/group), using double-labeling immunocytochemistry. Galanin gene expression in PF. Similar to the orexins (experiment 1), a rise in dietary fat from 10 to 50% produced a small, but significant, increase in the density of galanin-expressing cells in the PF (Fig. 5). This change was detected immediately dorsal to the fornix, in the same area where orexin neurons are expressed. Galanin and orexin peptide-ir in PF. With singlelabeling immunocytochemistry, a dense concentration of orexin-positive neurons and terminals was observed in the PF, both the dorsal and ventromedial areas around the fornix (Fig. 6, A and B). Galanin-positive neurons and terminals were also evident in these same areas of the PF (Fig. 6, C and D). While exhibiting Fig. 5. Density of galanin-expressing cells in the PF of rats maintained on LFD, MFD, or HFD in experiment 4 (n ⫽ 5/group). Data (means ⫾ SE) show a significant increase in galanin cell density in the PF as dietary fat content increases from 10 to 50%. * P ⬍ 0.05 vs. LFD; † P ⬍ 0.01 vs. LFD; ‡ P ⬍ 0.05 vs. MFD. anatomic overlap, the orexin-positive neurons were considerably larger than the galanin-containing neurons. They also had larger processes, suggesting that these peptides are expressed in separate neurons. Consistent with this, the double-labeling immunocytochemistry failed to reveal any coexistence of these two peptides in neurons of the PF. Whereas there was a suggestion at low magnification (⫻25) that these peptides may colocalize in a few neurons, a higher magnification (⫻63 or ⫻100) using laser confocal microscopy failed to reveal any double-labeled neurons, in either the dorsal or ventromedial areas of the PF (Fig. 6, E and F). This was true for each of the six rats examined, whether on an LFD or an HFD. This lack of colocalization agrees with the morphological differences of the orexin- and galanin-containing neurons in suggesting that they are distinct populations. Experiment 5: Orexin mRNA in Relation to a Propensity Toward Obesity in Rats Fig. 4. Density of orexin-expressing cells in the PF of lean C57L/J mice and obese AKR/J mice (n ⫽ 5/group) in experiment 3. Data (means ⫾ SE) show a reliable increase in orexin cell density in the PF of AKR/J mice. * P ⬍ 0.05 vs. C57L/J control mice. This next experiment provided a further test of the observed orexin-triglyceride relationship, specifically in a physiological state where triglyceride levels increase before significant changes in fat pad weights and leptin. Behavioral, hormonal, and metabolite measures. Obesity-prone rats, defined by their weight gain of 10.2 ⫾ 0.2 g/day on an HFD compared with 6.8 ⫾ 0.2 g/day for the obesity-resistant rats, exhibited an 8% (28 g) increase in body weight and were hyperphagic during the 5-day period (Table 4). With this small increase in body weight, the obesity-prone rats exhibited little change in fat pad weight, only a 1-g (⫹10%) increase, and they also had normal levels of leptin (Table 4). These rats, however, showed a similar pattern to the already-obese rats of experiment 2, in having elevated levels of triglycerides compared with the obesity- AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org R1460 OREXINS AND CIRCULATING TRIGLYCERIDES Fig. 6. Photomicrographs of immunofluorescent orexin- and galanin-staining neurons in the PF in experiment 4. A and B: immunofluorescent orexin cell bodies and terminals are shown in the dorsal and ventromedial areas around the fornix. C and D: galanin-positive neurons and terminals are shown in the same dorsal and ventromedial areas of the PF. E and F: double-labeling immunocytochemistry fails to reveal any coexistence of orexin and galanin in neurons of the PF. Magnification, ⫻25. resistant rats, as well as higher insulin and glucose (Table 4). Orexin gene expression. This endocrine profile of obesity-prone rats was accompanied by a small but significant increase in the density of orexin-expressing cells in the PF compared with obesity-resistant subjects (Fig. 7). Again, no difference in cell density was seen in the LH. Relation of orexin to circulating hormones and metabolites. Within-group analyses revealed, once again, a positive correlation between the density of orexin cells in the PF and levels of triglycerides (r ⫽ ⫹0.54, P ⬍ 0.05) across the group. Orexin mRNA showed no relation, in contrast, to insulin (r ⫽ ⫺0.10), glucose (r ⫽ ⫺0.12), or total kilocalorie intake (r ⫽ ⫹0.38). triglycerides. Acute intraperitoneal administration of this emulsion significantly increases circulating levels of triglycerides (1, 39). Behavioral, hormonal, and metabolite measures. Injection of Intralipid caused an ⬃29% increase in serum triglyceride levels (Table 5). This effect was seen in the absence of any change in leptin, insulin, or glucose, as well as in body weight and fat pad weight (Table 5). Orexin gene expression. Rats injected with Intralipid compared with saline rats showed a significant increase in the density of cells with detectable levels of orexin mRNA in the PF (Fig. 8). No change in orexin expression was seen in the LH. Experiment 6: Impact of Intralipid Injection on Orexin Expression in Rats The results of these experiments reveal a strong and consistent relationship, under physiological conditions, DISCUSSION This final experiment was conducted to test the possibility that triglycerides elevated in the blood have impact, direct or indirect, on orexin neurons in the PF. This was examined using the compound Intralipid, a commercially available lipid emulsion containing 20% Table 4. Measurements in obesity-resistant and obesity-prone rats on a HFD for 5 days in experiment 5 Final body weight, g Body fat, g Caloric intake, kcal/day Leptin, ng/ml Insulin, ng/ml Triglycerides, mg/dl Glucose, mg/dl Obesity Resistant Obesity Prone 350 ⫾ 3 12 ⫾ 0.5 113 ⫾ 3.1 12 ⫾ 1.2 0.6 ⫾ 0.07 117 ⫾ 12 116 ⫾ 8.6 378 ⫾ 3† 13 ⫾ 0.4 132 ⫾ 3.4† 12 ⫾ 1.4 1.1 ⫾ 0.21* 168 ⫾ 14† 145 ⫾ 8.4* Data are means ⫾ SE; n ⫽ 8/group. * P ⬍ 0.05, † P ⬍ 0.01 vs. obesity resistant. Fig. 7. Density of orexin-expressing cells in the PF or LH of obesityresistant and obesity-prone rats on HFD in experiment 5 (n ⫽ 8/group). Data (means ⫾ SE) reveal an increase in orexin cell density in the PF but not LH of obesity-prone rats. * P ⬍ 0.05 vs. obesityresistant rats. AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org OREXINS AND CIRCULATING TRIGLYCERIDES Table 5. Measurements in rats 4 h after intraperitoneal injection of saline or intralipid in experiment 6 Final body weight, g Body fat, g Leptin, ng/ml Insulin, ng/ml Triglycerides, mg/dl Glucose, mg/dl Saline Intralipid 322 ⫾ 3 4.0 ⫾ 0.35 1.20 ⫾ 0.14 2.0 ⫾ 0.21 56 ⫾ 4.6 162 ⫾ 13 323 ⫾ 4 4.3 ⫾ 0.33 1.43 ⫾ 0.16 1.9 ⫾ 0.16 72 ⫾ 6.4* 162 ⫾ 19 Data are means ⫾ SE; n ⫽ 8/group. * P ⬍ 0.05 vs. saline. between dietary triglycerides and orexin expression in the PF. Although anatomically overlapping in the PF and similarly responsive to dietary fat, the orexin and galanin peptide systems are distinct and likely function separately as they contribute to the physiological control and pathology of eating and body weight. Close Relation Between Orexin-Expressing Neurons and Circulating Triglycerides A main result of this report, revealed by in situ hybridization, is that consumption of an HFD in rats increases the density of orexin-expressing cells and that this effect occurs specifically in the PF. This dietinduced increase in orexin expression was confirmed with measurements of orexin mRNA in PF dissections or hypothalamus using quantitative PCR. This effect was not seen, however, in the LH, an area lying immediately adjacent to the PF. This high degree of site specificity, in addition to differences in experimental conditions, may explain why measurements of orexin mRNA or orexin A peptide in whole hypothalamus failed to reveal a significant change on an HFD (53, 65). Because orexin neurons are restricted spatially and other neurons are unlikely to have the necessary transcription factors required to turn on orexin expression, the rise in density of orexin-expressing cells in the PF may reflect a rise in expression from basal levels undetectable by digoxigenin-labeled probes. This is particularly likely because the animals were killed at the end of the light period, at a time when PF orexin is generally at its nadir (22, 64). Of particular note is the additional evidence that the HFD-induced increase in PF orexin cell density is accompanied invariably by a rise in circulating triglyceride levels. These parallel changes in orexin expression and metabolic fuels are characteristic of each of the rat feeding models tested. These models are the 25% fat MFD or 50% fat HFD rats, the already-obese rats on an HFD, and the obesity-prone rats in a preobese state on an HFD. A specific relationship between triglycerides and the orexins is substantiated by strong, positive correlations repeatedly detected between these measures in the different groups tested. Consistent with these findings in rats, the density of cells expressing orexin mRNA is also elevated in obese AKR/J mice, which compared with C57L/J mice consume greater fat R1461 and have higher circulating triglycerides, as well as body weight. These results confirm the basic finding that orexin expression rises with elevated lipids and obesity on a fat-rich diet, although it remains to be determined, with basal measurements independent of diet, whether the increased orexin mRNA in AKR/J mice reflects an inherent strain difference. The specificity of an orexin relationship to triglycerides is underscored by the finding, in experiment 5, that animals with a propensity toward obesity, but still with normal fat pad weights equivalent to controls, have elevated orexin cell density in the PF, together with higher triglycerides. Thus triglycerides or closely related metabolic fuels appear to be a necessary component of the enhanced orexin gene expression. Distinct Populations of Fat-Responsive Galanin and Orexin Neurons in the PF The results of the present and published studies reveal similarities between the orexins and galanin, suggesting that these two peptides may function as part of a common system regulating energy balance. Like the orexins, galanin expression is stimulated by an HFD in the PF, as shown here, and in the PVN (36), and it is elevated in obese rats (36). Moreover, preliminary studies indicate a possible coexistence of galanin and orexins in a few isolated neurons in the PF (27). Despite these similarities, there is little support for the possibility that these two peptides exist and function within the same neuronal population of the PF. Whereas the orexins and galanin at low magnification may appear to colocalize in a few PF neurons (27), this was not evident here at higher magnification, even in rats maintained on an HFD. Moreover, their neurons exhibit clear morphological differences, with the orexin-positive neurons and their processes considerably larger. Fig. 8. Density of orexin-expressing cells in the PF or LH of salineor Intralipid-treated rats (n ⫽ 10/group). Data (means ⫾ SE) reveal an increase in orexin cell density in the PF of Intralipid-treated rats in experiment 6. * P ⬍ 0.01 vs. saline-treated rats. AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org R1462 OREXINS AND CIRCULATING TRIGLYCERIDES Little Relation of Orexin-Expressing Neurons to Total Caloric Intake or Body Weight The increased density of orexin-expressing cells in the various models of dietary obesity raises the possibility that the orexins are responding to either a rise in total caloric intake or body weight. The present study, together with published results, however, is inconsistent with these possibilities. Although the HFD, obese, and obesity-prone rats exhibit hyperphagia during their initial exposure to the HFD, no significant correlations were seen between orexin cell density and caloric intake in any of these groups. These findings agree with published evidence, showing that orexin expression is actually inhibited by the presence of food in the gut (9), and it rises in association with a deprivation-induced reduction in caloric intake (11, 48). It is also unchanged in rats that overeat on a highly palatable diet compared with chow-fed rats (11) and is actually decreased in hyperphagic ob/ob mice and fa/fa rats (7, 20, 51, 62). Although the increased orexin gene expression in the three dietary models is accompanied by an elevation of body weight, several pieces of evidence dissociate these phenomena. In the present study, Intralipid stimulates orexin cell density in the absence of a change in body weight. Further, there were no significant correlations between orexin cell density and fat pad weights in the different groups tested, except the obese subjects of experiment 2. Although further tests are needed to provide direct evidence dissociating the orexins and body weight, published studies indicate that excess body weight per se does not increase and may, in fact, decrease orexin expression. For example, orexin mRNA is unchanged in obese rats fed a highly palatable diet (11), and it is actually reduced in ob/ob mice and fa/fa rats, which are profoundly obese (7, 20, 51, 62). Thus orexin neurons appear to be responsive specifically to a rise in dietary or circulating lipids, such as triglycerides, which occurs independently of changes in caloric intake or body weight. The orexins are, therefore, referred to as “fat-responsive” peptides. Little Association Between the Orexins and Circulating Levels of Insulin, Glucose, and Leptin In contrast to its association with triglycerides, orexin expression in the PF on an HFD shows little relation to circulating insulin and glucose, at least with food available ad libitum. The results demonstrate an increase in orexin expression in the PF of all models showing a rise in triglycerides, despite variable shifts in insulin and glucose. Consistent with these findings, the expression and production of the orexins are not affected by insulin-induced hypoglycemia in freely feeding animals (9, 11) or in insulin-injected animals given concurrent injections of glucose (25). This evidence argues against a functional link between orexin neurons and physiological changes in insulin or glucose. Under conditions of food deprivation, however, the orexins are shown to be stimulated in association with hypoglycemia (9, 11). Whereas previous studies demonstrate an inhibitory effect of leptin injection on orexin gene expression (6, 37), there is little evidence for a close association between this hormone and peptide system under natural feeding conditions. Increased orexin gene expression, while accompanied by a rise in leptin in HFD rats and in obese rats or mice on an HFD, can be similarly seen in the absence of a change in leptin, in the obesityprone rats. Further, investigations in genetically obese mutants with disrupted leptin signaling generally show no change (53) or a decrease in orexin peptides or gene expression (10, 51, 62). Thus endogenous leptin may not be important in the control of orexin neurons under physiological conditions. Direct Impact of Triglycerides on Orexin-Expressing Neurons in the PF The strong association between the orexins and triglycerides led us to test whether the orexin neurons in the PF are, in fact, responsive to experimentally induced changes in triglyceride levels. Such changes in triglycerides can be seen with acute injection of the compound, Intralipid (39). In experiment 6, this agent significantly raised circulating levels of triglycerides within 4 h after injection, without affecting insulin, glucose, or leptin levels. With this specific increase in triglycerides, Intralipid stimulated orexin gene expression in the PF, in precisely the same site affected by an HFD or dietary obesity. This finding supports the idea that circulating triglycerides, elevated under natural feeding conditions, contribute to the enhanced orexin gene expression. As for how triglycerides may impact on the brain, evidence indicates that these lipids are rapidly hydrolyzed in capillary endothelium and that fatty acids derived from triglycerides enter the brain from the plasma, through as yet undefined mechanisms (57). Although there is no direct evidence in the present study that fatty acids stimulate orexin neurons, it has long been known that they activate neurons in the LH in vitro (43). This suggests that fatty acids, derived from dietary triglycerides carried in chylomicrons, may actually stimulate neurons in this area in vivo. Whereas the mechanism of this response remains to be determined, it may involve both a direct, stimulatory effect of fatty acids on neuronal activity (43) or an indirect, inhibitory effect of fatty acids on glucose transport, in a manner similar to that observed in the periphery (24). The idea that neurons in the PF are responsive to a rise in lipids is supported by additional evidence that exposure to an HFD stimulates c-Fos expression in this area (54). Further experiments involving measurements of circulating fatty acids are needed to test this idea. Possible Function of PF Orexin Neurons on a Fat-Rich Diet Taken together, the above evidence suggests that orexin neurons in the PF are stimulated by a rise in circulating lipids during a state of positive energy AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org OREXINS AND CIRCULATING TRIGLYCERIDES balance, as well as by a decline in glucose levels during a state of negative energy balance. This suggests that the orexin peptides may have multiple functions depending on nutritional status, consistent with other evidence distinguishing functional subsets of orexin neurons (23). An interaction of the orexins with other obesity-related systems may provide the mechanisms for their multiple functions in energy balance. Among other areas, orexin neurons project to the arcuate nucleus (ARC) (42), where they innervate one set of neurons that expresses NPY and another set that expresses proopiomelanocortin (POMC) together with cocaine- and amphetamine-related transcript (CART) (26). Evidence showing a relation between orexin and NPY neurons demonstrates that both are stimulated by hypoglycemia (28, 47), and the feeding-stimulatory effect of orexin A is blocked by NPY antagonists (18, 33, 63). Thus, in states of negative energy balance, PF orexin neurons may be activated by a decline in glucose and, through ARC NPY neurons, trigger counterregulatory responses that maintain glucose levels and protect the body during starvation. In states of positive energy balance, in contrast, the orexin neurons in the PF, projecting to POMC/CART neurons, may function very differently. The orexins are similar to the POMC/ CART peptides in being stimulated by an HFD (46, 56) and implicated in the stimulation of lipid metabolism (46, 51) and SNS activity (19, 21, 49). Thus, with a fat-rich diet freely available, PF orexin neurons may be activated by the lipids and specifically target POMC/ CART neurons, which help the body deal with surplus lipid calories. This idea, relating the orexins to fat metabolism, receives support from the finding that orexin A stimulates lipid utilization during the light period but not at dark onset (38). At dark onset, NPY neurons are presumably the predominantly active neurons in the ARC, which initiate food intake and suppress POMC/CART neuronal activity (14). Thus injection of orexin A at this time may further stimulate NPY neurons, while having little impact on POMC/CART neurons and, thus, lipid utilization. In contrast, during the light period when NPY neurons are presumably quiescent, orexin A should be able to stimulate POMC/CART neurons and, thereby, increase lipid metabolism. This may help to explain why mice depleted of orexin neurons, which lack this excitatory input to both orexigenic NPY neurons and metabolism-promoting POMC/CART neurons, exhibit hypophagia in addition to obesity (29). In conclusion, the present results indicate that orexin neurons in the PF are fat-responsive, stimulated in parallel with a rise in triglycerides under both physiological and nonphysiological conditions. Although galanin in the same region also has properties of a fat-responsive peptide, these peptides most likely function as separate systems involved in lipid storage and utilization. Based on this together with published evidence, it is proposed that orexin neurons respond to elevated levels of circulating lipids, as well as to a decline in glucose. These neurons, in turn, project to different sets of downstream neurons, to maintain en- R1463 ergy homeostasis in states of both positive and negative energy balance. We thank Dr. L. de Lecea (The Scripps Research Institute) for the generous supply of orexin cDNA and antibody, and Clintech Nutrition for the supply of Intralipid. We are grateful to Prof. J. Arch (Univ. of Buckingham) for reading the manuscript and providing helpful comments and to F. Li and Dr. Z. Han (Rockefeller Univ.) for assistance with the quantitative PCR technique. We very much appreciate the help of K. Sepiashvili in the preparation of this manuscript. We thank Amgen for analysis of triglycerides and glucose levels. This research was supported by grants from the Wellcome Trust (058240) and the National Institutes of Health (MH-43422). REFERENCES 1. Adkins ES, Salman FT, and Fonkalsrud EW. Triglyceride absorption in transperitoneal alimentation. Am J Surg 159: 237–240, 1990. 2. American Physiology Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. 3. Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, and Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 281: R1801–R1807, 2001. 4. Bahceci M, Tuzcu A, Akkus M, Yaldiz M, and Ozbay A. The effect of high-fat diet on the development of obesity and serum leptin level in rats. Eat Weight Disord 4: 128–132, 1999. 5. Bayer L, Mairet-Coello G, Risold PY, and Griffond B. Orexin/hypocretin neurons: chemical phenotype and possible interactions with melanin-concentrating hormone neurons. Regul Pept 104: 33–39, 2002. 6. Beck B and Richy S. Hypothalamic hypocretin/orexin and neuropeptide Y: divergent interaction with energy depletion and leptin. Biochem Biophys Res Commun 258: 119–122, 1999. 7. Beck B, Richy S, Dimitrov T, and Stricker-Krongrad A. Opposite regulation of hypothalamic orexin and neuropeptide Y receptors and peptide expressions in obese Zucker rats. Biochem Biophys Res Commun 286: 518–523, 2001. 8. Bhathena SJ. Comparison of effects of decapitation and anesthesia on metabolic and hormonal parameters in Sprague-Dawley rats. Life Sci 50: 1649–1655, 1992. 9. Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, and Williams G. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes 50: 105–112, 2001. 10. Cai XJ, Lister CA, Buckingham RE, Pickavance L, Wilding J, Arch JR, Wilson S, and Williams G. Down-regulation of orexin gene expression by severe obesity in the rats: studies in Zucker fatty and Zucker diabetic fatty rats and effects of rosiglitazone. Mol Brain Res 77: 131–137, 2000. 11. Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, Tadayyon M, Clapham JC, Wilding J, and Williams G. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes 48: 2132–2137, 1999. 12. Chen Y, Bender RA, Frotscher M, and Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci 21: 7171–7181, 2001. 13. Commerford SR, Pagliassotti MJ, Melby CL, Wei Y, and Hill JO. Inherent capacity for lipogenesis or dietary fat retention is not increased in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 280: R1680–R1687, 2001. 14. Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, and Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001. 15. Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, and Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with auto- AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org R1464 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. OREXINS AND CIRCULATING TRIGLYCERIDES nomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96: 748–753, 1999. De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, Van Den Pol AN, Bloom FE, Gautvik KM, and Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322–327, 1998. Dobrian AD, Davies MJ, Prewitt RL, and Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension 35: 1009–1015, 2000. Dube MG, Horvath TL, Kalra PS, and Kalra SP. Evidence of NPY Y5 receptor involvement in food intake elicited by orexin A in sated rats. Peptides 21: 1557–1560, 2000. Dun NJ, Le Dun S, Chen CT, Hwang LL, Kwok EH, and Chang JK. Orexins: a role in medullary sympathetic outflow. Regul Pept 96: 65–70, 2000. Eberle AN, Baker BI, Kishida M, Baumann JB, and Girard J. Melanin concentrating hormone. IV. Development of a sensitive solid-phase radioimmunoassay for melanin-concentrating hormone. Life Sci 45: 1149–1154, 1989. Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, and Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385, 1998. Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, and Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci 21: 1656–1662, 2001. Fadel J, Bubser M, and Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci 22: 6742–6746, 2002. Gremlich S, Bonny C, Waeber G, and Thorens B. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem 272: 30261–30269, 1997. Griffond B, Risold PY, Jacquemard C, Colard C, and Fellmann D. Insulin-induced hypoglycemia increases preprohypocretin (orexin) mRNA in the rat lateral hypothalamic area. Neurosci Lett 262: 77–80, 1999. Guan JL, Saotome T, Wang QP, Funahashi H, Hori T, Tanaka S, and Shioda S. Orexinergic innervation of POMCcontaining neurons in the rat arcuate nucleus. Neuroreport 12: 547–551, 2001. Hakansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, and Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol 11: 653–663, 1999. Han S, Chen X, Wu YM, Naes L, and Westfall T. Elevated neuropeptide Y gene expression and release during hypoglycemic stress. Peptides 18: 1335–1340, 1997. Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, and Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30: 345– 354, 2001. Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, Clapham JC, and Arch JR. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept 104: 153–159, 2002. Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, and Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96: 45–51, 2000. Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, and Arch JR. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 20: 1099–1105, 1999. Jain MR, Horvath TL, Kalra PS, and Kalra SP. Evidence that NPY Y1 receptors are involved in stimulation of feeding by orexins (hypocretins) in sated rats. Regul Pept 87: 19–24, 2000. 34. Kilduff TS and Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci 23: 359–365, 2000. 35. Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thies F, Croset M, and Lecerf J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci 16: 201–204, 2001. 36. Leibowitz SF, Akabayashi A, and Wang J. Obesity on a high-fat diet: role of hypothalamic galanin in neurons of the anterior paraventricular nucleus projecting to the median eminence. J Neurosci 18: 2709–2719, 1998. 37. Lopez M, Seoane L, Garcia MC, Lago F, Casanueva FF, Senaris R, and Dieguez C. Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochem Biophys Res Commun 269: 41–45, 2000. 38. Lubkin M and Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun 253: 241–245, 1998. 39. Mahedero G, Moran JM, Salas J, and Blanco M. Absorption of Intralipid and interferences from nutrients infused into the peritoneal cavity of the rat. Am J Surg 164: 45–50, 1992. 40. Mortimer BC, Umeda Y, Elsegood CL, and Redgrave TG. Effects of anaesthesia on the removal from plasma of intravenously injected chylomicron-like lipid emulsions in rats and mice. Clin Exp Pharmacol Physiol 20: 51–58, 1993. 41. Muroya S, Uramura K, Sakurai T, Takigawa M, and Yada T. Lowering glucose concentrations increases cytosolic Ca2⫹ in orexin neurons of the rat lateral hypothalamus. Neurosci Lett 309: 165–168, 2001. 42. Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, and Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res 827: 243–260, 1999. 43. Oomura Y, Nakamura T, Sugimori M, and Yamada Y. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol Behav 14: 483–486, 1975. 44. Oomura Y, Ono T, Ooyama H, and Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature 222: 282–284, 1969. 45. Paxinos G and Watson C. The Rat Brain in Stereotaxic Coordinates (2nd ed.). Sydney: Academic, 1986. 46. Rohner-Jeanrenaud F, Craft LS, Bridwell J, Suter TM, Tinsley FC, Smiley DL, Burkhart DR, Statnick MA, Heiman ML, Ravussin E, and Caro JF. Chronic central infusion of cocaine- and amphetamine-regulated transcript (CART 55–102): effects on body weight homeostasis in lean and high-fat-fed obese rats. Int J Obes Relat Metab Disord 26: 143– 149, 2002. 47. Sahu A, Kalra PS, and Kalra SP. Food deprivation and ingestion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides 9: 83–86, 1988. 48. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, and Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585, 1998. 49. Shirasaka T, Nakazato M, Matsukura S, Takasaki M, and Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol Regul Integr Comp Physiol 277: R1780–R1785, 1999. 50. Shor-Posner G, Brennan G, Ian C, Jasaitis R, Madhu K, and Leibowitz SF. Meal patterns of macronutrient intake in rats with particular dietary preferences. Am J Physiol Regul Integr Comp Physiol 266: R1395–R1402, 1994. 51. Stricker-Krongrad A, Richy S, and Beck B. Orexins/hypocretins in the ob/ob mouse: hypothalamic gene expression, peptide content and metabolic effects. Regul Pept 104: 11–20, 2002. 52. Sweet DC, Levine AS, Billington CJ, and Kotz CM. Feeding response to central orexins. Brain Res 821: 535–538, 1999. 53. Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei MA, and Bloom SR. Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett 457: 157–161, 1999. AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org OREXINS AND CIRCULATING TRIGLYCERIDES 54. Wang H, Storlien LH, and Huang XF. Influence of dietary fats on c-Fos-like immunoreactivity in mouse hypothalamus. Brain Res 843: 184–192, 1999. 55. Wang J, Alexander JT, Zheng P, Yu HJ, Dourmashkin J, and Leibowitz SF. Behavioral and endocrine traits of obesityprone and obesity-resistant rats on macronutrient diets. Am J Physiol Endocrinol Metab 274: E1057–E1066, 1998. 56. Wang ZW, Zhou YT, Kakuma T, Lee Y, Higa M, Kalra SP, Dube MG, Kalra PS, and Unger RH. Comparing the hypothalamic and extrahypothalamic actions of endogenous hyperleptinemia. Proc Natl Acad Sci USA 96: 10373–10378, 1999. 57. Watkins PA, Hamilton JA, Leaf A, Spector AA, Moore SA, Anderson RE, Moser HW, Noetzel MJ, and Katz R. Brain uptake and utilization of fatty acids: applications to peroxisomal biogenesis diseases. J Mol Neurosci 16: 87–92, 2001. 58. Welch CC, Kim EM, Grace MK, Billington CJ, and Levine AS. Palatability-induced hyperphagia increases hypothalamic dynorphin peptide and mRNA levels. Brain Res 721: 126–131, 1996. 59. West DB, Boozer CN, Moody DL, and Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol Regul Integr Comp Physiol 262: R1025–R1032, 1992. 60. Wise RA. Addiction becomes a brain disease. Neuron 26: 27–33, 2000. R1465 61. Yamada H, Okumura T, Motomura W, Kobayashi Y, and Kohgo Y. Inhibition of food intake by central injection of antiorexin antibody in fasted rats. Biochem Biophys Res Commun 267: 527–531, 2000. 62. Yamamoto Y, Ueta Y, Date Y, Nakazato M, Hara Y, Serino R, Nomura M, Shibuya I, Matsukura S, and Yamashita H. Down regulation of the prepro-orexin gene expression in genetically obese mice. Mol Brain Res 65: 14–22, 1999. 63. Yamanaka A, Kunii K, Nambu T, Tsujino N, Sakai A, Matsuzaki I, Miwa Y, Goto K, and Sakurai T. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res 859: 404–409, 2000. 64. Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, and Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci 14: 1075–1081, 2001. 65. Ziotopoulou M, Mantzoros CS, Hileman SM, and Flier JS. Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 279: E838–E845, 2000. AJP-Regul Integr Comp Physiol • VOL 284 • JUNE 2003 • www.ajpregu.org