* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 5 & 6 Metabolism S11 Chpt. 6 for HO
Survey
Document related concepts
Nicotinamide adenine dinucleotide wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
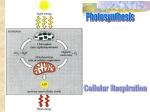
Photosynthesis wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Phosphorylation wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Transcript
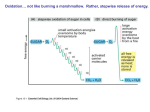
1/18/2011 Metabolism: Fueling Cell Growth Principles of Metabolism Cells (including your own) must: • Synthesize new components (anabolism/biosynthesis) • Harvest energy and convert it to a usable form (catabolism) Principles of Metabolism The role of ATP energy currency Adenosine triphosphate 1 1/18/2011 Principles of Metabolism The role of ATP energy currency Principles of Metabolism Harvesting energy - Oxidation of the chemical energy source releases energy (ex. glucose → CO2) •Oxidation/reduction reactions (redox reactions) electron donor electron acceptor OIL – Oxidation is loss of electrons RIG – Reduction is gain of electrons Principles of Metabolism The role of electron carriers “reducing power” In redox reactions, protons often follow electrons e- + H+ = H 2 1/18/2011 Principles of Metabolism The role of electron carriers 12 pairs of electrons (snatched by electron carriers) Glucose →→→→→→→6 CO2 •Passed to the electron transport chain ((used to create the proton p motive force); ultimately passed to a terminal electron acceptor (such as O2, making H2O) •Used in biosynthesis (to reduce compounds) Principles of Metabolism Synthesizing ATP •Substrate-level phosphorylation Principles of Metabolism Synthesizing ATP •Substrate-level phosphorylation •Oxidative phosphorylation - the energy of proton motive force is harvested; chemical energy is used to create the proton motive force (involves an electron transport chain) ATP synthase ADP + Pi → ATP 3 1/18/2011 Principles of Metabolism Synthesizing ATP •Substrate-level phosphorylation •Oxidative phosphorylation - the energy of proton motive force is harvested; chemical energy is used to create the proton motive force (involves an electron transport chain) •Photophosphorylation Photophosphorylation - the energy of proton motive force is harvested; radiant energy is used to create the proton motive force (involves an electron transport chain) Scheme of Metabolism energy source terminal electron acceptor Glucose (C6H12O6) + O2 Energy ATP (substrate-level phosphorylation) Energy NADPH NADH/FADH2 → electron transport chain ↓ proton motive force ↓ ATP (oxidative phosphorylation) Energy Energy Carbon dioxide (CO2) + H2 O Scheme of Metabolism energy source terminal electron acceptor Glucose (C6H12O6) + O2 Energy (heat) Carbon dioxide (CO2) + H2 O Energy Energy Energy Energy Carbon dioxide (CO2) + H2 O Figure 6.23 4 1/18/2011 Scheme of Metabolism Central Metabolic Pathways Central Metabolic Pathways 5 1/18/2011 Central Metabolic Pathways Glycolysis (aka Embden-Meyerhoff pathway, glycolytic pathway) glucose→ 2 pyruvate •2 ATP (net gain) •2 spent; 4 made •2 NADH •six different p precursor metabolites Central Metabolic Pathways Glycolysis (aka Embden-Meyerhoff pathway, glycolytic pathway) glucose→ 2 pyruvate •2 ATP (net gain) •2 spent; 4 made •2 NADH •six different p precursor metabolites Central Metabolic Pathways Glycolysis (aka Embden-Meyerhoff pathway, glycolytic pathway) glucose→ 2 pyruvate •2 ATP (net gain) •2 spent; 4 made •2 NADH •six different p precursor metabolites Pentose phosphate pathway (not pictured) glucose→ intermediate of glycolysis •NADPH (amount varies) •two different precursor metabolites 6 1/18/2011 Central Metabolic Pathways Central Metabolic Pathways Transition step pyruvate (3 C) → acetyl CoA (2 C) + CO2 (twice per glucose) •NADH •One precursor metabolite Central Metabolic Pathways TCA cycle (aka Kreb’s cycle, citric acid cycle) acetyl CoA (2 C) → 2 CO2 (twice per glucose) •ATP •3 NADH •FADH2 •two t different diff t precursor metabolites 7 1/18/2011 Central Metabolic Pathways Central Metabolic Pathways Central Metabolic Pathways 8 1/18/2011 Central Metabolic Pathways Review Which central metabolic pathway generates the most reducing power? Review How would a bacterium use protein as an energy source? 9 1/18/2011 Central Metabolic Pathways Scheme of Metabolism energy source terminal electron acceptor Glucose + O2 (C6H12O6) Energy ATP (substrate level phosphorylation) Energy Energy Energy Carbon dioxide NADH/FADH2 → electron transport chain ↓ proton motive force ↓ ATP (oxidative phosphorylation) (CO2) +H2O Glucose has been oxidized, but where do the electrons go??? Central Metabolic Pathways 10 1/18/2011 Electron Transport Chain of mitochondria Part of figure 3.55 Electron Transport Chain of mitochondria Terminal electron acceptor FADH2 → FAD Electron Transport Chain of mitochondria Creates the proton motive force FADH2 → FAD 11 1/18/2011 Electron Transport Chain The Mechanics 2e- 2H+ Electron Transport Chain Mitochondrial matrix (inside) NADH + H+ Intermembrane space (outside) Hydrogen carrier Electron carrier Hydrogen carrier Electron carrier 2H+ Hydrogen carrier 2H+ Electron carrier Electron Transport Chain of mitochondria FADH2 → FAD 12 1/18/2011 Electron Transport Chain of E. coli oxidase test Aerobic respiration (shown) Anaerobic respiration •NO3 as a TEA (different ubiquinol oxidase) •Quinone used provides humans with vitamin K FADH2 → FAD Pathway Eukaryote Prokaryote Glycolysis Cytoplasm Cytoplasm Intermediate step Cytoplasm Cytoplasm TCA cycle Mitochondrial matrix Cytoplasm ETC Mitochondrial inner membrane Plasma membrane Overall Maximum Energy Yield 13 1/18/2011 Overall Maximum Energy Yield Overall maximum energy yield of aerobic respiration (ignoring the pentose phosphate pathway): Complete oxidation of glucose 4 ATP 10 NADH 2 FADH2 Electron El t transport t t chain (oxidative phosphorylation) •3 ATP/NADH •2 ATP/FADH2 Overall Maximum Energy Yield Overall maximum energy yield of aerobic respiration (ignoring the pentose phosphate pathway): Complete oxidation of glucose 4 ATP 10 NADH 2 FADH2 Electron El t transport t t chain (oxidative phosphorylation) •3 ATP/NADH •2 ATP/FADH2 38 ATP (maximum theoretical) Overall Maximum Energy Yield Overall maximum energy yield of aerobic respiration (ignoring the pentose phosphate pathway): Complete oxidation of glucose 4 ATP 10 NADH 2 FADH2 Electron El t transport t t chain (oxidative phosphorylation) •3 ATP/NADH •2 ATP/FADH2 4 + 34= 38 ATP (maximum theoretical) 14 1/18/2011 Fermentation •Used when respiration is not an option • • Lack of TEA No electron transport chain •Oxidation of glucose stops at pyruvate Fermentation •Used when respiration is not an option • • Lack of TEA No electron transport chain •Oxidation of glucose stops at pyruvate •Passes electrons from NADH to pyruvate or a derivative NAD NADH The logic: •Oxidizes NADH, generating NAD for use in further rounds of glucose breakdown •Stops short of the transition step and the TCA cycle, which together generate 5X more reducing power 15 1/18/2011 Fermentation Fermentation Review 16 1/18/2011 Review Energy source versus terminal electron acceptor Glucose + 6 O2 → 6 CO2 + 12 H2O Enzymes • A specific, unique, enzyme catalyzes each biochemical reaction • Enzyme activity can be controlled by a cell • Enzymes can be exploited medically, industrially • Enzyme names usually reflect the function and end in -ase 17 1/18/2011 Enzymes Enzymes What are allosteric enzymes and why are they important? Enzymes Enzyme inhibition Non-competitive inhibition - Inhibitor/substrate act at different sites •Regulation (allosteric) •Enzyme poisons (example: mercury) Competitive inhibition - Inhibitor/substrate act at same site Ex.: → PABA → → folic acid → coenzyme Sulfa 18 1/18/2011 Enzymes Environmental factors influence enzyme activity temperature, pH, salinity Enzymes Cofactors act in conjunction with certain enzymes Coenzymes are organic cofactors 19