* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 acetyl CoA - WordPress.com
Lactate dehydrogenase wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Mitochondrion wikipedia , lookup
Metalloprotein wikipedia , lookup
Butyric acid wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Microbial metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
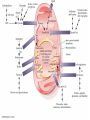
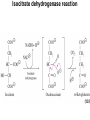
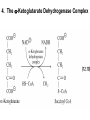
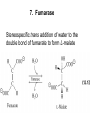
Citric Acid Cycle/Krebs Cycle citric acid cycle is used to harvest high energy electrons from carbon fuel. the central metabolic hub of the cell produces intermediates which are precursors for fatty acids, amino acids, nucleotide bases, and cholesterol named after Hans Krebs who was largely responsible for elucidating its pathways in the 1930s. Summary of the citric acid cycle acetyl CoA combines with oxaloacetate to form citrate. Ultimately, the oxaloacetate is recycled and the acetate is broken down to CO2. Each cycle produces 1 ATP, 3 NADH, and 1 FADH, per acetyl CoA. For each acetyl CoA which enters the cycle: (1) Two molecules of CO2 are released (2) Coenzymes NAD+ and FAD+ are reduced (3) One GDP is phosphorylated (4) The initial acceptor molecule (oxaloacetate) is reformed “All roads lead to Rome” To extract energy from nutrients: break down of large molecules into smaller ones Who? degradation by final products Polysaccarides Specific enzymes ex amylases sugar monomers Proteins proteases amino acids Fats (triacylglycerols) lipases glycerol to fatty acids then eventually acetyl-CoA “All roads lead to Rome” Mille viae ducunt homines per saecula Romam: A thousand roads lead men forever to Rome To extract energy from nutrients: break down of large molecules into smaller ones final products sugar monomers, amino acids fatty acids, acetyl-CoA “delivered” into CA cycle!! Catabolic pathways feed into CA cycle cross into mitochondria Just as “All roads lead to Rome”.. All pathways lead to CA cycle! PS opposite is true!!! “All roads lead to Rome” Catabolic pathways feed into CA cycle cross into mitochondria Just as “All roads lead to Rome”.. All pathways lead to CA cycle! ALL METABOLIC PATHWAYS ARE RELATED, AND ALL OPERATE SIMULTANEOUSLY! CA cycle... Generate energy from our food, and generate intermediates for our cellular processes.. Metabolic “hub” of our cells!! gluconeogenesis GLUCOSE glycolysis alanine aminotransferase Alanine lactate PYRUVATE dehydrogenase pyruvate carboxylase pyruvate dehydrogenase lipogenesis Oxaloacetate Acetyl CoA Fatty acids -oxidation citric acid cycle CO2 Lactate ketogenesis (liver only) ketone oxidation Ketone bodies cholesterol synthesis Cholesterol steroid hormones (endocrine glands) The Citric Acid Cycle Can Be a Multistep-Catalyst Oxaloacetate is regenerated The cycle is a mechanism for oxidizing acetyl CoA to CO2 by NAD+ and FAD The cycle itself is not a pathway for a net degradation of any cycle intermediates Cycle intermediates can be shared with other pathways, which may lead to a re-supply or net decrease in cycle intermediates Fates of carbon atoms in the cycle Carbon atoms from acetyl CoA (red) are not lost in the first turn of the cycle Citric Acid Cycle/Krebs Cycle citric acid cycle is used to harvest high energy electrons from carbon fuel. the central metabolic hub of the cell produces intermediates which are precursors for fatty acids, amino acids, nucleotide bases, and cholesterol The citric acid cycle may seem like an elaborate way to oxidize acetate into carbon dioxide, but there is chemical logic to the cycle. Citric Acid Cycle/Krebs Cycle The citric acid cycle may seem like an elaborate way to oxidize acetate into carbon dioxide, but there is chemical logic to the cycle. In order to directly oxidize acetate into two molecules of CO2 a C—C bond must be broken. Under the mild conditions found in cells, there is insufficient energy to break the bond. Cells often break C—C bonds between carbon atoms α and β to a carbonyl called cleavage Enzymes that catalyze: aldolases and transaldolases Another common type of C—C cleavage is α-cleavage of an αhydroxy-ketone carried out by ketolases and decarboxylases. Since neither of these common strategies for cleavage of C—C O bonds is available to acetate, the acetate is activated in the form acetyl-CoA, condensed with oxaloacetate to form citrate and then C CH3 Coenzyme A-SH + HO a β-cleavage is carried out in subsequent acetic steps. acid O Coenzyme A-S C acetyl-CoA CH3 + H2O 1. Citrate Synthase Citrate formed from acetyl CoA and oxaloacetate Only cycle reaction with C-C bond formation a synthase is an enzyme which catalyzes a synthesis process; makes covalent bonds Stereo views of citrate synthase (a) Open conformation (b) Closed conformation Product citrate (red) 2. Aconitase catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate Elimination of H2O from citrate to form C=C bond of cisaconitate Stereospecific addition of H2O to cis-aconitate to form 2R,3SIsocitrate 3. Isocitrate Dehydrogenase dehydrogenase is an enzyme that oxidizes a substrate by transferring one or more hydrides to an acceptor, NAD or FAD Oxidative decarboxylation of isocitrate to a-ketoglutarate (a-kg) (a metabolically irreversible reaction) One of four oxidation-reduction reactions of the cycle Hydride ion from the C-2 of isocitrate is transferred to NAD+ to form NADH Oxalosuccinate is decarboxylated to a-kg Isocitrate dehydrogenase reaction 4. The a-Ketoglutarate Dehydrogenase Complex 5. Succinyl-CoA Synthetase Free energy in thioester bond of succinyl CoA is conserved as GTP (or ATP in plants, some bacteria) GDP + Pi GTP Synthetase is an enzyme that catalyzes the linking together of two molecules especially by using the energy derived from the concurrent splitting off of a pyrophosphate group from a triphosphate (as ATP)— now called ligase 6. The Succinate Dehydrogenase (SDH) Complex Located on the inner mitochondrial membrane (other components are dissolved in the matrix) Dehydrogenation is stereospecific; only the trans isomer is formed Substrate analog malonate is a competitive inhibitor of the SDH complex Reaction of the succinate dehydrogenase complex 7. Fumarase Stereospecific trans addition of water to the double bond of fumarate to form L-malate 8. Malate Dehydrogenase Citric acid cycle inputs and outputs per glucose molecule Inputs: Outputs: 2 acetyl groups 6 NAD+ 2 FAD 2 ADP + 2 P 4 CO2 6 NADH 2 FADH2 2 ATP Energy conservation by the cycle Energy is conserved in the reduced coenzymes NADH, QH2 and one GTP NADH, QH2 can be oxidized to produce ATP by oxidative phosphorylation Reduced Coenzymes Fuel the Production of ATP Each acetyl CoA entering the cycle nets: (1) 3 NADH (2) 1 QH2 (3) 1 GTP (or 1 ATP) Oxidation of each NADH yields 2.5 ATP Oxidation of each QH2 yields 1.5 ATP Complete oxidation of 1 acetyl CoA = 10 ATP Fig. 19-8, p.526 3 control points in CA cycle PDH.. Covered Isocitrate dehydronase ADP/NAD+ allosteric activators a-ketogluterate dehydrogenase complex Guess who inhibits???? (same as always: ATP/NADH say No!)