* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download THE CITRIC ACID CYCLE
Mitochondrion wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Metalloprotein wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
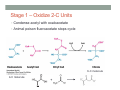
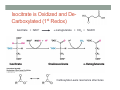
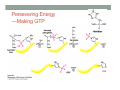
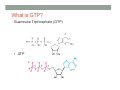
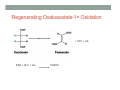
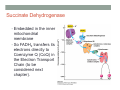
THE CITRIC ACID CYCLE aka Krebs cycle or Tricarboxylic acid (TCA) cycle Overview of Citric Acid Cycle • Feed to cycle is the acetyl group – note that this is already partially oxidized • In this cycle, we will complete its oxidation into two molecules of CO2. • The electrons lost by carbon will be captured by NAD+ and FAD and fed to oxidative phosphorylation Overview of Cellular Respiration The Citric Acid Cycle Start here Stage 1 – Oxidize 2-C Units • Condense acetyl with oxaloacetate • Animal poison fluoroacetate stops cycle 6-C molecule 4-C molecule + Why is Thioester bond so energetic? -20.9 kJ/mole -31.5 kJ/mole Citrate Synthesis Mechanism Origin of Oxaloacetate • Can be derived from pyruvate by carboxylation Pyruvate + CO2 + ATP + H2O Oxaloacetate + ADP + Pi + 2H+ Citrate Synthesis • Undesirable side reaction is to hydrolyze the acetyl group off of acetyl CoA before the acetyl is attached to the oxaloacetate • Catalyzed by Citrate Synthase Open form Closed form Citrate Synthesis • Oxaloacetate binds first and creates binding sites for • • • • acetyl CoA – so oxaloacetate is immediately ready for reaction The oxaloacetate then reacts with acetyl CoA forming the unstable compound, citryl CoA The formation of citryl CoA causes the enzyme to completely close and brings enzyme residues in close contact so that water can hydrolyze off the CoA After desorbing CoA and citrate, the enzyme returns to its open position Thus, induced fit minimizes side reactions such as hydrolysis of acetyl CoA Citrate Isomerized to Isocitrate • The isomerization is accomplished by dehydrating the citrate then hydrating the intermediate, cis-Aconitate • Why the reaction: easier to oxidize a 2o ROH than a 3o ROH (next step) • At equilibrium molecules in this reaction mainly exist as citrate • However, reaction is pulled to right as the exergonic oxidation following this step depletes the reaction below of isocitrate thus shifting it to the right 2o ROH 3o ROH At equilib: 90% 4% 6% Alcohols – 1o, 2o, 3o (Reminder) Citrate to Isocitrate mechanism Isocitrate is Oxidized and DeCarboxylated (1st Redox) Isocitrate + NAD+ -ketogluterate + CO2 + NADH Carboxylate Lewis resonance structures Detailed chemistry Second Oxidative De-carboxylation • Similar to the formation of acetyl CoA by de-carboxylating pyruvate, we have the same tri-enzyme type of complex for -ketoglutarate (both an -ketoacid) -ketoglutarate + CoA + NAD+ Succinyl CoA + CO2 + NADH -ketogluterate dehydrogenase complex Similar to Pyruvate Conversion to Acetyl CoA NAD+ NADH + H+ R R = CH2-CH2-COO- ketoglutarate and succinyl CoA R = CH3 for pyruvate and acetyl CoA Detailed Chemistry Analogous to TPP :B :B Flexible amide arm With disulfide bridge H:B+ :B :B B:H+ Uses FAD to reduce back to sulfide and NAD+ to oxidize back to FAD Stage 1 - 4 Steps to oxidize C to CO2 • So far we’ve had a 2-C moiety enter (acetyl) and we’ve converted that into 2 moles of CO2 • Generated 2 moles of NADH (high transfer potential electron carrier) Stage 2 – 4 Steps produce energy and regenerate oxaloacetate • In regenerating oxaloacetate pull more energy out of cycle. • In doing so, take 1 step to preserve the thioester bond energy by phosphorylating GDP to GTP • Then spend three steps in converting a methylene group back to a carbonyl carbon in oxaloacetate Taking Advantage of the Energetic Thioester Bond • ΔGo’ for hydrolysis of thioester bond in succinyl CoA is -33.5 kJ/mole • Compare to ATP phosphate hydrolysis at -30 kJ/mole • We preserve that energy by making GTP • This reaction utilizes a swinging histidine side chain to transfer the PO42- group from succinyl phosphate to GDP Persevering Energy —Making GTP ; What is GTP? • Guanosine Triphosphate (GTP) • ATP Regenerating Oxaloacetate-1st Oxidation • Overall, in order to get back to oxaloacetate need to convert a methylene group –CH2– by oxidizing to a carbonyl C=O. • In the first step FAD is reduced rather than using NAD+, since the free energy change is insufficient to reduce NAD+ • This enzyme is Complex II in the ETC Succinate dehydrogenase Carbon Oxid # Total Valence e- -2, -2 46 -1, -1 44 Regenerating Oxaloacetate-1st Oxidation + 2H+ + 2e- FAD + 2H+ + 2e- FADH2 Detailed Chemistry – Two Views Succinate Dehydrogenase • Embedded in the inner mitochondrial membrane • So FADH2 transfers its electrons directly to Coenzyme Q (CoQ) in the Electron Transport Chain (to be considered next chapter). Regenerating Oxaloacetate-2nd Oxidation • Fumerate is then hydrolyzed • Carbon oxid # -1 -1 0 -2 fumarase Regenerating Oxaloacetate-3rd Oxidation • Oxidation of the alcohol carbon gives oxaloacetate • ΔGo’ = +29.7 kJ/mole (endergonic) ; driven by consumption of oxaloacetate by converting acetyl CoA Maleate dehydrogenase Oxidation number Total valence e- 0 52 +2 50 Oxidation of L-Malate Energetics in Citric Acid Cycle Citric Acid Cycle Energetics • Including Glycolysis all 6 Glucose carbons are converted to CO2 – 1 in pyruvate to acetyl CoA and two in Citric Acid Cycle • And 4 moles of ATP • Go through cycle twice for each glucose Citric Acid Productivity • The 12 reduced species from oxidizing 6 Glucose carbons will eventually make ATP in the ETC • 2 NAD+ from Glycolysis • 2 NAD+ from pyruvate to acetyl CoA • 6 NAD+ from TCA • 2 FADH2 from TCA • Altogether, they will make 34 molecules of ATP • Together with 4 ATP made in Glycolysis gives theoretical total of 38 ATP Summary of Citric Acid Cycle • For each Pyruvate (3-C molecule) go through PDH and citric acid cycle once or twice/Glucose molecule • Take in partially oxidized 2-C molecule acetyl • From this we make in citric acid cycle • 2 molecules of CO2 • Convert one molecule of GDP to GTP • Reduce 3 molecules of NAD+ to NADH • Reduce 1 molecule of FAD to FADH2 • Enzymes in the cycle are closely association with one another • Citric acid cycle is anaerobic since no O2 is involved, however oxygen (ETC) needs to be present to re-oxidize NADH and FADH2 back to their oxidized forms. Overall Energy Summary – Theoretical ATP Yield Process ATP/GTP Reduced Species ATP Glycolysis 2 2 NADH 2 x 3 =6 2 NADH 2x3=6 6 NADH 2 FADH2 6 x 3 = 18 2x2=4 Preparation for TCA TCA 2 Total 4 Total theoretical yield of ATP from glucose is 38 ATP 34 Citric Acid Control • As we have seen, pyruvate dehydrogenase acts to control acetyl CoA entering the cycle. • However acetyl CoA from fat metabolism enters the cycle directly • Therefore, the cycle must also have its own means of control; these are at two points: • Allosteric enzymes • Isocitrate dehydrogenase • -ketogluterate dehydrogenase (like PDH) Citric Acid Control • Citric acid cycle has its own control points. Why? Because other energy sources (fats and proteins) enter metabolism via acetyl-CoA • Isocitrate dehydrogenase • Positively stimulated by ADP which means cell needs more ATP • Inhibitory are high levels of NADH and ATP • -ketogluterate dehydrogenase • Inhibited by its products (succinyl CoA and NADH) • Why these control points • When isocitrate dehydrogenase is inhibited, citrate builds up; citrate then transfers to cytoplasm where it allosterically inhibits phosphofructokinase to prevent glucose processing • -ketogluterate accumulation can be used for amino acids and purine bases When Energy Needs are Met – TCA used for Intermediate Generation