* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lipid rafts
Endogenous retrovirus wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Western blot wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Proteolysis wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Paracrine signalling wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biochemical cascade wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Endocannabinoid system wikipedia , lookup
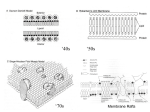
Receptors of fatty acids and endocannabinoids; lipid rafts [email protected] Receptors of fatty acids 1) Peroxisomal proliferator-activated receptors (PPAR) – nuclear 2) Free-fatty acid-activated receptors (FFAR) – on the cell surface, coupled to G proteins The PPAR family of receptors 3 isoforms: – PPAR – PPARδ/ – PPARγ (oxidized LDL) Regulation of lipid metabolism: they bind to response elements to stimulate transcription of FA oxidation and lipid synthesis genes They bind to DNA as heterodimers with the retinoid X receptor (RXR, activated by 9-cis retinoic acid) (PPAR responsive element) Ligands of PPARs Synthetic Natural Major natural ligands: free fatty acids with long chains (> 12C), particularly polyunsaturated FAs HODE=hydroxyoctadecadienoic acid Effects of PPAR PPARα regulates expression of various genes implicated in lipid oxidation, mainly in the liver and oxidative muscles, such as the heart PPARγ is involved in lipogenesis and terminal differentiation of adipocytes in white adipose tissue PPAR Expression is high in the tissues with high rates of FA oxidation: liver, heart, skeletal muscle, kidney, brown fat Function: regulation of the – cellular uptake – activation – β-oxidation of FA: stimulates the peroxisomal β-oxidation pathway profoundly, mitochondrial to a lesser extent PPAR expression is upregulated during fasting and stress (i.e. when FAs are released from the adipose tissue) Lipid-lowering drugs of the fibrate class (bezafibrate) are potent activators of PPAR PPARα target genes LPL fatty acid transport protein (FATP), fatty acid translocase (FAT) acyl-CoA synthetase enzymes of peroxisomal β-oxidation (acyl-CoA oxidase, thiolase) enzymes involved in mitochondrial β-oxidation (CPT1, acyl-CoA dehydrogenase) enzymes of ketogenesis: 3-hydroxy-3-methylglutaryl-CoA synthase PPAR/δ Ubiquitous; high expression in brain, adipose tissue, skin Particularly abundant during development (such as in CNS) Suggested to be involved in the differentiation of cells within the CNS, myelinization, and lipid metabolism in brain May be also implicated in basic cellular functions, such as membrane lipid synthesis and turnover Similarly to PPAR, it stimulates the expression of proteins of FA oxidation (heart) PPARγ 3 isoforms: – PPARγ1 – in wide range of tissues – PPARγ2 – predominantly in adipose tissue – PPARγ3 – only in adipose tissue, macrophages, colon Functions: – regulation of adipocyte differentiation – regulation of lipid anabolism: in the adipose tissue, they regulate the expression of LPL, FATP… PPARγ are targeted by anti-diabetic substances of the thiazolidinedione class (glitazones, e.g. rosiglitazone) that enhance the tissue sensitivity to insulin and reduce the plasma levels of FA and Glc The FFAR family of receptors Localized to the plasma membrane (unlike PPARs) and coupled to G proteins Ligand spectrum includes FA chain lengths down to one (unlike PPARs) 3 isoforms: – FFA1R – FFA2R – FFA3R Roles of FFARs FFA1R FFA2R FFA3R Expression pancreatic islets, liver, monocytes, adipose tissue, skeletal muscle, heart neutrophils pancreas, immune cells Ligands medium- (down to 10C) to long-chain FA short-chain FA (C1-C6) similar to FFA2R Effects regulation of insulin secretion control of control of leptin chemotaxis production Endocannabinoids Biologically active lipophilic substances that activate cannabinoid receptors Derivatives of arachidonic acid, which are generated from membrane phospholipids in response to stimuli Two best-characterized: Synthesis of anandamide (PE) The reaction is initiated by activating neurotransmitter receptors and/or by elevated intracellular Ca2+ Synthesis of 2-arachidonoylglycerol a) b) Also linked to elevated intracellular Ca2+ 2 pathways: – phospholipase C + diacylglycerol lipase – phospholipase A1 + phospholipase C (specific for lyso-PI) Function Paracrine mediators (rapidly eliminated through uptake into cells and enzymatic hydrolysis) Produced mainly in the: – nervous system – immune system Anandamide levels in tissues – very low and in some cases, it does not act as a full agonist physiological significance questionable On the other hand, 2-AG is a full agonist at the CB1 as well as CB2 receptor and the levels in tissues are much higher Cannabinoid receptors Targeted by THC (Δ9-tetrahydrocannabinol) THC – CB1 – most abundant in the CNS, also present in immune cells, lung, small intestine, uterus, testis… – CB2 – found mostly in the immune system (leukocytes, spleen, lymph nodes) 7-transmembrane, coupled to Gi/Go proteins inhibition of adenylyl cyclase… Physiological roles 2-AG from stimulated neurons attenuates neurotransmitter release by decreasing intracellular Ca2+ (?calming the excitation of neurons to prevent cell death?) 2-AG suppresses long-term potentiation ?Role in immune response? (CB2 – in the immune system) CB1 stimulation inhibits proliferation of human breast cancer cells Lipid rafts Plasma membrane: NOT absolutely homogeneous! Lipid rafts Resistant to mild detergents Highly dynamic, densely organized (10-200 nm) Sphingolipid- and cholesterol-rich microdomains of the plasma membrane containing a variety of signalling and transport proteins This environment fits for transport, conformational changes of signal transducers, but also for pathogen entry into the host cell (HIV, Ebola) A model of a lipid raft Cylindrical glycerophospholipids (GPLs) form the disordered Lc phase of the membrane Ordered Lo phase – raft: in the outer leaflet, cholesterol fills the voids between sphingolipids (SM); in the inner leaflet, chol. fills the voids between selected (pyramidal) GPLs This organization rigidifies the membrane Proteins in lipid rafts Raft resident proteins are often GPI (glycosylphosphatidylinositol) anchored: Rafts contain: – receptors (Fas, MHCI, II) – signalling molecules (tyrosin kinases, G proteins) – transporters (GLUT4, FAT) Lipid rafts facilitate TCR-mediated T cell activation TCR moves to rafts during T-cell activation Formation of the TCR-MHC complexes and aggregation of co-stimulatory molecules in lipid rafts, raft aggregation Tyr phosphorylation and recruitment of signalling proteins Caveolae Types of rafts that are rich in proteins of the caveolin family (caveolin-1,-2,-3) Caveolin-1, integral membrane protein, forms oligomers that associate with each other and form a pit in the membrane Proposed roles: – signalling – transport – pathogen entry (SV40, E. Coli) Raft-mediated HIV entry into the host cell – a model gp120 (viral), CD4, and sphingolipids (SLs) form a complex in the raft area; SLs stabilize HIV on the cell surface The raft floats on the cell surface to the co-receptor for the CD4–gp120 complex SLs facilitate the conformational changes of gp120 that lead to the shedding of gp120 → release of the N-terminus of viral gp41 (initially buried in a gp120 pocket) This „fusion peptide“ penetrates into the plasma membrane Rafts in prion infection The interaction of PrPC with lipid rafts (sphingolipids) might stabilize the ‘normal’ conformation of PrPC These interactions should be destabilized when exogenous (shed from an infected cell) PrPSc is inserted in the vicinity of PrPC Formation of PrPC/PrPSc complex (probably by coalescence of both rafts), PrPC → PrPSc conversion Propagation on the cell surface