* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download the ask1-map kinase cascades in mammalian stress response
Biochemical switches in the cell cycle wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Tyrosine kinase wikipedia , lookup
Programmed cell death wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Signal transduction wikipedia , lookup
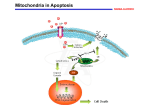
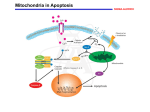
THE ASK1-MAP KINASE CASCADES IN MAMMALIAN STRESS RESPONSE Dr. R.A.SIDDIQUE M.V.Sc., PhD Scholar National Dairy Research Institute, Karnal, Haryana, 132001 INDIA E-mail: [email protected] STRESS: It is a medical term for wide range of strong stimuli which can cause a physiological response, first described in 1936 by Hans Selye. It include three stages: 1. Alarm reaction: body detects the external stimuli. 2. Adaptation : body engages defensive countermeasures against the stressor. 3. Exhaustion: body begins to run out of defences. Here I will mainly concentrate on oxidative stresses and ER stresses, MAP Kinase cascade transmit stimuli from outside the cell to the nucleus. Three MAP Kinase cascades in mammals : ERKs, c-Jun N-terminal Kinases(JNKs), p38 MAP Kinases. Each consists of three classes of Ser/Thr Kinases MAP Kinase, MAP Kinase Kinase( MAPKK) - --MEK, MAP Kinase Kinase Kinase( MAPKKK) MAP KKK Phosphorylate and activate MAP KK and this activated MAP KK phosphorylate and activate MAP Kinase. Different MAP Kinases involved in ASK1 Activation Stimulus MAPKKK Inflammatory ASK1 cytokines MAPKK MAPK Response MKK3/6 p38 MAPK Inflammation, MKK4/7 SAPK/JNK Apoptosis, Diffentiation ERKs activated by Cytokines &growth factors and role in Cell growth and Diffrentiation On the other hand JNKs and p38 MAP Kinases activated by: chemical and physical stressors: UV radiation X-ray heat shock osmotic shock proinflammatory cytokines : TNFα control : stress adaptation, cell death and survival. ASK1 (Apoptosis Signal Regulating Kinase1) 160 KDa Ser/Thr Protein Kinase Member of MAP KKK family Activates both JNK and p38 pathway by phosphorylating and activating SEK1(MKK4)/ MKK7 and MKK3/Mkk6. ASk1 activated with death receptor ligands ( TNFα and Fas ligand) Also activated by cytotoxic stresses: H2O2, anticancer drugs and growth factor deprivation. New mechanism by ER stress, calcium signaling, GPCR signaling. Overexpression of wild types ASK1 causes mitochondria dependent apoptosis. In this cascade Yamamoto et al. 2000 demonstrated ASK1 mediated JNK activation Phosphorylates Bcl-2 Reduced Antiapoptotic activity Note: However the detailed molecular mechanism that link mitochondria dependent apoptosis and ASK1-p38/JNK activation remains unknown. ASK1 JNK Pathway in Bcl-2 Phosphorylation Structure of ASK1 Mechanism of ASK1 Activation By Homo-oligomerisation It was demonstrated that synthetic ASK1-ASK1 fusion construct activate JNK and p38pathway In resting stage ASK1 = Homo- oligomer through its C-terminal coiled-coil domain. H2O2 Stimuli Additional interface created on pre-formed ASK1 oligomer Autophosphorylation of Thr 845 in Mouse Activation of ASK1 In Human at Thr838 Thr residue located in activation loop of kinase domain Oxidative Stress and ASK1 interacting Proteins ROS --- super oxide and H2O2 produced through cellular processes or derived from exogenous sources play imp role in normal cell-proliferation, survival and immune response. Excessive Production of ROS causes: Severe damage to cellular components. Loss of cell functions Ultimately apoptosis or necrosis Heart failure, myocardial infarction and neuronal cell death ASK1 strongly activated in cells exposed to oxidants and involved in oxidative stress induced apoptosis Negative regulation of oxidative-stress induced apoptosis by Trx (ASK1 repressor Thioredoxin) Trx directly bind to N-terminal Of ASK1 and inhibits Kinase activity. Contd… Binding of Trx to ASK1 require Reduced form of disulphide bridge between two residues in catalytic site of TRx, Cys32 and Cys35. Oxidized Trx could bind or inhibit ASK1 This binding also involved in TNFα signaling TNFα ROS Dissociate Trx from ASK1 Glutaredoxin Another intracellular redox- signaling molecule Inhibits Glucose deprivation induced ASK1 activation PP5 dephosphorylate Thr845 and inactivate kinase activity in vivo and in vitro. PP5 negative feedback of ASK1 activation In resting stage 14-3-3 protein bind to ASK1 through Ser 967 and reduce ASK1-induced apoptosis. Goldman et al. demonstrated : H2O2 induce Ser 967 dephosphorylation and increased activation Contd… • Necessity of ASK1 activation in ROS induced Apoptosis Tobiume et al. (2001) Generated ASK1 null mice MEFs isolated from ASK1-/- mice resistant to H2O2 apoptosis JNK and p38 activation also suppressed So essentiality of ASK1-p38/JNK cascade. Death Receptor mediated ASK1 activation Death receptor- Fas Fas assembles as DISC upon activation by FasL or agonistic antibodies. DISC--- FADD and caspase -8 DISC-----acute execution of apoptosis Alternate Pathway for JNK activation Activated Fas + Daxx Daxx binds N-terminal of ASk1 Activate JNK Apoptosis Note: Mechanism is still not clear Contd… TNFα regulate immune response, inflammation and apoptosis TRAF2 couples to TNFα receptor TRAF2 directly interact to C-terminal domain of ASk1 . TNFα induces dissociation of Trx . Activated ASk1 ------- apoptosis Moreover it has been reported that TRAF2 over expression or treatment leads to the production of ROS Therefore, Trx negative regulator of both H2O2 induced as well as TNFα induced activator of ASK1. MEFs from ASK1-/- resistant to TNFα induced apoptosis reduced activation of JNK and p38, indicates ASK1 is required for TNFα –induced apoptosis. ER Stress-induced Apoptosis and ASK1 activation Accumulated unfolded protein in lumen of ER UPR UPR regulated by IRE1, PERK, ATF6. PERK and IRE1: ER resident transmembrane Ser/Thr protein kinase. PERK and IRE1 phosphorylated and activated in response to UPR. ATF = leucine zipper transcription factor , cleaved and activated in Golgi apparatus. Activation of above molecules “Adaptive Response” reduction of nascent protein in ER If , adaptive response not sufficient apoptosis. IRE1 activate JNK signaling mediated via TRAF2 Contd.. ER stress induced apoptosis in human pathological cases: Amyloidosis Hypercholesterolemia Neurodegenerative diseases: Huntington’s diseases Huntington's diseases : expansion of CAG repeats code for expanded polyglutamine (Poly Q). Long poly Q ER stress activates IRE1 which recruit TRAF2 , and interacts directly with the ASK1 and activate SEK1-JNK pathway pathway. Poly Q dependent ER stress due to proteasome dysfunction. Bence et al., 2001 and Zhou et al., 2003. IRE-TRAF2-ASK1 Cascade in Pathogenesis of Poly Q disease G-protein coupled receptor (GPCR) signaling and ASK1 activation in cardiomyocytes McDonald et al. first reported β-arrestin 2 key molecule in rec. desensitization and internalization also functions as a scaffold protein in ASK1-SEK1-JNK3 cascade( related to angiotensin II-induced JNK3 activation. Physiological role unknown Recently GPCR mediated ASK1 activation cardiac dysfunction ROS production ASk1 is essential for angiotensin II- induced cardiac hypertrophy by using ASK1-/- mice Izumiya et al., 2003 ASk1 seems to be promising therapeutic target for cardiac dysfunction. Calcium signaling and ASK1-p38 cascade activation Ca play role in Neuronal Functions and Ca dependent activation of MAP kinase in Synaptic plasticity. Enters in neuron via NMDA receptor or voltage-gated Ca channels. Binds to CaM and activate ERK pathway In this pathway CaMBPs (Ras-GRF and CaMKIV positively modulate ERK1/2 activation induced by NGF However relationship between Ca signaling and JNK/p38 activation has not been well defined. Conclusion ASK1 causes apoptosis in response to common proapoptotic stresses, such as oxidative stress and death rec. ligands. Moreover pathogenic stress (ER stress, GPCR induced ROS production) also causes apoptosis via ASK1-JNK/p38 cascades in neurons and cardiomyocytes. ASK1 may be therapeutic target for treatment of Neurodegenerative diseases and cardiac dysfunction. CaMKII phosphorylates ASK1 and activate ASK1-p38 pathway in neurons and play important role in synaptic plasticity Further knowledge of its regulatory mechanism –more promising therapeutic target for apoptosis based incurable diseases. In addition, an understanding of novel physiological roles of ASK1 may shed light on diverse cellular processes regulated by this important Protein Kinase