* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Who Gets Lupus?
Anti-nuclear antibody wikipedia , lookup
Germ theory of disease wikipedia , lookup
Drosophila melanogaster wikipedia , lookup
Globalization and disease wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Adaptive immune system wikipedia , lookup
Immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Complement system wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Innate immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Systemic lupus erythematosus wikipedia , lookup
Autoimmunity wikipedia , lookup
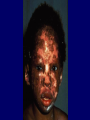
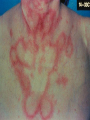
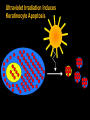
“SLE: Fighting Self Sabotage” Feb. 2005 Immunology in health and Disease Susan Manzi, MD, MPH Associate Professor of Medicine and Epidemiology Co-Director Lupus Center of Excellence Objectives I. Epidemiology II. Pathogenesis Genetic Sources of autoantibodies Environmental triggers Defective immune regulation Gender/hormonal factors III. Clinical and laboratory features Diagnosis/natural history Autoantibodies Treatment Who Gets Lupus? Females > Males – Childbearing – Children, elderly 7:1 12:1 2:1 African-American (3-4x) > Caucasian Asian Hispanic Female Preponderance of Autoimmune Disease Disease Female/ Male Ratio Thyroid diseases Diffuse lymphocytic thyroiditis Goitrous, struma lymphomatosa (Hashimoto), Hypercellular variant, adult onset 25-50:1 Hypercellular variant, juvenile onset 4-7:1 Fibrous variant 4:1 Non goitrous Severe atrophic (myxedema) 6:1 Mild atrophic (asymptomatic) 8:1 Primary hyperthyroidism (Graves Basedow disease) With benign or no exophthalmos 4-8:1 With progressive ophthalmopathy 2:1 Female Preponderance of Autoimmune Disease Disease Female/Male Ratio Systemic lupus erythematosus Rheumatoid arthritis Sjogren’s syndrome Idiopathic adrenal insufficiency (autoimmune adrenal disease) Scleroderma Myasthenia gravis Multiple sclerosis 9:1 2-4:1 9:1 2-3:1 3-4:1 2:1 1-5:1 Prevalence of SLE • • • • African-American women: Caucasian women: African-American men: Caucasian men: 56-283 17-71 3-53 3-19 Range of prevalence figures per 100,000 persons Systemic lupus erythematosus in “Women and Health” (Goldman and Hatch, eds.), pp 704-723, 2000 Observations to Support Genetic Factors in Lupus 1. Clustering in families 2. Concordance - monozygotic (identical twins) 25-30% - dizygotic 5% 3. Other autoimmune conditions in family members Pathogenesis of SLE Pathogenesis of SLE Environmental Genetic Ag T HELPER Autoantibodies B Hormonal B and T Cell Hyperactivity Defective Immune Regulation Immune Complexes Tissue Damage Mode of Inheritance Polygenic (>95%) VS Monogenic (<5%) Homozygous deficiency of: C1q 38/41 (93%) C4 14/16 (88%) C2 38/66 (58%) Paradox Complement activation plays a critical role in the inflammatory process and tissue damage in SLE, but early complement deficiencies cause SLE. Possible Explanations 1. C1q clears immune complexes 2. C1q binds to and clears apoptotic blebs (sources of autoantigens) 3. Absence of C1q permits sustained infections that could trigger autoimmune response. Genes increase susceptibility to SLE In the major histocompatibility complex (MHC) C2,C4 deficiency DR2,DR3 TNF- polymorphisms In non-MHC C1q deficiency (rare, but greatest risk!!) Chromosome 1 region 1q41-43 (PARP) region 1q23 (FcRIIA, RIIIA) Polymorphisms in IL-10, IL-6 and mannose-binding protein Sources of Autoantigens 1. Apoptotic cells 2. Activated cells (antigens move to cell membrane) 3. Modification of proteins during apoptosis 4. Infectious agents Sources of Autoantigens 4. Infectious agents - molecular mimicry - epitope spreading - nonspecific activation of B/T cells - infection induced apoptosis Environmental Triggers Ultraviolet light (photosensitivity) Drug-induced lupus milder, male =female, older ages Infectious agents (EBV, CMV) Ultraviolet Irradiation Induces Keratinocyte Apoptosis Ultraviolet Irradiation Induces Pro-Inflammatory Cytokines TNF IL1 GMCSF IL8 PGE2 LTB4 Complement Mediates Clearance of Apoptotic Blebs TNF IL1 GMCSF IL8 PGE2 LTB4 C4 C3 C3 C4 Tolerance C4 C3 C4 C4 C4 C3 C4 C4 C3 C3 C4 C3 C4 C3 C4 C3 Impaired Clearance of Apoptotic Blebs in SLE TNF IL1 GMCSF IL8 PGE2 LTB4 C4 C3 C3 Lymph Nodes Spleen Auto-Antibody C4 C4 C3 C4 C4 C4 C3 C4 C4 C3 C3 C4 C3 C4 C3 C4 C3 Defective Immune Regulation B and T-cell hyperactivity Sustained autoantigens/impaired clearance of apoptotic cells Epitope spreading due to lack of “turn off” Exaggerated intracellular response to activation Defective Immune Regulation B and T-cell hyperactivity Increased production of pro-inflammatory cytokines Decreased clearance of immune complexes Increased expression of surface molecules that increase B/T- cell activation (CD40L) CD40L-CD40 Interactions TCR B Cell T-cell CD3 CD40 CD40L (gp39) CD40: B-cells, endothelial cells, macrophages, Ag-presenting cells, renal parenchymal, tubular, etc cells CD40L: T-cells, platelets