* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download cell division control
Spindle checkpoint wikipedia , lookup
Endomembrane system wikipedia , lookup
Tissue engineering wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Signal transduction wikipedia , lookup
Programmed cell death wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell growth wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
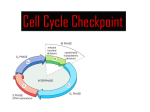
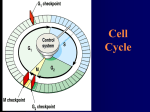
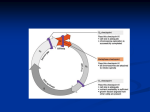
Wed 11/27 • Collect HW: Bozeman Biology Mitosis Video • Cancer Video/Powerpoint • Finish Cell Cycle/Mitosis Control Booklet • HOMEWORK: “But I’m Too Young” Cancer Case Study – follow link on website, complete 8 questions • DUE MONDAY!!! • Cell Cycle, Mitosis, & Control (Chp.12) Quiz will be TUESDAY (short Quiz ~12 questions) Review • Cells must either reproduce or they die. • Cells that can not reproduce and are destined to die are terminal cells (red blood, nerve cells, muscles cells etc.). • The "life of a cell" is termed the cell cycle as there are distinct phases. • They are G1, S, G2, M Frequency of cell division • Frequency of cell division varies by cell type – embryo • cell cycle < 20 minute – skin cells • divide frequently throughout life • 12-24 hours cycle – liver cells • retain ability to divide, but keep it in reserve M metaphase anaphase • divide once every year or two telophase prophase – mature nerve cells & muscle cells • do not divide at all after maturity • permanently in G0 C G2 S interphase (G1, S, G2 phases) mitosis (M) cytokinesis (C) G1 Checkpoint control system • Checkpoints – cell cycle controlled by STOP & GO chemical signals at critical points – signals indicate if key cellular processes have been completed correctly Checkpoint control system • 3 major checkpoints: –G1/S • can DNA synthesis begin? –G2/M • has DNA synthesis been completed correctly? • commitment to mitosis • MPF (mitosis promoting factor) –spindle checkpoint • are all chromosomes attached to spindle? • can sister chromatids separate correctly? • APC (anaphase promoting complex) G0 phase – if cells do not pass G1/S checkpoint – non-dividing, differentiated state – most human cells in G0 phase liver cells M Mitosis G2 Gap 2 S Synthesis in G0, but can be “called back” to cell cycle by external cues G1 Gap 1 G0 Resting nerve & muscle cells highly specialized arrested in G0 & can never divide Internal control of the cell cycle: •Controlled by “signal molecules”. • must be phosphorylated in order to work. •Below is a simple model of how this could occur. Kinases are proteins (enzymes) that phosphorylate these chemical signals & trigger the cell cycle phases. This kinase represents the inactive form. •This kinase has two active forms • S-form or the M-form • phosphorylate different chemical signals. OR Cell cycle kinases must be activated by molecules called cyclins. inactive kinase (no cyclin attached) The kinases are called cyclin-dependentkinases (Cdk) because it needs cyclin to be phosphorylated. two different cyclins This represents what happens when cyclins are present. As the cell goes through the cell cycle: •Different cyclins are made to activate the various Cdks. •Once the kinase is activated, the cyclin is destroyed which deactivates the kinase. •Kinases are not destroyed, they are only activated or deactivated. The cell cycle begins. The cell has a certain amount of cyclin-dependent kinases (Cdks). The cell begins to make the S cyclin. The S-cyclin activates the Cdk. The Cdk complex phosphorylates the S-signal which initiates the S-phase to start once it gets to a critical level. • Once the S-signal is phosphorylated, it leaves. • the S-cyclin is destroyed • the kinase returns to the inactive state. • When there is enough S-signal, then the S-phase will begin. Now the Cdk is inactive, and the cell begins to make the Mcyclin. The M-cyclin activates the Cdk. • Cdk complex phosphorylates the M-signal • initiates the M-phase to start once it gets to a critical level. • This complex is called the mitosispromotingfactor (MPF). • Once the Msignal is phosphorylated, it leaves. • M-cyclin is destroyed • kinase returns to the inactive state. • When there is enough Msignal, then the M-phase will begin. Various cyclins are made & destroyed throughout the cell cycle whereas the level of cell division kinases remain constant. Kinases however are activated by various cyclins and the activity are mirrored by the rise and fall of cyclins. Fluctuations in concentration of cyclins allow for cell cycle checkpoints. The three major check points are G1/S , G2/M and Spindle checkpoints. These checkpoints have build-in-stop signals that hold the cell cycle at the checkpoint until overridden by go-ahead signals. This is a textbook’s diagram of how cyclins and kinases in the cell cycle work. Often the G1 check point or "restriction point" in mammalian cells seems to be the most important one. If a cell receives a goahead signal at this check-point, it will complete the cell cycle and divide. However, if the cell does not receive the go-ahead signal in G1, the switches to a nondividing state called G0. How Cdks actually work is not well understood but the Cdks seem to activate other proteins and enzymes that affect particular steps in the cycle. External Signals-This include certain chemical and physical factors that affect cell division. Mammalian cells need certain nutrients and regulatory proteins or growth factors are needed for cell division. For example, when the skin has been damage (wound), platelets release a substance called platelet-derived growth factor (PDGF). This growth factor stimulate fibroblast cells to start to reproduce and make scar tissue. External signals can effect how cells grow in culture. Density-dependent inhibition- cells in culture stop dividing when they become crowded forming a single layer of cells. It seems that when crowded, there is insufficient growth factor produced and nutrients for cell division to continue. Anchorage dependence- mammalian cells need to be attached to substratum like the inside of a culture jar or other tissue in order to reproduce. This phenomenon is linked to a control system attached to the plasma membrane proteins and the cytoskeleton. These phenomenon keep the growth of tissue in check. Cancer cells do not exhibit density-dependent inhibition or anchorage dependence. External signals • Growth factors – coordination between cells – protein signals released by body cells that stimulate other cells to divide • density-dependent inhibition – crowded cells stop dividing – each cell binds a bit of growth factor » not enough activator left to trigger division in any one cell • anchorage dependence – to divide cells must be attached to a substrate » “touch sensor” receptors Growth factor signals growth factor nuclear pore nuclear membrane P P cell division cell surface receptor protein kinase cascade Cdk P P E2F chromosome P cytoplasm nucleus Example of a Growth Factor • Platelet Derived Growth Factor (PDGF) – made by platelets in blood clots – binding of PDGF to cell receptors stimulates cell division in connective tissue • heal wounds Growth Factors and Cancer • Growth factors can create cancers – proto-oncogenes • normally activates cell division (positive control) – growth factor genes – become oncogenes (cancer-causing) when mutated • if switched “ON” can cause cancer • example: RAS (activates cyclins) – tumor-suppressor genes • normally inhibits cell division (negative control) • if switched “OFF” can cause cancer • example: p53 Cancer & Cell Growth • Cancer is essentially a failure of cell division control – unrestrained, uncontrolled cell growth • What control is lost? – lose checkpoint stops – gene p53 plays a key role in G1/S restriction point • p53 protein halts cell division if it detects damaged DNA – options: p53 is the » stimulates repair enzymes to fix DNA Cell Cycle » forces cell into G0 resting stage Enforcer » keeps cell in G1 arrest » causes apoptosis of damaged cell • ALL cancers have to shut down p53 activity p53 discovered at Stony Brook by Dr. Arnold Lev p53 — master regulator gene NORMAL p53 p53 allows cells with repaired DNA to divide. p53 protein DNA repair enzyme p53 protein Step 1 Step 2 Step 3 DNA damage is caused by heat, radiation, or chemicals. Cell division stops, and p53 triggers enzymes to repair damaged region. p53 triggers the destruction of cells damaged beyond repair. ABNORMAL p53 abnormal p53 protein Step 1 Step 2 DNA damage is caused by heat, radiation, or chemicals. The p53 protein fails to stop cell division and repair DNA. Cell divides without repair to damaged DNA. cancer cell Step 3 Damaged cells continue to divide. If other damage accumulates, the cell can turn cancerous. Development of Cancer • Cancer develops only after a cell experiences ~6 key mutations (“hits”) – unlimited growth • turn on growth promoter genes – ignore checkpoints • turn off tumor suppressor genes (p53) – escape apoptosis • turn off suicide genes – immortality = unlimited divisions • turn on chromosome maintenance genes – promotes blood vessel growth • turn on blood vessel growth genes – overcome anchor & density dependence • turn off touch-sensor gene It’s like an out-of-control car with many systems failing! What causes these “hits”? • Mutations in cells can be triggered by UV radiation chemical exposure radiation exposure heat cigarette smoke pollution age genetics Tumors • Mass of abnormal cells – Benign tumor • abnormal cells remain at original site as a lump – p53 has halted cell divisions • most do not cause serious problems & can be removed by surgery – Malignant tumor • cells leave original site – lose attachment to nearby cells – carried by blood & lymph system to other tissues – start more tumors = metastasis • impair functions of organs throughout body Traditional treatments for cancers • Treatments target rapidly dividing cells – high-energy radiation • kills rapidly dividing cells – chemotherapy • stop DNA replication • stop mitosis & cytokinesis • stop blood vessel growth New “miracle drugs” • Drugs targeting proteins (enzymes) found only in cancer cells – Gleevec • treatment for adult leukemia (CML) & stomach cancer (GIST) • 1st successful drug targeting only cancer cells Novartes without Gleevec with Gleevec Any Questions?? ***Cell Cycle & Cancer Movie 2008-2009 Extra slides Three major checkpoints 1. G1/S (R point) checkpoint is the primary determining factor for cell division to take place. Growth factors are affecting the cell cycle, and cells are growing. Once the R point is passed the DNA is going to be replicated. If a cell receives a go-ahead signal at this check-point, it will complete the cell cycle and divide. However, if the cell does not receive the go-ahead signal in G1, the switches to a nondividing state called G0. 2. This checkpoint represents the commitment for starting the process of mitosis. This checkpoint also ensures that the DNA has been replicated correctly. If the DNA has been damaged, then the cell does not continue to mitosis. Once the Cdk and cyclin combine, it is called “mitosis promoting factor” or MPF. 3. The M/ spindle check point ensures that all the chromosomes are attached to the spindle in preparation of mitosis. The separation of the chromatids are irreversible. Once chromatids are replicated they are held together by a protein substance called cohesion protein. Another protein called seperase will destroy this protein. Seperase is inhibits or unable to destroy cohesion because of third protein called securin. So in effect the APC (anaphase promoting complex) activates securin, which actives an enzyme seperase to destroy during anaphase, however in vertebrates, all of the cohesion is removed during chromatid condensation except the cohesion at the centromeres. Once the cohesion is completely removed, then the tension of the microtubules cause the separation of the chromatids. APC also destroys cyclins in order to drive the cell out of mitosis.