* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Forward Genetics
Koinophilia wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Minimal genome wikipedia , lookup
Ridge (biology) wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Pathogenomics wikipedia , lookup
Oncogenomics wikipedia , lookup
Frameshift mutation wikipedia , lookup
X-inactivation wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Public health genomics wikipedia , lookup
Population genetics wikipedia , lookup
Genomic imprinting wikipedia , lookup
RNA interference wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Genome evolution wikipedia , lookup
Gene expression profiling wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene expression programming wikipedia , lookup
History of genetic engineering wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Point mutation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Designer baby wikipedia , lookup
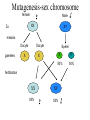
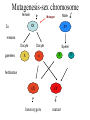
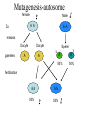
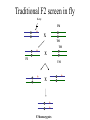
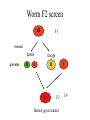
Phenotype (Function) Genetics Forward Genetics Gene A Gene B Gene C P Proteins A B C Survey In classical or forward genetics, we commonly use chemicals or radiation to generate mutations in model organisms such as yeast, worm, fly or mouse. In doing so, we typically try to generate mutations in A: somatic cells B: germline cells (gametes, sperm or oocytes) C: not sure Mutagenesis-sex chromosome female 2n Male XX XY meiosis Oocyte gametes Oocyte X Sperm X Y 50% 50% X fertilization XX 50% XY 50% Mutagenesis-sex chromosome female 2n Male Mutagen XX XY meiosis Oocyte gametes Oocyte X Sperm X x fertilization xX xY heterozygote mutant Y Mutagenesis-autosome female 2n Male A A A A meiosis Oocyte gametes Oocyte A A Sperm x A A 50% 50% fertilization AA 50% y AA 50% Mutagenesis-autosome female 2n X-ray Male A A A A meiosis Oocyte gametes Oocyte A Sperm x y A A 50% 50% A some a fertilization AA 50% AA 50% Aa Heterzygous mutant Your opinions When you mutagenize the gametes of P0 animals, you usually do not get homozygous mutants in the F1 generation. A: Yes B: No C: not sure. Do you usually get homozygous mutants in the F2? A: Yes B: No C: not sure. F1 screens vs. F2 screens Po WT + X + F1 mutant m + Po WT + X + + + F1 WT m X + m + + + F2 mutant m m But, how do we get the same m in two F1 and let them mate? Balancer chromosomes Chromosomes that suppress crossover. Homozygous of the chromosome is either lethal or with a visible phenotype. Usually contain inversions and translocations A B wt balancer B A A B Resulting abnormal chromosomes B A Neither can be paired with WT Traditional F2 screen in fly X-ray TM * X TM * TM X F1 TM * * X * * F3 homozygotes F2 screens in the worm Hermaphrodite Po XX meiosis Sperm gametes Oocyte X X 100% 100% x fertilization XX 100% Self-progeny xX F1 Worm F2 screen xX F1 meiosis Sperm gametes X Oocyte x X X xx F2 Homozygous mutant 1/4 summary Po WT + X + + + Po WT mutagen F1 WT m X + + + F1 WT m X + m + F3 mutant m m Fly, mouse, … + + mutagen F1 WT m + F2 mutant m m worm Basic mutagenesis in the worm Treat with 50mM EMS for 4 hrs F1 each F1 carries two mutagenized chromosomes m/+ heterozygotes for each mutation Recovering for a few hours P0 F1 eggs Several L4 or young adult worms/plate. Multiple large plates F2 eggs F2 Remove P0 parents after laying ~100 eggs Total genomes screened = 2X # of F1 animals x # of F1 plates Remove F1 worms after laying ~ 2000 eggs for 20 hrs Worms with m/m genotype for each mutation are mixed with m/+ and +/+ animals mutant worms (m/m) are individually picked on to a fresh small plate. Question If you plan to mutagenize and screen for a mutation in a tumor suppressor gene that may leads to tumorigenesis, would you do F1 screen or F2 screen? A: F1 B: F2 Genetic and physical map Genetic map nDf41 mDf4 1.8 map unit Cloned genes rme-2 sup-23 Non-cloned genes evl-7 2.0 unc-5 him-3 unc-77 mor-2 fem-1 2.2 skn-1 sup-41 Physical map YACs cosmids Named genes eP14 rme-2unc-5 fem-1 skn-1 nhr-48 Genetic map and physical map will completely unified when every gene has been mutated. A: yes. B: No. C: not sure. Figure 8.15. Linkage analysis between two mutations. +; dpy +; + + dpy unc; + unc + unc; + unc + dpy + + dpy X A few wild type A few Dumpy (Dup) Pick several male progeny unc unc dpy + X Uncoordinate (Unc) Wild type 50% unc ; + + + unc + + + Segregate no dumpy progeny, discard 50% Pick several wild-type hermaphrodites and place each to one pate If the two genes are unlinked unc ; dpy + + If the two genes are linked unc + + dpy Segregate dumpy progeny, continue mapping with the plates dpy ; unc + + Situation 1. The two genes are unlinked Wild type 9/16 Phenotypes of worms In the plate genotypes dpy ; unc + + dpy ; + + + + + ; unc + + + ; + + 4/16 2/16 Unc 3/16 Dpy 3/16 dpy ; unc unc + 2/16 dpy ; unc + dpy ; unc unc 1/16 dpy ; dpy + + A single plate + + 2/16 1/16 Pick 20 Unc animals If ~ 2/3 of these Unc worm segregate Dpy-and-Unc animals, non-linkage between the two genes is deduced. 2/16 1/16 Dpy-and-Unc 1/16 dpy ; unc dpy unc 2/16 + genes + dpy Situation 2. unc The two are linked on one of the six chromosomes unc + + dpy unc + + dpy unc dpy A single plate Self fertilizing Wild type 1/2 Unc 1/4 Dpy 1/4 Dpy-and-Unc 0 Phenotypes + dpy unc + Non-recombinant Genotypes + + unc + unc + unc dpy unc + Rare recombinants dpy dpy unc unc dpy dpy unc dpy + dpy Pick 20 Unc animals majority unc unc + + linkage between the genes is indicated by segregation of no Dpy animals from all or the majority of Unc animals. Rare unc unc dpy dpy The frequency of rare recombinants that segregate Dpy animals is correlated with the genetic distance between the two genes. Figure 8.16. Example of genetic three point mapping phenotype genotype dpy unc mapping strain Wild type hermaphrodite Most of the gametes egl meiosis Rare gametes from a recombination event dpy unc egl dpy Sperms or eggs unc Sperms or eggs egl Progeny from combination of the common gametes Wild type Unc and Dpy Progeny from combination between common gametes and recombinant gametes Self-fertilizing unc + dpp + egl + Dpy non-Unc unc + dpp + egl dpy Unc non-Dpy unc + dpp unc + + unc + dpp unc + dpy Wild type Egl + egl + + egl + Egl unc + + + egl + + egl dpy + egl + dpy unc Recombination occurs to the left of egl egl to the right of the egl dpy dpy egl unc egl Recognizable recombinants Progeny with Egl phenotype unc unc + dpp + + dpy unc + dpp + egl dpy unc + dpp unc egl + unc + dpp unc + + Dpy non-Unc Unc non-Dpy No Yes unc egl dpy Map position a b Dpy non-Unc Unc non-Dpy a = b Yes No # of recombinations occurred to the left of egl # of recombinations occurred to the right of egl Figure 8.17. An example of genetic mapping using SNPs egl unc X unc egl A C. elegans strain from Hawaii. SNPs between this strain and the Bristol strain have been determined. Genetic mutant derived from the strain from Bristol, England. The egl mutation is being mapped. egl unc X X X *1 2* *3 *4 *5 *6 The hybrid strain. Stars indicate SNPs in the region Select Unc but non-Egl recombinants B A egl unc * *2 *3 *4 *5 *6 unc unc unc C egl unc * * * *4 *5 *6 unc egl * * * * * *6 Determine SNP #4 for all recombinant worms by sequencing or digestion. Worms A and B have #4 SNP from the Hawaii strain Determine SNP #5 and #6 for those that have lost SNP#4 (worm C only) Worm C has SNP #6 but not #5: the egl gene maps to the right of SNP#5 Common steps involved in cloning C. elegans genes defined by mutations. Genetic mutation SNP mapping RNAi of candidate genes Mapping using marker mutations Microinjection of cosmid/YAC clones Injection of subclones sequencing mutant DNA What would be the flow chart for cloning in yeast? Fly? Human? Which is the strongest evidence for claiming the cloning of the gene defined by the mutation? A. The transgene put back into the animal can rescue the mutant phenotype. B. You find a missense mutation in this gene by sequencing. C. Reducing the gene activity by RNAi mimics the mutant phenotype. D. The gene is expressed in the tissue with the mutant phenotype. Figure 8.19. Microinjection transformation in C. elegans. DNA solution is injected to the distal arms of the gonad Injection Select F1 transgenic animals, most are unstable F2 transgenic animals, stable lines Three types of markers unc-119(-) mutant unc-119(+) gene as a marker Wild type Wild type A dominant rol6 mutant gene as a marker Roller Wild type a strongly expressed GFP gene as a marker Green worm Figure 8.21. The prevailing model for the mechanism of RNAi dsRNA Introduced into cells Dicer Bind to Dicer-RDE1enzyme complex dsRNA is cut to ~22 nt siRNA Incorporated into RISC nuclease complex Multiple-protein components of RISC Unwinding siRNAs, activation of RISC Target mRNA AAAAAAAA 5’ Cleavage of target mRNA Figure 8.22. RNAi methods in C. elegans. A. Injecting ds RNA into intestine or gonad dsRNA intestine Transfer to plates Observe phenotype in progeny B. Soak worms with dsRNA solution Soak for 24 hours Transfer to plates Observe phenotype in progeny C. Feed the worms a bacterial strain that expresses dsRNA Feed worms the bacterial strain Grow the bacterial strain containing the vector expressing dsRNA Observe phenotype in progeny