* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Anatomy & Physiology
Survey
Document related concepts
Western blot wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Biological aspects of fluorine wikipedia , lookup
Endomembrane system wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Citric acid cycle wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Genetic code wikipedia , lookup
Protein adsorption wikipedia , lookup
Expanded genetic code wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Metalloprotein wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Transcript
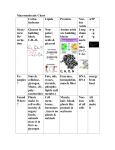
Anatomy & Physiology Ch. 2 BIOCHEMISTRY What is biochemistry? Biochemistry is the study of the chemical composition and reactions of living matter. Organic compounds contain carbon and are covalently bonded, such as starch. Inorganic compounds (not organic) include chemicals such as water, salts, acids, and bases. Water Water is the most abundant and important inorganic compound in organisms. 60-80 % of cellular volume. Water has many properties essential to an organisms survival. Properties of Water High heat capacity- absorbs or releases a large amount of heat before changing its temperature. Important for temp. regulation. High heat of vaporization- large amount of energy is needed for water to evaporate. Provides cooling effect when we sweat. Polarity- water is a polar molecule which makes it an excellent solvent. Many important nutrients, gases, and other compounds are in solution. Properties of water, cont. Reactivity- water is an important in many chemical reactions. (Hydrolysis and dehydration synthesis) Cushioning- fluid surrounding body organs and tissues provides protection from physical trauma and reduces friction. What is a salt? Salts are ionic compounds that dissociate into ions in solution. Salts found in the body include NaCl, KCl,and calcium carbonate. Calcium phosphates make up bones and teeth. K+ and Na+ ions are important for nerve transmission and muscle contraction. Electrolytes Acids, bases, and salts are electrolytes because they ionize and dissociate in water. These solutions can then conduct an electric current. Acids Acids and bases are covalently bonded molecules that dissociate in solution. Acids release H+ ions in solution. They are sour and corrosive. pH<7 – Acids in the body include HCl (gastric juice), uric acid, amino acids, lactic acid, carbonic acid, Vitamin C,fatty acids, vaginal fluid Bases Bases are proton acceptors, some are hydroxides (OH-) Bases are slippery and bitter, pH > 7. Bases in the body include blood, semen, ammonia, and bicarbonate ion. pH The relative concentrations of hydrogen [H+] and hydroxyl [OH-] ions in body fluids is measured in pH units. The pH scale runs from 0 to 14. pH of 7 is neutral, below 7 is considered to be acidic, and above 7 is basic. Each unit of pH represents a 10x change. For example, a pH of 2 is ten times more acidic than a pH of 3. Neutralization Neutralization reactions occur when acids and bases are mixed to form water and salt. Ex) HCl + NaOH -> H2O + NaCl Buffers Buffers are weak acids or bases that prevent extreme changes in pH. One buffer system helps maintain pH homeostasis of the blood: carbonic acid/bicarbonate ion Carbohydrates Carbohydrates contain carbon, hydrogen, and oxygen in a ratio of 1:2:1. Carbohydrates include sugars and starches, and are important for energy. A few carbohydrates are used for structural purposes. Larger molecules are less soluble in water. Carbs, cont. Monosaccharides (simple sugars) are single-chain or single ring structures containing 3-7 carbon atoms. Ex) glucose, fructose, and galactose Disaccharides are made up of two sugar units. Ex) lactose and maltose Polysaccarhides- chains of monosaccharides forming a polymer. Ex) starch, cellulose, glycogen Lipids Contain carbon, hydrogen,and oxygen. Structure often includes fatty acids and glycerol. Phosphorus is found in some more complex lipids. Insoluble in water, but dissolve in other lipids and organic solvents. Neutral fats Formed by fatty acids and glycerol in a 3:1 ratio. Also called triglycerides. Concentrated source of energy. Fats when solid, and oils when liquid. Saturated fats have only single bonds. Meat fat and butter fat are examples. Unsaturated fats contain double bonds. Olive oil and peanut oil are examples. Usually considered to be healthier. Phospholipids Modified triglyceride with phosphorus containing group. Has hydrophobic tail (nonpolar) and hydrophilic (polar) head. Amphipathic molecule. Chief component of cell membranes, also found in nervous tissue Steroids Flat molecules made up of four interlocking hydrocarbon rings. Fat soluble and contain little oxygen. Examples: cholesterol, sex hormones, bile salts, and important for cell membranes Fat-Soluble vitamins Some vitamins are not water-soluble. These nonpolar vitamins can accumulate in fat tissues if consumed in excess. Vitamin A- found in orange fruits and vegetables, part of the photoreceptor pigment involved in vision Vitamin E- found in green leafy vegetables, important in wound healing, has antioxidant properties Vitamin K- made available by the action of intestinal bacteria, prevalent in many foods, necessary for blood clotting Prostaglandins Diverse group of lipids formed from a 20-carbon fatty acid found in cell membranes. play a role in blood clotting, inflammatory response, and labor contractions Lipoproteins Protein-based substances that transport fatty acids and cholesterol in the bloodstream; major varieties are highdensity lipoproteins (HDLs) and lowdensity lipoproteins (LDLs) Proteins Contain carbon, hydrogen, oxygen, and nitrogen. Many contain phosphorus and sulfur. Composed of chains of 20 amino acid types, joined by peptide bonds Basic structural material of the body, making up 10-30% of cell mass. Includes enzymes, hemoglobin, and contractile proteins of muscles Amino Acids Amino acids are the building blocks of protein. Protein structure Proteins have a complex structure on four levels: Primary-sequence of amino acids Secondary- chain bends or twists (B-pleated sheet or alpha-helix) Tertiary- secondary structure folds to produce a compact, globular structure Quaternary- two or more polypeptide chains group to form a complex protein such as hemoglobin Fibrous vs. Globular proteins Overall structure of a protein determines its biological function. Fibrous proteins (structural proteins) are extended and thread-like. Water insoluble, have secondary structure Ex) collagen, keratin, elastin Globular proteins (functional proteins) are spherical and compact. Water soluble with tertiary structure. Ex) enzymes and antibodies Types of Globular proteins Antibodies-proteins important in the immune response Hormones-chemical messengers carried in the blood that stimulate target cells. Transport proteins-carry materials in the blood (hemoglobin) and across cell membranes Catalysts (Enzymes)-act as biological catalysts, to regulate and accelerate the rate of biochemical reactions without being used up in the process. Enzymes When enzymes and other proteins are exposed to conditions outside the optimum temperature and pH range, their three dimensional shape changes (denaturation). Since enzymes are shape-specific, any changes prevent them from carrying out their function. Nucleic Acids (DNA and RNA) Nucleic acids are composed of C,H,O, N, and P. The structural unit of nucleic acids are nucleotides. A nucleotide consists of a nitrogen containing base, a five carbon sugar, and a phosphate group. DNA DNA contains deoxyribose, is double stranded, and contains the bases adenine, thymine, adenine, and guanine. Base pairing: A—T and G—C DNA stores genetic information RNA RNA is single stranded, contains ribose, the base uracil instead of thymine RNA helps carry out its instructions. Adenosine triphosphate (ATP) • Although glucose is the most important cellular fuel, its energy cannot be used directly by cells. • Glucose catabolism is coupled with ATP synthesis, with energy being stored in the bonds of ATP. • ATP is the universal energy compound of body cells. How ATP Drives Cellular Work • ATP has 3 phosphates attached (P) • Removal of a P releases energy from the bond, leaving ADP • Removal of another P releases less energy, leaving AMP http://www.euronet.nl/users/warnar/atp.gif