* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 14 (Part 1)
Signal transduction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Proteolysis wikipedia , lookup
Photosynthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Coenzyme Q10 wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Phosphorylation wikipedia , lookup
Metalloprotein wikipedia , lookup
Mitochondrion wikipedia , lookup
Western blot wikipedia , lookup
Citric acid cycle wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
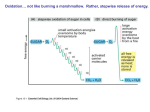
Chapter 14 (Part 1) Electron transport Chemiosmotic Theory • Electron Transport: Electrons carried by reduced coenzymes are passed through a chain of proteins and coenzymes to drive the generation of a proton gradient across the inner mitochondrial membrane • Oxidative Phosphorylation: The proton gradient runs downhill to drive the synthesis of ATP • Electron transport is coupled with oxidative phosphorylation • It all happens in or at the inner mitochondrial membrane Outer Membrane – Freely permeable to small molecules and ions. Contains porins with 10,000 dalton limit Inner membrane – Protein rich (4:1 protein:lipid). Impermeable. Contains ETR, ATP synthase, transporters. Cristae – Highly folded inner membrane structure. Increase surface area. Matrix- “cytosol” of the mitochondria. Protein rich (500 mg/ml) Contains TCA cycle enzymes, pyruvate dehydrogenase, fatty and amino acid oxidation pathway, DNA, ribosomes Intermembrane Space – composition similar to cytosol Reduction Potentials • High Eo' indicates a strong tendency to be reduced • Crucial equation: Go' = -nF Eo' • Eo' = Eo'(acceptor) - Eo'(donor) • NADH + ½ O2 + H+ NAD++ H+ + H2O NAD++ H+ + 2e- NADH Eo’ = -0.32 ½ O2 + 2e- + 2H+ H2O Eo’ = 0.816 Go‘= -nF(Eo'(O2) - Eo'(NADH)) Go‘= -nF(0.82 –(-0.32)) = -nF(1.14) = -2(96.5 kJ mol-1V-1)(1.136) = -220 kJ mol-1 Electron Transport • Four protein complexes in the inner mitochondrial membrane • A lipid soluble coenzyme (UQ, CoQ) and a water soluble protein (cyt c) shuttle between protein complexes • Electrons generally fall in energy through the chain - from complexes I and II to complex IV Standard reduction potentials of the major respiratory electron carriers. Complex I • • • • • • NADH-CoQ Reductase Electron transfer from NADH to CoQ More than 30 protein subunits - mass of 850 kD 1st step is 2 e- transfer from NADH to FMN FMNH2 converts 2 e- to 1 e- transfer Four H+ transported out per 2 e- NADH + H+ NAD+ FMN FMNH2 Fe2+S Fe3+S CoQ CoQH2 Complex II • • • • • Succinate-CoQ Reductase aka succinate dehydrogenase (from TCA cycle!) four subunits Two largest subunits contain 2 Fe-S proteins Other subunits involved in binding succinate dehydrogenase to membrane and passing e- to Ubiquinone • FAD accepts 2 e- and then passes 1 e- at a time to Fe-S protein • No protons pumped from this step Succinate Fumarate FAD FADH2 Fe2+S Fe3+S CoQ CoQH2 Q-Cycle • • • • Transfer from the 2 e- carrier ubiquinone (QH2) to Complex III must occur 1 e- at a time. Works by two single electron transfer steps taking advantage of the stable semiquinone intermediate Also allows for the pumping of 4 protons out of mitochondria at Complex III Myxothiazol (antifungal agent) inhibits electron transfer from UQH2 and Complex III. UQ UQ.- UQH2 Complex III • CoQ-Cytochrome c Reductase • CoQ passes electrons to cyt c (and pumps H+) in a unique redox cycle known as the Q cycle • Cytochromes, like Fe in Fe-S clusters, are oneelectron transfer agents • cyt c is a water-soluble electron carrier • 4 protons pumped out of mitochondria (2 from UQH2) CoQH2 CoQ cyt b ox cyt b red Fe2+S Fe3+S cyt c1 ox cyt c1 red cyt c red cyt c ox Complex IV • Cytochrome c Oxidase • Electrons from cyt c are used in a fourelectron reduction of O2 to produce 2H2O • Oxygen is thus the terminal acceptor of electrons in the electron transport pathway the end! • Cytochrome c oxidase utilizes 2 hemes (a and a3) and 2 copper sites • Complex IV also transports H+ (2 protons) cyt c red cyt c ox cyt a ox cyt a red cyt a3 red cyt a3 ox O2 2 H2O Inhibitors of Oxidative Phosphorylation • Rotenone inhibits Complex I - and helps natives of the Amazon rain forest catch fish! • Cyanide, azide and CO inhibit Complex IV, binding tightly to the ferric form (Fe3+) of a3 • Oligomycin and DCCD are ATP synthase inhibitors Shuttling Electron Carriers into the Mitochondrion • The inner mitochondrial membrane is impermeable to NADH. • Electrons carried by NADH that are created in the cytoplasm (such as in glycolysis) must be shuttled into the mitochondrial matrix before they can enter the ETS Glycerol phosphate shuttle malate/aspartate shuttle system Electron transport is coupled to oxidative phosphorylation Uncouplers • Uncouplers disrupt the tight coupling between electron transport and oxidative phosphorylation by dissipating the proton gradient • Uncouplers are hydrophobic molecules with a dissociable proton • They shuttle back and forth across the membrane, carrying protons to dissipate the gradient • w/o oxidative-phosphorylation energy lost as heat • Dinitrophenol once used as diet drug, people ran 107oF temperatures H NO2 O2N OH NO2 O 2N O