* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Substrate and oxidative phosphorylation
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Mitochondrion wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
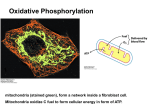
Substrate and oxidative phosphorylation • Substrate-level phosphorylation is a type of chemical reaction that results in the formation and creation of adenosine triphosphate (ATP) by the direct transfer and donation of a phosphoryl (PO3) group to adenosine diphosphate (ADP) from a reactive intermediate. While technically the transfer is PO3, or a phosphoryl group, convention in biological sciences is to refer to this as the transfer of a phosphate group. In cells, it occurs primarily and firstly in the cytoplasm (in glycolysis) under both aerobic and anaerobic conditions. • Unlike oxidative phosphorylation, here the oxidation and phosphorylation are not coupled or joined, although both types of phosphorylation result in ATP. • It should be noted that there is an oxidation reaction coupled to phosphorylation, however this occurs in the generation of 1,3bisphosphoglycerate from 3phosphoglyceraldehyde via a dehydrogenase. ATP is generated in a separate step (key difference from oxidative phosphorylation) by transfer of the high energy phosphate on 1,3-bisphosphoglycerate to ADP via a kinase. ATP is synthesized when protons flow back to the mitochondrial matrix through an enzyme complex ATP synthase. The oxidation of fuels and the phosphorylation of ADP are coupled by a proton gradient across the inner mitochondrial membrane. Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers. OXIDATIVE PHOSPHORYLATION IN EUKARYOTES TAKES PLACE IN MITOCHONDRIA Two membranes: outer membrane inner membrane (folded into cristae) Two compartments: (1) the intermembrane space (2) the matrix The outer membrane is permeable to small molecules and ions because it contains pore-forming protein (porin). The inner membrane is impermeable to ions and polar molecules. Contains transporters (translocases). Location of mitochondrial complexes • Inner mitochondrial membrane: Electron transport chain ATP synthase • Mitochondrial matrix: Pyruvate dehydrogenase complex Citric acid cycle Fatty acid oxidation THE ELECTRON TRANSPORT CHAIN Series of enzyme complexes (electron carriers) embedded in the inner mitochondrial membrane, which oxidize NADH2 and FADH2 and transport electrons to oxygen is called respiratory electron-transport chain (ETC). The sequence of electron carriers in ETC NADH FMN Fe-S succinate FAD Fe-S Co-Q Fe-S cyt b cyt c1 cyt c cyt a cyt a3 O2 High-Energy Electrons: Redox Potentials and Free-Energy Changes In oxidative phosphorylation, the electron transfer potential of NADH or FADH2 is converted into the phosphoryl transfer potential of ATP. Phosphoryl transfer potential is G°' (energy released during the hydrolysis of activated phosphate compound). G°' for ATP = -7.3 kcal mol-1 Electron transfer potential is expressed as E'o, the (also called redox potential, reduction potential, or oxidation-reduction potential). E'o (reduction potential) is a measure of how easily a compound can be reduced (how easily it can accept electron). All compounds are compared to reduction potential of hydrogen wich is 0.0 V. The larger the value of E'o of a carrier in ETC the better it functions as an electron acceptor (oxidizing factor). Electrons flow through the ETC components spontaneously in the direction of increasing reduction potentials. E'o of NADH = -0.32 volts (strong reducing agent) E'o of O2 = +0.82 volts (strong oxidizing agent) NADH FMN Fe-S succinate FAD Fe-S Co-Q Fe-S cyt b cyt c1 cyt c cyt a cyt a3 O2 Important characteristic of ETC is the amount of energy released upon electron transfer from one carrier to another. This energy can be calculated using the formula: Go’=-nFE’o n – number of electrons transferred from one carrier to another; F – the Faraday constant (23.06 kcal/volt mol); E’o – the difference in reduction potential between two carriers. When two electrons pass from NADH to O2 : Go’=-2*96,5*(+0,82-(-0,32)) = -52.6 kcal/mol