Clinical Study The Relationship between
... severity of disease [20]. The cut-off point of plasma coenzyme Q10 (0.52 µmol/L) in this study is also similar with the CORONA (Controlled Rosuvastatin Multinational Study in Heart Failure) trial conducted by McMurray et al. [21], the mortality was significantly increased in the lowest level of coen ...
... severity of disease [20]. The cut-off point of plasma coenzyme Q10 (0.52 µmol/L) in this study is also similar with the CORONA (Controlled Rosuvastatin Multinational Study in Heart Failure) trial conducted by McMurray et al. [21], the mortality was significantly increased in the lowest level of coen ...
ATP Synthesis
... of Krebs cycle) by virtue of its succinate dehydrogenase activity coupled with the reduction of its FAD cofactor to FADH2 - Next, Complex II facilitates the transfer of electrons from FADH2 to CoQ—in a series of redox steps (from FADH2 via three iron-sulfur clusters) culminating with the overall rea ...
... of Krebs cycle) by virtue of its succinate dehydrogenase activity coupled with the reduction of its FAD cofactor to FADH2 - Next, Complex II facilitates the transfer of electrons from FADH2 to CoQ—in a series of redox steps (from FADH2 via three iron-sulfur clusters) culminating with the overall rea ...
The Electron Transport Chain
... Shown above is a mitochondrian. The mitochondrian is enclosed by an outer membrane and a more complex inner mitochondrial membrane. The space between the inner and outer mitochondrial membranes is called the intermembrane space. With in this space we find enzymes that utilize ATP such as creatine k ...
... Shown above is a mitochondrian. The mitochondrian is enclosed by an outer membrane and a more complex inner mitochondrial membrane. The space between the inner and outer mitochondrial membranes is called the intermembrane space. With in this space we find enzymes that utilize ATP such as creatine k ...
Electron-Transport Chain and ATP production
... Electron-Transport Chain and ATP production Occurs in the inner mitochondrial membrane where NADH and FADH2 are oxidized back to NAD+ and FAD. They transfer their e- in a series of steps and ultimately to O2: O2 + 4e- + 4H+ → 2H2O The energy released in these e- transfers is used to pump H+ (protons ...
... Electron-Transport Chain and ATP production Occurs in the inner mitochondrial membrane where NADH and FADH2 are oxidized back to NAD+ and FAD. They transfer their e- in a series of steps and ultimately to O2: O2 + 4e- + 4H+ → 2H2O The energy released in these e- transfers is used to pump H+ (protons ...
ETC_2012 Quiz
... Free Energy of ATP • Transport of e-s down the ETC from NADH to O2 produces 52.8 kcal of energy • Converting one ADP to ATP requires 7.3 Kcal/mol • __ 3 ATPs are produced/molecule of NADH oxidized in the ETC 2 ATPS /mol of FADH2 oxidized • __ • The remaining energy is lost as heat or used for anci ...
... Free Energy of ATP • Transport of e-s down the ETC from NADH to O2 produces 52.8 kcal of energy • Converting one ADP to ATP requires 7.3 Kcal/mol • __ 3 ATPs are produced/molecule of NADH oxidized in the ETC 2 ATPS /mol of FADH2 oxidized • __ • The remaining energy is lost as heat or used for anci ...
Fe-S
... runs downhill to drive the synthesis of ATP • Electron transport is coupled with oxidative phosphorylation ...
... runs downhill to drive the synthesis of ATP • Electron transport is coupled with oxidative phosphorylation ...
The Benefits of Immunocal Platinum
... times to CoQ10 /ˌkoʊ ˌkjuː ˈtɛn/, CoQ, Q10, or Q, is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenyl chemical subunits in its tail. This oil-soluble, vitamin-like substance is present in most eukaryotic cells, primarily in the mitochondria. ...
... times to CoQ10 /ˌkoʊ ˌkjuː ˈtɛn/, CoQ, Q10, or Q, is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenyl chemical subunits in its tail. This oil-soluble, vitamin-like substance is present in most eukaryotic cells, primarily in the mitochondria. ...
Solving Biochemistry`s Biggest Mystery: How We Produce Energy
... Part 1: The discovery of coenzyme Q-10. An Interview with Dr. Fred L Crane by Richard A. Passwater, Ph.D. More than half of the people in the United States take a daily vitamin supplement. Most of these individuals don’t even realize that this was not possible not too awfully long ago. Thanks to a s ...
... Part 1: The discovery of coenzyme Q-10. An Interview with Dr. Fred L Crane by Richard A. Passwater, Ph.D. More than half of the people in the United States take a daily vitamin supplement. Most of these individuals don’t even realize that this was not possible not too awfully long ago. Thanks to a s ...
Document
... below 5 mM, F6P inhibits it. This way liver does not compete with muscle for glucose ...
... below 5 mM, F6P inhibits it. This way liver does not compete with muscle for glucose ...
X - The Nutrition Investigator
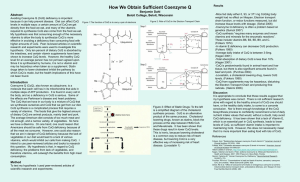
... multiple steps of ATP production. It is found in every cell in the body, and so a deficiency in CoQ is serious. Some of the effects are Parkinson’s disease and heart problems. The CoQ that we have in our body is a mixture of CoQ that we synthesize ourselves and CoQ that we get from our diet. CoQ syn ...
... multiple steps of ATP production. It is found in every cell in the body, and so a deficiency in CoQ is serious. Some of the effects are Parkinson’s disease and heart problems. The CoQ that we have in our body is a mixture of CoQ that we synthesize ourselves and CoQ that we get from our diet. CoQ syn ...
Coenzyme Q10
Coenzyme Q10, also known as ubiquinone, ubidecarenone, coenzyme Q, and abbreviated at times to CoQ10 /ˌkoʊ ˌkjuː ˈtɛn/, CoQ, or Q10 is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenyl chemical subunits in its tail.This oil-soluble, vitamin-like substance is present in most eukaryotic cells, primarily in the mitochondria. It is a component of the electron transport chain and participates in aerobic cellular respiration, generating energy in the form of ATP. Ninety-five percent of the human body’s energy is generated this way. Therefore, those organs with the highest energy requirements—such as the heart, liver and kidney—have the highest CoQ10 concentrations. There are three redox states of CoQ10: fully oxidized (ubiquinone), semiquinone (ubisemiquinone), and fully reduced (ubiquinol). The capacity of this molecule to exist in a completely oxidized form and a completely reduced form enables it to perform its functions in the electron transport chain, and as an antioxidant, respectively.