* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 24. Amines
Elias James Corey wikipedia , lookup
George S. Hammond wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Discodermolide wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Ene reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Homoaromaticity wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Aromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Organosulfur compounds wikipedia , lookup
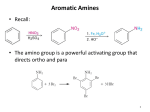
Stille reaction wikipedia , lookup
Aromatization wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Chapter 24. Amines and Heterocycles Based on McMurry’s Organic Chemistry, 7th edition Amines – Organic Nitrogen Compounds Organic derivatives of ammonia, NH3, Nitrogen atom with a lone pair of electrons, making amines both basic and nucleophilic Occur in plants and animals 2 Why this Chapter? Amines and carbonyl compounds are the most abundant and have rich chemistry In addition to proteins and nucleic acids, a majority of pharmaceutical agents contain amine functional groups 3 Common Names of Heterocyclic Amines If the nitrogen atom occurs as part of a ring, the compound is designated as being heterocyclic Each ring system has its own parent name 4 24.2 Properties of Amines Bonding to N is similar to that in ammonia N is sp3-hybridized C–N–C bond angles are close to 109° tetrahedral value 5 Chirality Is Possible (But Not Observed) An amine with three different substituents on nitrogen is chiral (in principle but not in practice): the lone pair of electrons is the fourth substituent Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion 6 24.3 Basicity of Amines The lone pair of electrons on nitrogen makes amines basic and nucleophilic They react with acids to form acid–base salts and they react with electrophiles 7 8 24.5 Biological Amines and the Henderson-Hasselbalch Equation What form do amines exist at physiological pH inside cells 9 Selective Preparation of Primary Amines: the Azide Synthesis Azide ion, N3 displaces a halide ion from a primary or secondary alkyl halide to give an alkyl azide, RN3 Alkyl azides are not nucleophilic (but they are explosive) Reduction gives the primary amine 10 Gabriel Synthesis of Primary Amines A phthalimide alkylation for preparing a primary amine from an alkyl halide The N-H in imides (CONHCO) can be removed by KOH followed by alkylation and hydrolysis 11 Reductive Amination of Aldehydes and Ketones Treatment of an aldehyde or ketone with ammonia or an amine in the presence of a reducing agent 12 Mechanism of Reductive Amination 13 Reducing Step Sodium cyanoborohydride, NaBH3CN, reduces C=N but not C=O Stable in water 14 Hofmann and Curtius Rearrangements Carboxylic acid derivatives can be converted into primary amines with loss of one carbon atom by both the Hofmann rearrangement and the Curtius rearrangement 15 24.7 Reactions of Amines Alkylation and acylation have already been presented 16 Hofmann Elimination Converts amines into alkenes NH2 is very a poor leaving group so it converted to an alkylammonium ion, which is a good leaving group 17 Orientation in Hofmann Elimination We would expect that the more highly substituted alkene product predominates in the E2 reaction of an alkyl halide (Zaitsev's rule) However, the less highly substituted alkene predominates in the Hofmann elimination due to the large size of the trialkylamine leaving group The base must abstract a hydrogen from the most sterically accessible, least hindered position 18 24.8 Reactions of Arylamines Amino substituents are strongly activating, ortho- and para-directing groups in electrophilic aromatic substitution reactions Reactions are controlled by conversion to amide 19 Arylamines Are Not Useful for Friedel-Crafts Reactions The amino group forms a Lewis acid–base complex with the AlCl3 catalyst, preventing further reaction Therefore we use the corresponding amide 20 Diazonium Salts: The Sandmeyer Reaction Primary arylamines react with HNO2, yielding stable arenediazonium salts 21 Uses of Arenediazonium Salts The N2 group can be replaced by a nucleophile 22 Reduction to a Hydrocarbon By treatment of a diazonium salt with hypophosphorous acid, H3PO2 23 Mechanism of Diazonium Replacement Through radical (rather than polar or ionic) pathways 24 Diazonium Coupling Reactions Arenediazonium salts undergo a coupling reaction with activated aromatic rings, such as phenols and arylamines, to yield brightly colored azo compounds, ArN=NAr 25 How Diazonium Coupling Occurs The electrophilic diazonium ion reacts with the electron-rich ring of a phenol or arylamine Usually occurs at the para position but goes ortho if para is blocked 26 Azo Dyes Azo-coupled products have extended conjugation that lead to low energy electronic transitions that occur in visible light (dyes) 27 24.9 Heterocycles A heterocycle is a cyclic compound that contains atoms of two or more elements in its ring, usually C along with N, O, or S 28 Pyrole and Imidazole Pyrole is an amine and a conjugated diene, however its chemical properties are not consistent with either of structural features 29 Chemistry of Pyrole Electrophilic substitution reactions occur at C2 b/c it is position next to the N A more stable intermediate cation having 3 resonance forms At C3, only 2 resonance forms 30 Polycyclic Heterocycles 31 24.10 Spectroscopy of Amines Infrared Characteristic N–H stretching absorptions 3300 to 3500 cm1 Amine absorption bands are sharper and less intense than hydroxyl bands Protonated amines show an ammonium band in the range 2200 to 3000 cm1 32 Nuclear Magnetic Resonance Spectroscopy N–H hydrogens appear as broad signals without clear-cut coupling to neighboring C–H hydrogens In D2O exchange of N–D for N–H occurs, and the N– H signal disappears 33 13C NMR Carbons next to amine N are slightly deshielded - about 20 ppm downfield from where they would absorb in an alkane 34