* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Proteins - Downtown Magnets High School
Ancestral sequence reconstruction wikipedia , lookup
Magnesium transporter wikipedia , lookup
Genetic code wikipedia , lookup
Expanded genetic code wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Bottromycin wikipedia , lookup
Circular dichroism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein domain wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Protein folding wikipedia , lookup
Point mutation wikipedia , lookup
Biosynthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Western blot wikipedia , lookup
Protein adsorption wikipedia , lookup
List of types of proteins wikipedia , lookup
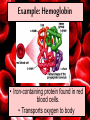
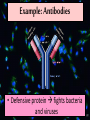
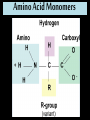
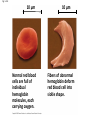
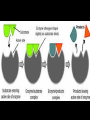
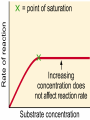
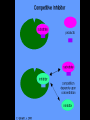
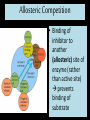
Proteins Big Idea 4: Biological Systems Interact Essential Knowledge • Essential knowledge 4.B.1: Interactions between molecules affect their structure and function. • a. Change in the structure of a molecular system may result in a change of the function of the system. • b. The shape of enzymes, active sites, and interaction with specific molecules are essential for basic functioning of the enzyme. Protein Functions! • Structural support, storage, transport, cellular communications, movement, and defense against foreigners • Make up more than 50% of dry mass of cells Example: Hemoglobin • Iron-containing protein found in red blood cells. • Transports oxygen to body Example: Antibodies Antibodies • Defensive protein fights bacteria and viruses Example: Lactase, an Enzyme • Enzyme that helps break down sugar lactose into galactose and glucose. Speeds up reactions rates: • Lactose intolerant: Mutation of Chrom. 2. • Cramps, bloating, flatulence Example: Insulin • Hormonal protein: regulates sugar in blood (tells cells to take it in), pancreas Polypeptides • Polymers built from same set of 20 amino acids • A protein consists of one or more polypeptides Amino Acid Monomers Amino Acid Polymers • Amino acids are linked by peptide bonds Protein Structure and Function • Consists of 1/more polypeptides twisted, folded, and coiled into a unique shape (determined by amino acid sequence) Four Levels of Protein Structure • Primary, Secondary, Tertiary, Quartenary! • Watch Videos! • Chaperonins are protein molecules that assist the proper folding of other proteins Polypeptide Correctly folded protein Cap Hollow cylinder Chaperonin (fully assembled) Steps of Chaperonin 2 The cap attaches, causing the Action: cylinder to change shape in such a way that it creates a 1 An unfolded polyhydrophilic environment for peptide enters the the folding of the polypeptide. cylinder from one end. 3 The cap comes off, and the properly folded protein is released. Sickle-Cell Disease: A Change in Primary Structure • A change in primary structure can affect a protein’s structure and ability to function • Ex: Sickle-cell disease: results from a single amino acid substitution in protein hemoglobin Fig. 5-22a Normal hemoglobin Primary structure Val His Leu Thr Pro Glu Glu 1 Secondary and tertiary structures 2 3 4 5 6 7 subunit Quaternary structure Normal hemoglobin (top view) Function Molecules do not associate with one another; each carries oxygen. Fig. 5-22b Sickle-cell hemoglobin Primary structure Secondary and tertiary structures Val His Leu Thr Pro Val Glu 1 2 Exposed hydrophobic region Quaternary structure Sickle-cell hemoglobin Function Molecules interact with one another and crystallize into a fiber; capacity to carry oxygen is greatly reduced. 3 4 5 6 7 subunit Fig. 5-22c 10 µm Normal red blood cells are full of individual hemoglobin molecules, each carrying oxygen. 10 µm Fibers of abnormal hemoglobin deform red blood cell into sickle shape. Messing Up Proteins? • Alterations in pH, salt concentration, temp., or other environmental factors can cause a protein to unravel denaturation inactive protein Enzyme Proteins! • Acts as a catalyst to speed up chemical reactions • Can perform functions repeatedly workhorses! Cofactors • A non-protein chemical compound required for enzyme activity Ex: Fe • “Helper Molecules" that assist in biochemical transformations. Coenzymes • A protein chemical compound required for enzyme activity • “Helper Molecules" that assist in biochemical transformations. Cofactors and Coenzymes • Work together to regulate enzyme function. • Usually the interaction relates to a structural change that alters the activity rate of the enzyme Competitive Inhibitors • Binding of inhibitor molecule to active site of enzyme prevents binding of the substrate and vice versa. Allosteric Competition • Binding of inhibitor to another (allosteric) site of enzyme (rather than active site) prevents binding of substrate Model Interpretations • The change in function of an enzyme can be interpreted from data regarding the concentrations of product or substrate as a function of time. These representations demonstrate the relationship between an enzyme’s activity, the disappearance of substrate, and/ or presence of a competitive inhibitor.