* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 5th Lecture 1433
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of TRPV1 antagonists wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
CCR5 receptor antagonist wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Theralizumab wikipedia , lookup
Drug design wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
NMDA receptor wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Drug interaction wikipedia , lookup
Toxicodynamics wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Psychopharmacology wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Neuropharmacology wikipedia , lookup
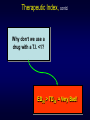
Pharmacology-1 PHL 313 Fifth Lecture By Abdelkader Ashour, Ph.D. Phone: 4677212 Email: [email protected] Drug Receptor Interactions, The two-state model of receptor activation The receptor is in two conformational states, ‘resting’ (R) and ‘active’ (R*), which exist in equilibrium Normally, when no ligand is present, the equilibrium lies far to the left, and a few receptors are found in the R* state For constitutively active receptors, an appreciable proportion of receptors adopt the R* conformation in the absence of any ligand Agonists have higher affinity for R* than for R and thus shift the equilibrium from the resting state (R) to the active (R*) state and hence, produce a response (Activated state) (Resting state) (Active state) Drug Receptor Interactions, Inverse agonist Inverse agonist “An agent which binds to the same receptor binding-site as an agonist for that receptor but exerts the opposite pharmacological effect” Difference from Antagonist: Antagonist binds to the receptor, but does not reduce basal activity Agonist positive efficacy Antagonist zero efficacy Inverse agonist negative efficacy Inverse agonists are effective against certain types of receptors (e.g. certain histamine receptors and GABA receptors) which have constitutive activity Example 1: The agonist action of benzodiazepines on the benzodiazepine receptor in the CNS produces sedation, muscle relaxation, and controls convulsions. b-carbolines (inverse agonists) which also bind to the same receptor cause stimulation, anxiety, increased muscle tone and convulsions Example 2: The histamine H2 receptor has constitutive activity, which can be inhibited by the inverse agonist cimetidine. On the other hand, burimamide acts as a neutral antagonist Drug Receptor Interactions, The two-state model of receptor activation & Inverse Agonist An inverse agonist has higher affinity for R than for R* and thus will shift the equilibrium from the active (R*) to resting state (R) state A neutral antagonist has equal affinity for R and R* so does not by itself affect the conformational equilibrium but reduces by competition the binding of other ligands In the presence of an agonist, partial agonist or inverse agonist, the antagonist restores the system towards the constitutive level of activity Inverse Agonist Antagonist (Activated state) (Resting state) (Active state) Drug Receptor Interactions, The two-state model of receptor activation & Inverse Agonist, contd. An inverse agonist has higher affinity for R than for R* and thus will shift the equilibrium from the active (R*) to resting state (R) state A neutral antagonist has equal affinity for R and R* so does not by itself affect the conformational equilibrium but reduces by competition the binding of other ligands In the presence of an agonist, partial agonist or inverse agonist, the antagonist restores the system towards the constitutive level of activity Drug-Receptor Bonds 1. Covalent Bond -Very strong -Not reversible under biologic conditions unusual in therapeutic drugs Example: Phenoxybenzamine at a adrenergic receptors The rest of pharmacology is concerned with weak, reversible, electrostatic attractions: 2. Ionic bond -Weak, electrostatic attraction between positive and negative forces -Easily made and destroyed 3. Dipole - dipole interaction -A stronger form of dispersion forces formed by the instantaneous dipole formed as a result of electrons being biased towards a particular atom in a molecule (an electronegative atom). -Example: Hydrogen bonds Drug-Receptor Bonds, 4. Hydrophobic interactions “The tendency of hydrocarbons (or of lipophilic hydrocarbon-like groups in solutes) to form intermolecular aggregates or intramolecular interactions in an aqueous medium” -usually quite weak -Important in the interactions of highly lipidsoluble drugs with the lipids of cell membranes and perhaps in the interaction of drugs with the internal walls of receptor “pockets” 5. Dispersion (Van der Waal) forces -Attractive forces that arise between particles as a result of momentary imbalances in the distribution of electrons in the particles -These imbalances produce fluctuating dipoles that can induce similar dipoles in nearby particles, generating a net attractive force contd. Drug-Receptor Bonds and Selectivity Drugs which bind through weak bonds to their receptors are generally more selective than drugs which bind through very strong bonds This is because weak bonds require a very precise fit of the drug to its receptor if an interaction is to occur Only a few receptor types are likely to provide such a precise fit for a particular drug structure To design a highly selective short acting drug for a particular receptor, we would avoid highly reactive molecules that form covalent bonds and instead choose molecules that form weaker bonds Selectivity: Preferential binding to a certain receptor subtype leads to a greater effect at that subtype than others -e.g. salbutamol binds at β2 receptors (lungs) rather than at β1 receptors (heart) Lack of selectivity can lead to unwanted drug effects. -e.g. salbutamol (b2-selective agonist ) vs isoprenaline (non-specific b-agonist) for patients with asthma. Isoprenaline more cardiac side effects (e.g., tachycardia) Therapeutic Index (T.I.) A measure of drug safety The ratio of the dose that produces toxicity to the dose that produces a clinically desired or effective response in a population of individuals Therapeutic Index = TD50/ED50 or LD50/ED50 where TD50 is the dose that produces a toxic effect in 50% of the population, LD50 is the dose that is lethal in 50% of the population and ED50 is the dose that produces therapeutic response in 50% of the population In general, a larger T.I. indicates a clinically safer drug TD50 Therapeutic Index, contd. Why don’t we use a drug with a T.I. <1? ED50 > TD50 = Very Bad! Therapeutic Index (T.I.), • High therapeutic index – NSAIDs • Aspirin • Tylenol • Ibuprofen – Most antibiotics – Beta-blockers contd. • Low therapeutic index – Lithium – Neuroleptics • Phenytoin • Phenobarbital – Digoxin – Immunosuppressives Spare Receptors In some systems, full agonists are capable of eliciting 50% response with less than 50% of the receptors bound (receptor occupancy) Maximal effect does not require occupation of all receptors by agonist Low concentrations of competitive irreversible antagonists may bind to receptors and a maximal response can still be achieved Pool of available receptors exceeds the number required for a full response Common for receptors that bind hormones and neurotransmitters if [R] is increased, the same [DR] can be achieved with a smaller [D] A similar physiological response is achieved with a smaller [D] Economy of hormone or neurotransmitter secretion is achieved at the expense of providing more receptors