* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download agonist - Buffalo State
CCR5 receptor antagonist wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
Toxicodynamics wikipedia , lookup
NMDA receptor wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
Drug discovery wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Theralizumab wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Drug interaction wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Drug Chemistry and
Toxicology
CHE 618
Alexander Nazarenko
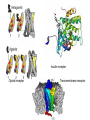
The Receptor Concept
The Nature of Drug Receptors
Drug Parameters: Affinity and Efficacy
The Langmuir Isotherm equation
Insulin receptor
Opioid receptor
Transmembrane receptor
Occupation theory
Drug effect is directly proportional to number of receptors occupied
Drug effect ceases as drug-receptor complex dissociate
Ariens & Stephenson theory introduced terms of "affinity" & "efficacy"
Affinity: ability of the drug to combine with receptor to create drug-receptor complex
Efficacy: ability of the drug-receptor complex to initiate a response
Affinity “drug-receptor interaction” is governed by the law of mass action.
In this theory
Agonist: drug with high affinity & high intrinsic activity Partial agonist: drug with high
affinity & low intrinsic activity
Antagonist: drug with high affinity & low intrinsic activity
Rate theory
The activation of receptors is directly proportional to the total number of encounters of
the drug with its receptors per unit time
Pharmacological activity is directly proportional to the rate of dissociation & association
not number of receptors occupied
Agonist: drug with fast association & fast dissociation
Partial agonist: drug with intermediate association & intermediate dissociation
Antagonist: drug with fast association & slow dissociation
Induced fit theory
As the drug approaches the receptor the receptors alters the conformation of its binding
site to produce drug—receptor complex
An agonist is a chemical that binds to a receptor and
triggers a response. Whereas an agonist causes an action,
an antagonist blocks the action of the agonist and
an inverse agonist causes an action opposite
to that of the agonist.
From the Greek αγωνιστής (agōnistēs),
contestant; champion; rival
< αγων (agōn), contest, combat; exertion,
struggle
< αγω (agō), I lead, lead towards,
conduct; drive
Drug + Receptor
[Drug/Receptor Complex]
K
Action
The Langmuir Isotherm
C
C
1 C K C
β = 100
K = 1/100 = 0.01
1
0.8
0.6
0.4
0.2
0
0
0.05
0.1
0
0.0001
0.001
0.01
1
1
0.8
0.8
0.6
0.6
0.4
0.4
0.2
0.2
0
0
0.005
0.01
0.015
0.02
0.025
0.15
0.2
0.1
1
a
Various plots
1
0.8
0.6
1/a
0.4
12
0.2
C
y = 0.01x + 1
10
0
0
0.005
0.01
0.015
0.02
0.025
8
6
0.04
C/a
0.035
4
0.03
2
1/C
0
0
500
1000
0.025
0.02
y = x + 0.01
0.015
0.01
Lineweaver–Burk plot
C
0.005
0
Hanes–Woolf plot
0
0.02
Michaelis–Menten kinetics
Lineweaver–Burk or double-reciprocal plot of kinetic data,
showing the significance of the axis intercepts and gradient.
Vmax S
V
K S
Michaelis-Menten Plot relating the reaction
rate V to the substrate concentration [S].
Agonists
Full agonists are able to activate the receptor and result in a
maximal biological response.
Partial Agonist
Partial agonists do not activate receptors thoroughly, causing
responses which are partial compared to those of full agonists
Antagonist
Antagonists bind to receptors but do not activate them.
This results in receptor blockage, inhibiting the binding of other agonists.
Inverse Agonist
Inverse agonists reduce the activity of receptors by inhibiting
their constitutive activity.
Full Agonist
+1
Functional Response
Partial Agonist
Neutral Antagonist
0
Full Inverse Agonist
-1
Log[Drug]
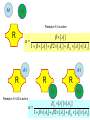
Competitive Antagonism
A1 + Receptor
A2 + Receptor
{A1 Receptor}
effect
{A2 Receptor}
[ A1 ]
1 [ A1 ] 2 [ A2 ]
A1
A2
Receptor-A1 is active
R
[ A1 ]
1 [ A1 ] 2 [ A2 ] 12 [ A1 ] [ A2 ]
A1
A1
R
R
R
A2
Receptor-A1-A2 is active
A2
12 [ A1 ] [ A2 ]
1 [ A1 ] 2 [ A2 ] 12 [ A1 ] [ A2 ]
Similar:
In coordination chemistry
In enzyme kinetics
Metal Ion + Ligand 1 + Ligand 2
Enzyme + Substrate + Inhibitor
Dose-response curve
What is there that is not poison?
All things are poison and nothing
without poison
Solely the dose determines that
a thing is not a poison