* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Visualizing expression patterns of Shh and Foxf1 genes
Ridge (biology) wikipedia , lookup
X-inactivation wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Microevolution wikipedia , lookup
Genome evolution wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Epigenetics of depression wikipedia , lookup
Gene nomenclature wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Genomic imprinting wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Designer baby wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Gene expression profiling wikipedia , lookup
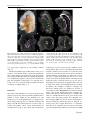
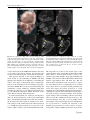
Pediatr Surg Int (2008) 24:3–11 DOI 10.1007/s00383-007-2036-1 ORIGINAL ARTICLE Visualizing expression patterns of Shh and Foxf1 genes in the foregut and lung buds by optical projection tomography Hideaki Sato Æ Paula Murphy Æ Shay Giles Æ John Bannigan Æ Hajime Takayasu Æ Prem Puri Published online: 26 October 2007 Ó Springer-Verlag 2007 Abstract Congenital malformations of the foregut are common in humans. The respiratory and digestive tubes are both derived by division of the foregut primordium. Sonic hedgehog (Shh) and Fork head box F1 (Foxf1) genes encode regulatory molecules that play a pivotal role in gut and lung morphogenesis and are therefore important candidate genes to be examined in models of foregut developmental disruption. Optical projection tomography (OPT) is a new, rapid and non-invasive technique for three-dimensional (3D) imaging of small biological tissue specimens that allows visualization of the tissue distribution of RNA in developing organs while also recording morphology. To explore the application of OPT in this context, we visualized Shh and Foxf1 gene expression patterns in the mouse foregut and lung buds at several stages of development. Time-mated CBA/Ca mice were harvested on embryonic days 9–12. The embryos were stained following whole mount in situ hybridization with labelled RNA probes to detect Shh and Foxf1 transcripts at each stage. The embryos were scanned by OPT to obtain 3D representations of gene expression domains in the context of the changing morphology of the embryo. H. Sato H. Takayasu P. Puri (&) Children’s Research Centre, Our Lady’s Children’s Hospital, Crumlin, Dublin, Ireland e-mail: [email protected] P. Murphy Department of Zoology, University of Dublin, Trinity College, Dublin 2, Ireland S. Giles J. Bannigan School of Medicine and Medical Science, University College Dublin, Belfield, Dublin, Ireland OPT analysis of Shh and Foxf1 expression in the foregut and lung buds revealed extra details of the patterns not previously reported, particularly in the case of Foxf1 where gene expression was revealed in a changing pattern in the mesenchyme around the developing lung. Shh expression was also revealed in the epithelium of the lung bud itself. Both genes were detected in complementary patterns in the developing bronchi as late as E12, showing successful penetration of molecular probes and imaging at later stages. OPT is a valuable tool for revealing gene expression in an anatomical context even in internal tissues like the foregut and lung buds across stages of development, at least until E12. This provides the possibility of visualizing altered gene expression in an in vivo context in genetic or teratogenic models of congenital malformations. Keywords Oesophageal atresia Foregut Sonic hedgehog Foxf1 Optical projection tomography Introduction Congenital malformations of the foregut such as the oesophageal atresia/tracheo-oesophageal fistula (OA/TOF) are common in humans. Until recently, little was known about the pathogenesis of these anomalies and there was little opportunity to study them. The accidental finding that the anthracycline antibiotic Adriamycin has teratogenic effects on rats, producing tracheo-oesophageal malformations has provided a reproducible model [1]. This Adriamycin rat model has contributed to investigating the mechanisms of OA/TOF congenital anomalies [2–4]. The mouse is the foremost mammalian model of development, offering an expanding wealth of genetic and molecular 123 4 knowledge and scientific research techniques. Our group has confirmed that the Adriamycin mouse model (AMM) produces a spectrum of tracheo-oesophageal malformations [5, 6]. Sonic hedgehog (Shh) is a secreted glycoprotein that acts as a cell signalling molecule and has multiple patterning roles in the developing embryo. The Shh gene is expressed in various organizing centers, such as the notochord, floor plate of the neural tube and polarizing region of the limbs [7]. It is involved in dorso-ventral foregut patterning [7, 8], presumably through notochord localization. The later expression of Shh in lung buds suggests that it plays a role in the morphogenesis of various epithelial appendages of the respiratory system [8, 9]. Homozygous Shh mutant mouse embryos are characterized by a number of development defects; especially in the respiratory tract that include failure of the trachea to develop as a separate structure from the oesophagus [8, 10]. In Adriamycin treated rats, the level of Shh protein expression is very low, without any time-dependent changes [11, 12]. The transcription factor Fork head box F1 (Foxf1) gene encodes a regulatory molecule that plays a pivotal role in gut and lung morphogenesis, presumably through the regulation of cell-specific target genes and is therefore an important candidate gene to be examined in models of foregut developmental disruption [13, 14]. Foxf1 is expressed in the foregut mesoderm and its expression is upregulated by Shh [15]. In mice, tracheo-oesophageal morphogenesis is highly sensitive to the dosage of Foxf1. Heterozygous Foxf1 embryos show variable phenotypes that include narrowing of the oesophagus and trachea, oesophageal atresia, tracheo-oesophageal fistula, and lung hypoplasia [14, 16]. Similar anomalies were also demonstrated in mice lacking in Shh, and it has been suggested that Foxf1 is involved in the Shh signalling pathway, downstream of Shh [15]. Recently, various tools for obtaining three-dimensional (3D) information on biological tissues have been investigated and improved [17–19]. Optical projection tomography (OPT) is a new, rapid and non-invasive technique for 3D imaging of small biological specimens that allows visualization of the tissue distribution of RNA, protein or histological stains in developing organs while also recording morphology [20]. To explore the application of OPT to the study of a congenital model, such as AMM, we visualized Shh and Foxf1 gene expression patterns in the mouse foregut and lung buds at several stages of development. This enabled us to visualize not only morphological development, but underlying molecular changes, such as gene expression. Here, the strengths and limitations of the approach are presented. 123 Pediatr Surg Int (2008) 24:3–11 Materials and methods Animals Male and female CBA/Ca mice (Harlan UK, Bicester, England) were accurately time-mated over a 4 h period starting at 8 a.m. Identification of a vaginal plug at the end of the mating period was taken to be the start of gestation. The dams were humanely killed by swift cervical dislocation between embryonic day (E) 9 and E13. The embryos were washed with phosphate buffered saline (PBS) and then fixed in 4% paraformaldehyde in Rnasefree PBS overnight. The embryos to be used for in situ hybrydization were stored at –20°C following dehydration through a methanol series. Whole mount in situ hybridization Before staining, E12 embryos were dissected to include only the trunk region from the level of the first branchial arch to the base of the liver. Whole mount in situ hybridization was performed on embryos using digoxygeninlabelled riboprobes for Shh and Foxf1 (generous gift of Dr L.Lundh). This technique involved embryo pretreatment followed by overnight hybridization with the probe, application of the antidigoxygenin antibody (Roche, Mannheim, Germany) and signal development using the NBT/BCIP solution (Roche, Mannheim, Germany). Whole embryos were examined and photographed to view the externally visible pattern of gene expression. Optical projection tomography OPT was carried out as described in Sharpe et al. (20). Stained embryos were embedded in a 1.0% low melting point agarose in H2O and attached to a metal mount. The mounted embryos were then dehydrated through 100% methanol prior to clearing by immersion in a 2:1 benzyl alcohol:benzyl benzoate solution. The specimen was then loaded into a prototype OPT scanner built at the Medical Research Council Human Genetics Unit and installed in the Zoology Department, Trinity College, Dublin. A total of 400 projection images were captured using a Leica MZFLIII stereo-fluorescent microscope through a full 360° rotation of the specimen. Images were captured either in visible light (to capture the colorimetric staining of the gene expression pattern) or in UV light with a Texas Red filter to capture embryo morphology. The captured data were transferred to a Linux computer where a series of Pediatr Surg Int (2008) 24:3–11 programmes (provided by the Edinburgh Mouse Atlas Project EMAP) performed a back projection algorithm to reconstruct 3D representations of the specimens. Reconstructed data were viewed and analysed using software provided by EMAP. All experiments were carried out in compliance with the current European Union regulations for animal investigation (ED86/609/EC), with prior ethical approval under licence from the Department of Health, Ireland. Results Analysis of computer reconstructed specimens following OPT allowed the Shh and Foxf1gene expression patterns to be viewed in 3D, either externally as ‘‘volume rendered’’ (vr) 3D movies (still shots from these movies are shown in Figs. 1, 2, 3, 4), or following virtual sectioning to reveal the patterns of transcript distribution through the developing tissues. The following is a description of the patterns revealed across stages E9–E12, with emphasis on foregut and lung bud expression. This shows the advantage of viewing the full pattern in the context of the changing morphology of the tissues at each stage and the potential of revealing possibly altered expression in the context of altered morphology in a congenital model system. Shh expression In E9 embryos (Fig. 1a–e), OPT revealed the expression of Shh in the floor plate of the neural tube, the notochord and the foregut where transverse sections (Fig. 1c, d) clearly show localization of Shh transcripts to the ventral aspect of the gut invagination, shown here at the level of the pharyngeal arches. The precise domain of the ventral localization could not be ascertained from whole mount preparations (Fig. 1a), showing the advantage of 3D computer representation where the data can be virtually sectioned and analysed in multiple orientations. At E10, Shh expression persisted in the floor plate of the neural tube, notochord, foregut and hindgut, as is visible in the raw whole mount in situ hybridization image and the 3D computer representation (Fig. 1f, g). In the foregut, OPT analysis of the specimen clearly showed extra details of the pattern; restriction to the ventral endoderm of the laryngotracheal groove and through the emerging lung buds (Fig. 1h–j), including the site of division of the respiratory groove from the foregut (Fig. 1g). At E11, external views of in situ hybridized embryos showed Shh expression again in the floor plate of the neural tube, and now also detectable in the polarizing regions of the fore and hind limb buds (Fig. 1k, l). Expression in the 5 gut was no longer clearly visible externally in the raw specimen (Fig. 1k) as the size of the embryo increased, but was clearly visible following OPT scanning (Fig. 1l). Section analysis of the OPT reconstruction clearly showed Shh expression through the foregut, the branching division of the trachea and in the lung buds (Fig. 1n–q), with the ventral restriction in the endoderm of the laryngotracheal groove (Fig. 1o) extending into the dorsal territory in more caudal sections (Fig. 1p), and lung buds (Fig.1q). At E12, embryos were enlarged considerably and the complexity of the tissues increased so that successful whole mount in situ hybridization was challenged due to reduced penetration of the RNA probe and components of the detection method. To ensure penetration, the embryos were dissected to include only the trunk region from the first branchial arch to the base of the liver. In such preparations, internal expression cannot be viewed easily in raw specimens, although part of the floor plate and stomach are visibly stained (Fig. 2a). Following OPT scanning and reconstruction, details of the expression in the endoderm of the oesophagus and lung buds could be seen (Fig. 2b–e). Foxf1 expression External analysis of whole mount preparations showing Foxf1 expression in E9 embryos (Fig. 3a) showed strong staining in the anterior mesenchyme in the region of the foregut and in the ventral part of the neural tube (Fig. 3a). The superficial pattern appeared very similar to that of Shh. OPT analysis clearly revealed the precise distribution of Foxf1 transcripts in the floor plate of the neural tube and the notochord, similar in this respect to Shh expression, and in the mesenchyme associated with the foregut (Fig 3c–e), adjacent to the expression of Shh in the ventral foregut endoderm. Foxf1 expression in E10 embryos was again visible in whole-mount preparations in the ventral CNS, particularly strongly in the hindbrain, midbrain and diencephalons, and in the viscera at the level of the foregut. It was also visible in the posterior fore-limb buds (Fig. 3f). From OPT analysis, the distribution in the viscera became clear, again in the mesenchyme around the foregut, most intense in the ventral aspect and around the lung buds (Fig. 3g–k). In addition, there was particularly strong expression of Foxf1 in a posterior enlargement of the foregut where the stomach was developing (Fig. 3k). Superficial analysis of E11 embryos, hybridized to reveal Foxf1 expression (Fig. 3l), showed staining again in the ventral neural tube, but appeared to be restricted to the brain region and the trunk between the levels of the limb buds. However, OPT analysis showed that ventral neural tube expression was continuous (Fig. 3m) and that there was an apparent discontinuity is an artefact of viewing 123 6 Pediatr Surg Int (2008) 24:3–11 Fig. 1 The expressions of Shh at E9–E11. a–e E9, f–j E10, k–q: E11. a, f and k Photomicrographs of lateral views of whole mount Shh in situ hybridized specimens. b, g and l Still shots taken from movies of the externally visible patterns of 3D computer reconstructions following optical projection tomography (OPT) scanning (vr views). Note the enhanced visualization of the expression patterns, especially of more deeply stained tissue, such as the foregut and lung buds. c, h and n Virtual sections taken through the computer reconstructions in coronal planes. m A virtual section in a sagittal plane. d, e, i, j, o, p, q Transverse virtual sections taken in the planes indicated by lines on (c), (h) and (n). Arrowheads indicate foregut expression and arrows indicate respiratory tract expression. The arrowheads in (d) and (e) highlight the ventral restriction of Shh expression in the foregut. The arrow in (g) indicates the beginning of division of the respiratory groove from the foregut. Arrows in (h) and (j) indicate Shh expression in the lung buds. Arrowheads in (i) indicate ventral expression in the anterior foregut. m The expression of Shh in the division of trachea from foregut is visible (arrow). In (o)–(q), the restriction of Shh expression to ventral endoderm in the laryngotracheal groove (arrowhead in (o)), extending into the dorsal territory in more caudal sections (arrowhead in (p)) and lung buds (arrows in (q)) is visible. Scale bar a = 0.3 mm, f = 0.5 mm, l = 0.8 mm. a anterior, d dorsal, nt neural tube, nc notochord, h heart, fg foregut, hg hindgut, mb midbrain, fl forelimb, hl hindlimb, lb lung bud internal expression through an enlarged embryo, highlighting the need for OPT reconstruction and analysis. Expression increased in the posterior limb buds. OPT analysis showed the distribution of transcripts with respect to the division of trachea from the foregut and in the mesenchyme surrounding the lung buds (Fig. 3m–s). There 123 Pediatr Surg Int (2008) 24:3–11 7 Fig. 2 Shh expression at E12 in the dissected embryonic trunk from the first branchial arch to the base of the liver. a Photomicrograph of the raw specimen. Part of the floor plate and stomach are visibly stained. b A still from a vr movie showing the externally visible pattern in the 3D computer reconstruction of the specimen following OPT, enabling clear visualization of Shh expressions in the floor plate of the neural tube and in the endoderm of the oesophagus (arrowhead) and lung bud (arrow). c A virtual midline sagittal section through (b); Shh expression in the endoderm of the oesophagus is clearly visible. d A coronal section through (b); Shh expression in the endoderm of the lung buds is visible. e, f Transverse sections through (b) in planes indicated on (d). The OPT clearly visualized Shh expressions in the endoderm of the oesophagus (arrowhead in (e)) and lung buds (arrowhead in (f)). Scale bar a = .1.5 mm. a anterior, d dorsal, h heart, fl forelimb was still strong expression in the forming stomach (Fig. 3s). The dissected trunk region of E12 embryos (Fig. 4a), as in Fig. 2, showed little detail on superficial examination due to tissue density, but virtual sections showed expression in the splanchnic mesoderm surrounding the lung buds in a complementary pattern to the expression of Shh in the lung bud endoderm (compare Fig. 2c–f with Fig. 4c–f). Foxf1 is also now expressed in the splanchnic mesoderm around the oesophagus (Fig. 4c, f). from images of serial sections presents a number of technical problems. First of all, it is extremely labour intensive and time consuming, and, secondly, loss or distortion of the sections can cause inaccuracies in the 3D reconstruction. Another challenge for 3D imaging of embryonic events is the need to capture fluorescent dyes that are now commonly used to stain for the distribution of molecules in the embryonic tissues. Confocal microscopy can be used to image fluorescent stains with very high resolution, but only in small tissue samples several hundred microns in depth. In addition, this technique is limited to imaging only fluorescent staining and is not suitable for analysis of coloured dyes such as BCIP/NBT detection that are widely used to stain for the distribution of gene transcripts following whole mount in situ hybridization: a technique that adds very valuable information about gene activity in the context of the developing embryo [18]. Magnetic resonance imaging (MRI) can also be used for 3D imaging of internal morphology without the need for physical sectioning, but cannot capture fluorescent or colorimetric stains and is also limited in the size range it can feasibly cope with. Despite improvements to the resolution of MRI Discussion The need for 3D visualization of events in the developing embryo has previously been recognized and addressed in a number of recent studies [17–22]. Williams et.al. and Sasaki et.al. [21, 22] described tracheo-oesophageal separation during embryogenesis by compiling sequential 2D images from H & E stained sections at several stages of foregut development. They clearly demonstrated the developmental events in great detail, but reconstruction 123 8 Pediatr Surg Int (2008) 24:3–11 Fig. 3 The expressions of Foxf1 at E9–E11. a–e E9, f–k E10, l–s E11. a, f, l Photomicrographs of lateral views of whole mount Foxf1 in situ hybridized specimens. b, g, m Still shots taken from movies of the externally visible patterns of 3D computer reconstructions following optical projection tomography (OPT) scanning (vr views). c, h, o, p Virtual sections taken through the computer reconstructions in coronal planes. n A virtual section in a sagittal plane. d, e, i, j, k, q, r, s Transverse virtual sections taken in the planes indicated by lines on (c), (h), (o) and (p). Arrowheads indicate foregut expression and arrows indicate respiratory tract expression. The arrowheads in (d) and (e) highlight Foxf1 expression in the mesenchyme of the foregut. Arrows in (j) indicate Foxf1 expression in the mesenchyme around the lung buds. Arrowhead in (k) indicates Foxf1 expression in mesenchyme of the foregut where the stomach is developing. q–s The expression of Foxf1 to mesenchyme surrounding the laryngotracheal groove (arrowhead in (p)), extending into the mesenchyme around lung buds in more caudal sections (arrows in (s)). Scale bar a = 0.3 mm, f = 0.5 mm, l = 0.8 mm. a anterior, d dorsal, nt neural tube, nc notochord, h heart, fg foregut, hg hindgut, mb midbrain, fl forelimb, hl hindlimb, lb lung bud where it is possible to image specimens in the size range of mammalian embryos, it requires very special magnets that are extremely expensive and not widely available [19]. OPT is a relatively new imaging technique that is custom built to visualize specimens in the size range (0.5–10 mm) of mammalian embryos with high resolution, revealing details of developing organs within [20]. The technique is non-invasive (does not require sectioning), avoiding the problem of section loss and distortion, allows capture of coloured or fluorescent stained tissue to show gene 123 expression patterns and the distribution of other molecules within the context of the rapidly changing morphology of the developing embryo. OPT fills an important imaging gap between confocal microscopy and MRI, and gives important information on the distribution of developmentally active molecules. In this study, we show the use of OPT to visualize the distribution of the transcripts of two genes, Shh and Foxf1, which are prime candidates for involvement in the congenital malformations associated with OA/ TOF, across stages of development when malformations Pediatr Surg Int (2008) 24:3–11 9 Fig. 4 Foxf1 expression at E12 in the dissected embryonic trunk from the first branchial arch to the base of the liver. a Photomicrograph of the raw specimen. Part of the floor plate and lung are visibly stained. b A still from a vr movie showing the externally visible pattern in the 3D computer reconstruction of the specimen following OPT, enabling clear visualization of Foxf1 expressions in the floor plate of the neural tube and in the mesenchyme of the lung. c A virtual midline sagittal section through B; Foxf1 expression in splanchnic mesoderm around the oesophagus is clearly visible. d A coronal section through (b); Foxf1 expression in splanchnic mesoderm around the lung buds is visible. e, f Transverse sections through (b) in planes indicated on (d); the OPT clearly visualized Foxf1 expressions in the splanchnic mesoderm around the lung buds (arrowhead in (e), (f)) and oesophagus (arrow in (f)). Scale bar a = .1.5 mm. a anterior, d dorsal, h heart, fl forelimb occur in the mouse model AMM. This illustrates the value of our current approach in analysing the molecular and morphological alterations that occur in this mouse model. This approach depends on the coupled techniques of whole mount in situ hybridization (WISH) and OPT imaging. One limitation is the difficulty of extending the study to later stages of development, due to reduced penetration of the molecular probes and staining components involved in WISH. Here, we show successful staining and visualization of deep staining by combining with OPT scanning up to E12. It is as yet unknown how much more we can extend the method by further dissection of the tissue. This is currently under investigation. In normal development, the respiratory and digestive tubes are derived by division of the foregut primordium that is surrounded by splanchnic mesoderm [23]. In mice, the bronchi are the first respiratory structures to be generated as bulges on the ventrolateral wall of the foregut at E 9.5. At E10, the laryngo-tracheal groove gives rise to the trachea, which connects to the left and right main bronchi. The lung buds are located on the ventral aspect of the gastric dilatation of the foregut. The tracheal and bronchial stalks extend to form the main bronchi, a process completed by approximately E10.5. Stereotypic branching and budding occurs as the bronchial and bronchior tubules form between approximately E11 and E16 [24]. But the embryological development of the foregut into a respiratory and a digestive part is a complicated process, of which many aspects have not yet been clarified, particularly the factors that trigger the normal progression of events described above. In OA/TOF conditions, it is thought that a combination of genetic and environmental factors plays a role in the aetiology of foregut anomalies. Some morphological changes, such as branching of the notochord, have been noted in the Adriamycin treated model [25]. The notochord therefore may play a role in guiding normal development of the foregut in this respect. It will therefore be of great interest to visualize any alterations to the morphological progression of events as well as of expression patterns of prime candidate genes in the mouse model. 123 10 Shh plays multiple crucial roles as a signalling molecule during development in both invertebrate and vertebrate species. The localized expression of Shh plays a role in the morphogenesis of various epithelial structures such as hair, teeth, the respiratory system and the gut [26]. Specifically, in the respiratory system, Shh expression in the respiratory primordium from E9.5 in the mouse has an important role in lung branching [27]. In the foregut, the dorso-ventral restriction of Shh expression from E10 to E12 has previously been reported by several researchers [8, 28, 30], and mice in which the Shh gene has been inactivated display abnormal foregut morphogenesis resulting in oesophageal atresia, failed separation of the trachea and oesophagus, and lungs that arise from a single tracheo-oesophageal tube [8]. This indicates the important role of Shh in normal gut and respiratory tract development and the importance of Shh as a candidate gene for involvement in OA/TOF malformations. Ioannides et al. investigated the involvement of Shh in malformations of the trachea and oesophagus in the AMM. They revealed a lack of dorsoventral patterning in the foregut of the AMM, and suggest that the disturbance of this pattern may underline abnormal organogenesis [30]. Williams et.al. [21] showed the morphological changes in the notochord in the AMM and suggested that the proximity of the notochord to the foregut or its associated mesenchyme may be key to the induction of abnormalities. Arsic et al. [29] further suggested that a possible consequence of the abnormal proximity of the notochord to the foregut in Adriamycin treated embryos is that the foregut expression of Shh may be repressed by signals from the notochord. Shh is therefore a prime and topical candidate to be examined in this model. Our preliminary work presented here captures the complete 3D distribution of Shh transcripts during normal development, showing important time-dependent spatial changes in the pattern. In particular, it captured the details of dorso-ventral restriction in the foregut and enabled us to visualize the expression of Shh in the developing respiratory system, especially as the primordia separate from the foregut. This is a framework for the comparison of Adriamycin and control treated embryos in an ongoing work. Foxf1, previously known as Hfh8 or FREAC1, is a member of the forkhead family that is involved in foregut development. Foxf1-null mutant mice die in utero before embryonic day 10, due to extra-embryonic mesoderm defects. A study by Mahlapuu et al. [15] showed that heterozygous mice have a high perinatal mortality and exhibit multiple defects in foregut-derived structures, including lung hypoplasia, lung immaturity and lobulation defects as well as OA/TOF. Similar anomalies were also demonstrated in mice lacking the Shh gene and it has been suggested that Foxf1 acts downstream in the Shh pathway [15]. Hence, Foxf1 plays an important role in the development of the 123 Pediatr Surg Int (2008) 24:3–11 foregut. The expression of Foxf1 in the developing embryo is less comprehensively described in literature then that of Shh. Expression of Foxf1 has been reported in the splanchnic mesoderm surrounding the gut endoderm at E9.5, in the lung diverticulum, developing intestine and allantois at E10.5, and in the developing lung, stomach, intestine and intersomitic arteries at E12 [16]. In our study, OPT analysis enabled us to not only represent the patterns in 3D, but to add significant details to the known expression patterns. In the lung bud comparison with Shh expression (compare Figs. 1 and 3 and 2 and 4) highlighted the complementarity of the two patterns, supporting the idea of Foxf1 responding downstream of Shh signalling. In the developing digestive tract, particularly strong expression in the developing stomach was noted for the first time. These results support the use of WISH coupled with OPT imaging as a valuable tool for revealing gene expression in an anatomical context, even in internal tissues like the foregut and lung buds across stages of development, at least until E12. This provides the possibility of visualizing altered gene expression in an in vivo context in genetic or teratogenic models of congenital malformations. References 1. Diez-Pardo JA, Baoquan Q, Navarro C, Tovar JA (1996) A new rodent experimental model of esophageal atresia and tracheoesophageal fistula: preliminary report. J Pediatr Surg 31:498–502 2. Merei JM, Farmer P, Hasthorpe S, Qi BQ, Beasley SW, Myers NA, Hutson JM (1997) Timing and embryology of esophageal atresia and tracheo-esophageal fistula. Anat Rec 249:240–248 3. Possogel AK, Diez-Pardo JA, Morales C, Navarro C, Tovar JA (1998) Embryology of esophageal atresia in the Adriamycin rat model. J Pediatr Surg 33:606–612 4. Crisera CA, Connelly PR, Marmureanu AR, Colen KL, Rose MI, Li M, Longaker MT, Gittes GK (1999) Esophageal atresia with tracheoesophageal fistula: suggested mechanism in faulty organogenesis. J Pediatr Surg 34:204–208 5. Dawrant MJ, Giles S, Bannigan J, Puri P (2007) Adriamycin mouse model: a variable but reproducible model of tracheooesophageal malformations. Pediatr Surg Int 23:469–472 6. Dawrant MJ, Giles S, Bannigan J, Puri P (2007) Abnormal separation of the respiratory primordium in the adriamycin mouse model of tracheoesophageal malformations. J Pediatr Surg 42:375–380 7. Johnson RL, Riddle RD, Laufer E, Tabin C (1994) Sonic hedgehog: a key mediator of anterior–posterior patterning of the limb and dorso-ventral patterning of axial embryonic structures. Biochem Soc Trans 22:569–574 8. Litingtung Y, Lei L, Westphal H, Chiang C (1998) Sonic hedgehog is essential to foregut development. Nat Genet 20:58–61 9. van Tuyl M, Post M (2000) From fruitflies to mammals: mechanisms of signaling via the Sonic hedgehog pathway in lung development. Respir Res 1:30–35 10. Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383:407–413 Pediatr Surg Int (2008) 24:3–11 11. Arsic D, Keenan J, Quan QB, Beasley S (2003) Differences in the levels of Sonic hedgehog protein during early foregut development caused by exposure to Adriamycin give clues to the role of the Shh gene in oesophageal atresia. Pediatr Surg Int 19:463–466 12. Gillick J, Mooney E, Giles S, Bannigan J, Puri P (2003) Notochord anomalies in the adriamycin rat model: a morphologic and molecular basis for the VACTERL association. J Pediatr Surg 38:469–473 13. Costa RH, Kalinichenko VV, Lim L (2001) Transcription factors in mouse lung development and function. Am J Physiol Lung Cell Mol Physiol 280:L823–L838. Review 14. Mahlapuu M, Ormestad M, Enerback S, Carlsson P (2001) The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 128:155–166 15. Mahlapuu M, Enerback S, Carlsson P (2001) Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 128:2397– 2406 16. Lim L, Kalinichenko VV, Whitsett JA, Costa RH (2002) Fusion of lung lobes and vessels in mouse embryos heterozygous for the forkhead box f1 targeted allele. Am J Physiol Lung Cell Mol Physiol 282:L1012–L1022 17. Weninger WJ, Mohun T (2002) Phenotyping transgenic embryos: a rapid 3-D screening method based on episcopic fluorescence image capturing. Nat Genet 30:59–65 18. Potter SM, Pine J, Fraser SE (1996) Intravital imaging of green fluorescent protein using two-photon laser-scanning microscopy. Gene 173:25–31 19. Jacobs RE, Fraser SE (1994) Magnetic resonance microscopy of embryonic cell lineages and movements. Science 263:681–684 20. Sharpe J, Ahlgren U, Perry P, Hill B, Ross A, Hecksher-Sorensen J, Baldock R, Davidson D (2002) Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296:541–545 11 21. Williams AK, Quan QB, Beasley SW (2003) Three-dimensional imaging clarifies the process of tracheoesophageal separation in the rat. J Pediatr Surg 38:173–177 22. Sasaki T, Kusafuka T, Okada A (2001) Analysis of the development of normal foregut and tracheoesophageal fistula in an adriamycin rat model using three-dimensional image reconstruction. Surg Today 31:133–139 23. Merei JM, Hutson JM (2002) Embryogenesis of tracheo-esophageal anomalies: a review. Pediatr Surg Int 18:319–326 24. Maeda Y, Dave V, Whitsett JA (2007) Transcriptional control of lung morphogenesis. Physiol Rev 87:219–244 25. Williams AK, Qi BQ, Beasley SW (2001) Demonstration of abnormal notochord development by three-dimensional reconstructive imaging in the rat model of esophageal atresia. Pediatr Surg Int 17:21–24 26. Chuong CM, Patel N, Lin J, Jung HS, Widelitz RB (2000) Sonic hedgehog signaling pathway in vertebrate epithelial appendage morphogenesis: perspectives in development and evolution. Cell Mol Life Sci 57:1672–1681 27. Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, Mailleux A, Tefft D, Unbekandt M, Wang K, Shi W (2005) Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res 57:26R–37R 28. Ioannides AS, Henderson DJ, Spitz L, Copp AJ (2003) Role of Sonic hedgehog in the development of the trachea and oesophagus. J Pediatr Surg 38:29–36 29. Arsic D, Cameron V, Ellmers L, Quan QB, Keenan J, Beasley S (2004) Adriamycin disruption of the Shh-Gli pathway is associated with abnormalities of foregut development. J Pediatr Surg 39:1747–1753 30. Ioannides AS, Chaudhry B, Henderson DJ, Spitz L, Copp AJ (2002) Dorsoventral patterning in oesophageal atresia with tracheo-oesophageal fistula: evidence from a new mouse model. J Pediatr Surg 37:185–191 123