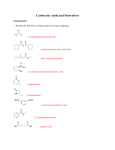
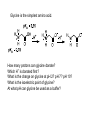* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Objective Reaction Type Structural Feature How to figure out how reactants react?
Bottromycin wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Elias James Corey wikipedia , lookup
George S. Hammond wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hydroformylation wikipedia , lookup
Petasis reaction wikipedia , lookup
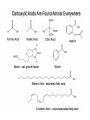
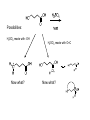
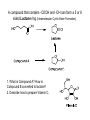
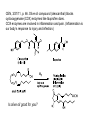
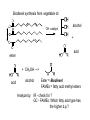
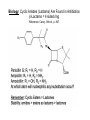
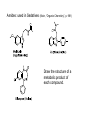
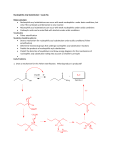
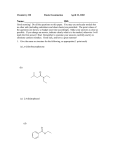
Objective: Determine products of a reaction How to figure out how reactants react? Reaction Type Structural Feature H+ transfer Addition electrophilic addition pi bond nucleophilic addition carbonyl C Acidic H+ Objective: Determine products of a reaction How to figure out how reactants react? Reaction Type Elimination Structural Feature H bonded to β-C; LG Substitution nucleophilic substitution LG bonded to α-C electrophilic aromatic substitution aromatic pi bond nucleophilic acyl substitution carbonyl C; LG Acids are Prepared from Different Functional Groups Identify the reaction conditions for each reaction. HBr HCN NaBH4 KMnO4 O3 CO2 Carboxylic Acids are Weak Acids The acid strength depends on the group bonded to the carbonyl carbon. Rank the acids from strongest to weakest: (i) (ii) (iii) A>B>C>D B>C>A>D D>A>C>B Objective: Determine the charge of acid based on pH Every Acid (HA) has a Conjugate Base (A-): HA <==> H+ + A- Biology: pH determines the form (acid or conjugate base) and charge. See pKa and a titration curve. [A-] _______ Henderson-Hasselback equation: pH = pKa + log [HA] Weak Acids Can be used in Buffers Objective: Determine the charge of acid based on pH What is the charge on acetic acid at pH 3? What is the charge at pH = 9? pH 4.7 Volume of base Glycine is the simplest amino acid. How many protons can glycine donate? Which H+ is donated first? What is the charge on glycine at pH 2? pH 7? pH 10? What is the isoelectric point of glycine? At what pH can glycine be used as a buffer? Glycine titration curve pH 9.78 2.35 Volume of base Carbonyl Carbon is Electrophilic ==> reacts w/ Nucleophiles. Lone pair on O is Nu:- ==> reacts with Electrophiles (H+). At which atom will the Nu:- react first? (i) Carbonyl carbon (ii) H bonded to O Which carbonyl carbon is more reactive (electrophilic)? (iii) On acid (iv) On aldehyde/ketone Objective: Predict product of reactions of acids Acids React To Form A Variety of Functional Groups Acid-Base and Nucleophilic Acyl Substitution 1. What do 4 of the 5 reactants have in common? (Nu:-, E+) What atom in the acid reacts? What is the reaction type? (acid-base, addition, elimination, substitution) What is the intermediate formed in each reaction? 2. What are acyl chlorides used for? (see Ch. 19) Objective: Describe Nu: acyl substitution mechanism Nucleophilic Acyl Substitution (Nu:- subs for OH) involves a Tetrahedral Intermediate NEED a LG! E.g., Nu:- = ROH (esterification) RCOOH + ROH -- H+ catalyst --> RCOOR + H2O Describe the mechanism of ester formation. Objective: Predict product of Nu:- acyl substitution reaction E.g., Nu:- = RNH2 (amide formation) RCOOH + RNH2 -- H+ catalyst --> RCONHR + H2O E.g., Nu:- = H:- (hydride from LiAlH4 or NaBH4) RCOOH + H- -- H+ catalyst --> RCHO or RCHOH + H2O E.g., Nu:- = Cl- (from SOCl2) RCOOH + Cl- -- H+ catalyst --> RCOCl + H2O 1. Synthesize the following compound from the indicated starting material and any necessary organic or inorganic reagents: 2. Predict the product of the reaction of pentanoic acid with each of the following reagents: a. NaOH b. Benzyl alcohol, H2SO4 (catalytic amount) c. LiAlH4, then hydrolysis 3. Predict the product: Possibilities: H2SO4 reacts with -OH Now what? H2SO4 reacts with O=C Now what? A compound that contains -COOH and -OH can form a 5 or 6 sided Lactone ring (Intramolecular Cyclic Ester Formation). 1. What is Compound A? How is Compound B converted to lactone? 2. Describe how to prepare Vitamin C. Mevalonolactone is an intermediate in the biosynthesis of terpenes and steroids. a. For (1), would you use HCN or NaCN? Give reasons. b. Suggest a synthesis of (2). Use ethylene or propylene as your source of carbon atoms and any necessary inorganic reagents. c. On standing in dilute aqueous acid, Compound A is smoothly converted to Mevalonolactone. Suggest a reasonable mechanism for this reaction. For each step, use curved arrow to show bonds breaking and forming. What other organic product is formed? Compound A Mevalonolactone Some Foods interfere with drug activity Found in grapefruit juice, which inactivates a cytochrome P450 enzyme http://cen.acs.org/articles/88/i39/ Foods-Drugs-Collide.html Reaction Roadmap: What group undergoes Nu:- acyl substitution? And what group is produced? What are β-keto acids used for? alkyne alkane alkene alkyl halide alcohol R-M epoxide acid amide w/ ROH aldehyde ketone ester Ether acetal Diols, cyanohydrins, imines 1. Synthesize the following compound from the indicated starting material and any necessary organic or inorganic reagents: 2. Predict the product: a. b. c. ID structure or reaction conditions. Which reactions involve a carbonyl carbon? Which reactions make a C-C bond? CEN, 3/21/11, p. 46. Olive oil compound (oleocanthal) blocks cyclooxygenase (COX) enzymes like Ibuprofen does. COX enzymes are involved in inflammation and pain. (Inflammation is our body’s response to injury and infection.) Is olive oil good for you? “Curbing Inflammation with Aspirin and Omega-3s” (CEN, 2/25/13, p. 29) Inflammation causes or exacerbates heart, lung, and kidney disease Resolvins serve as a signal our bodies use to start shutting down inflammation. Aspirin helps trigger “aspirin-triggered resolvin D3.” Omega-3 fatty acids are required as building blocks to create the signal. CEN, 3/14/11, p. 40. “Inflammation Stokes Cancer” Inflammation -- signal --> neutrophils and macrophages -- release --> reactive O, N, and X species -- damage --> biomolecules Oxidation of guanosine in DNA leads to 8-oxo7,8-dihydroguanosine, which can be further oxidized to form spiroiminodihydantoin and guanidinohydantoin. R = ribose. http://www.news-medical.net/news/2005/05/02/9722.aspx When highly unsaturated vegetable oils are heated at frying temperature (365oF) for extended periods--or even for half an hour--a highly toxic compound, HNE (4-hydroxytrans-2-nonenal) forms in the oil. May 2, 2005, What is the reaction type? Match the atoms in linoleic acid with HNE. Unsaturated fats, especially polyunsaturated fats, go rancid Three pathways: http://en.wikipedia.org/wiki/Rancidification 1. Microbial 2. Oxidative - the double bonds of an unsaturated fatty acid can undergo cleavage, releasing volatile aldehydes and ketones. 3. Hydrolytic - ester hydrolysis triglyceride (ester) --> ROH + 3 RCOOH Which chain is unsaturated? Polyunsaturated? Is the same acid formed when this triglyceride hydrolyzes? 1. Predict the products: 2. How are these two reactions similar? Acids and Acid Derivatives Can Be Converted From One to Another (Nu:- Acyl Substitution at carbonyl C (E+)) Reference: Virtual Textbook of Organic Chemistry http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/crbacid2.htm#react Nucleophilic Acyl Substitution Involves A Tetrahedral Intermediate How is nucleophilic acyl substitution reaction similar to a nucleophilic addition reaction of aldehydes and ketones? Where is the LG? Reference: Virtual Textbook of Organic Chemistry http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/crbacid2.htm#react Objective: Convert one acid derivative to another Identify the Reaction Conditions to Convert One Functional Group to Another (Hint: What Nu:- to use?) Many Types of Nylon for Many Uses (CEN, 2/18/13, p. 28) Carpets, fabric, food packaging, balloons Flexible tubing in autos As # of C’s increase, chemical resistance and flexibility increase, specific gravity decreases Brake and gas lines in cars Nylon is a common plastic. (See Chang, 6th ed., Fig. 22.4, p. 765) 1. Identify the functional group(s) in nylon. 2. How is nylon made? Or How is this functional group made? a. Condensation (or reverse) b. alkene addition c. alcohol oxidation Another way to make Nylon: use adipoyl chloride instead of adipic acid. Which reaction is faster? Predict the product: Hint: A is soap (Klein, “Organic Chemistry”, p. 1002) CEN, 2/14/11, p. 11 “Race To The Pump: Biofuel technologies vie to provide a sustainable supply of transportation fuels.” CEN, 2/14/11, p. 11 “Biofuel Technologies” 2nd generation 1st generation 3rd generation See also CEN, 8/15/11, p. 10 “Biofuels Policy” Biodiesel Cetane Number measures Combustion Quality of fuel during compression ignition (CEN, 6/13/11, p. 30) Fuel Cetane Number US biodiesel from soybean oil 47 Europe biodiesel from rapeseed and canola oil 54 * More efficient Difference in cetane number is due to number of C=C bonds Biodiesel synthesis from vegetable oil OH- catalyst alcohol + acid ester + CH3OH ---> acid alcohol Ester = Biodiesel FAMEs = fatty acid methyl esters Analyze by: IR - check for ? GC - FAMEs. Which fatty acid type has the higher b.p.? Prodrugs: inactive form of a drug that makes the active form in the body E.g., epinephrine is used in the treatment of glaucoma. What is the structure of epinephrine? Biology: Cyclic Amides (Lactams) Are Found in Antibiotics β-Lactams = 4 sided ring Reference: Carey, 8th ed., p. 847 β-Lactam antibiotics work by deactivating a transpeptidase enzyme, which is required for biosynthesis of bacterial cell walls. Draw the structure of the product. Amides: used in Sedatives (Klein, “Organic Chemistry”, p. 981) Draw the structure of a metabolic product of each compound. Sprayed by a Skunk! Should I use tomato juice or hydrogen peroxide? 1. The compound shown was subjected to the following series of reactions to give a product having the molecular formula, C9H9ClO3. What is this product? 2. Write a structural formula for the principle product or products of the following reaction: propanoyl chloride and sodium propanate Carey, 8th ed. Problem 19.40 Ambrettolide (C16H28O2) has a musk-like odor and is obtained from hibiscus. Its synthesis consists of 7 steps: Step Reactant Reagents Product 1 A H2O, H+, heat C16H32O5 (B) 2 B HBr C16H29Br3O2 (C) 3 C Ethanol, H2SO4 C18H33Br3O2 (D) 4 D Zinc, ethanol (converts vicinal C18H33BrO2 (E) dibromides to alkenes) 5 E NaCH3COO, CH3COOH C20H36O4 (F) 6 F KOH, C2H5OH, then H+ C16H30O3 (G) 7 G Heat Ambrettolide Determine the structures of Compounds B through G. Objective: predict reactions of nitriles Carey, 8th ed., Problem 19.29t, u, and v Klein, Ch. 21.50e and h, 65e Reaction Roadmap: Acid derivatives: acyl chlorides, anhydrides, esters, amides What group makes nitriles? What group is made from nitriles? alkyne alkane alkene alkyl halide alcohol R-M epoxide acid w/ ROH aldehyde ketone ester amide Ether acetal Diols, cyanohydrins, imines Knowing Reactive Sites of a Functional Group Helps ID Product How are the compounds in the top row similar? What reaction type do these compounds undergo? How are the compounds in the bottome row similar? What reaction type do these compounds undergo? Knowing How to Make a Functional Group Helps You Design a Synthesis Synthesize (convert) a compound from the top row to a compound in the bottom row. Synthesize (convert) a compound from the bottom row to a compound in the top row. 1. Predict the product in each reaction: 2. Using toluene, sodium cyanide, and carbon dioxide as the sources of carbon atoms, along with any necessary inorganic reagents, show how you would prepare the following: Benzoyl chloride Acid or Acid Derivative + Nu:- --> reaction occurs at carbonyl C Nu:- = H- (from LiAlH4 or NaBH4), N (from amine), O (from ROH), etc. Acid or Acid Derivative + HCl (or other acid) --> reaction occurs at carbonyl O Tetrahedral intermediate product 1. Compound A is a derivative of the carbohydrate perosamine, which is found in the antibiotic perimycin. When A is treated with excess acetic anhydride in methanol, a mono-acyl derivative B (C9H17NO5) in 73% yield. Draw the structure of B. (Hint: consider that methanol reacts with acetic anhydride. 2. Using toluene, sodium cyanide, and carbon dioxide as the sources of carbon atoms, along with any necessary inorganic reagents, show how you would prepare the following: phenylacetic acid Anatomy of a tear gas (http://pubs.acs.org/cgi-bin/bottomframe.cgi?7751gov1box) Police in Seattle released copious quantities of tear gas in their efforts to disperse demonstrators at the World Trade Organization meeting. The tear gas they used contained o-chlorobenzylidene malononitrile, according to a Seattle police spokeswoman. The substance is called CS after the two men who developed it in 1928, Ben B. Corson and Roger W. Stoughton. CS, a white crystalline solid at room temperature, is classified as an "antiriot agent" by the U.S. military. Law enforcement agencies in the U.S. began using CS tear gas in the 1960s, and now police around the world use it. U.S. sales of CS powder and munitions containing it, such as grenades and projectiles, are limited to law enforcement agencies. However, small aerosol cans containing a formulation of CS are available to the U.S. public. According to the Army, exposure to CS causes pain and burning in the eyes and skin within seconds. The chemical is an alkylating agent that reacts readily at nucleophilic sites, and sulfhydryl-containing enzymes such as those found in the eye are a prime target, the Army says. Effects of CS exposure include an extreme burning sensation in the eyes with a copious flow of tears, coughing, sneezing, a perception of chest tightness, and dizziness. Most of these effects subside within 30 minutes of exposure. High concentrations may trigger nausea and vomiting. A study by the National Toxicology Program found no evidence of carcinogenic activity in mice and rats exposed to CS for up to two years.