* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download NaBH4 Reduction of Vanillin
Marcus theory wikipedia , lookup
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Discodermolide wikipedia , lookup
Ene reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Stille reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Petasis reaction wikipedia , lookup
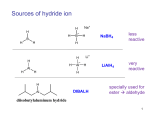
CH421 Experiment 1 NaBH4 Reduction of Vanillin Reading: Organic Chemistry by John McMurry, 8e, Chapter Section 17.4 Techniques: thin layer chromatography, melting point, IR, NMR Introduction The reduction of carbonyl compounds is an important synthetic method for the generation of alcohols. Compounds one oxidation level above alcohols, such as aldehydes and ketones, can be reduced by a variety of reagents to yield the corresponding alcohols. In general, most synthetic chemists employ one of two reagents for this transformation: sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4 or LAH). While either of these reagents can be employed for the reduction of aldehydes and ketones, there are vast differences between the reactivites of the LAH and NaBH4. Sodium borohydride is a rather mild reducing agent, reducing aldehydes and ketones selectively in the presence of more highly oxidized functional groups. LAH, on the other hand, is highly reactive and will readily reduce not only aldehydes and ketones, but esters, carboxylic acids, amides, acyl chlorides, and nitriles as well, with almost no selectivity. Aside from a lack of selectivity, LAH reacts violently with water and other hydroxylic compounds, and reductions using this reagent must be carried out under non‐protic, anhydrous conditions. This not only limits the solvents with which LAH can be used, but it presents greater challenges in the safe handling of LAH. Sodium borohydride, on the other hand, reacts only slowly with water and alcohols and can be used in a wide range of solvents, including water and alcohols, without consequence. It should also be noted that when using water as solvent, NaBH4 is relatively stable at pH 10 or higher. However, if a weakly acidic proton is present (i.e. a carboxylic acid or even a phenol), NaBH4 will react with the acid, which would thus destroy your reagent. The reduction reaction is usually exothermic, but only mildly, and can be easily controlled using a cooling bath of ice water. Taken together, NaBH4 is much safer to handle than is LAH, making it a safe and less expensive choice whenever the functional group to be reduced is an aldehyde or ketone. In a protic solvent (such as water or an alcohol), NaBH4 ionizes to form Na+ and –BH4. The negatively charged borohydride complex, –BH4, reacts with the carbonyl as illustrated below. The alcohol (in this example, the alcohol is ethanol, EtOH) facilitates the reduction by hydrogen bonding interactions between the carbonyl oxygen and the acidic hydroxyl group of the alcohol. Experiment 1: NaBH4 Reduction of Vanillin The borate, –BH3‐OEt, formed from the Lewis acid‐base reaction of –OEt and BH3, has three remaining B—H bonds, each of which, in turn, reduces another molecule of the starting ketone, as shown below. Note that the theoretical stoichiometry of the overall reaction is the following: one (1) mole of NaBH4 reduces four (4) moles of aldehyde or ketone. Thus, this should require 0.25 equivalents of NaBH4 for every 1.0 equivalents of aldehyde or ketone. In practice, however, we typically add at least 0.5 or even 1.0 equivalent of NaBH4 due to the fact that the reagent does slowly react with the protic solvent that is typically used (i.e. water or an alcohol). At the end of the reaction, any excess NaBH4 must be destroyed, or quenched, prior to the reaction workup and product isolation. In the following procedure, dilute, aqueous HCl is added to quench any unreacted NaBH4. In today’s experiment, sodium borohydride will be used to reduce the aldehyde in vanillin (500 mg scale) to the corresponding primary alcohol, vanillyl alcohol. Note that we will run the reaction in 1 M NaOH. We will monitor the reaction progress by TLC, and once the reaction is complete, we will quench the excess NaBH4 with dilute HCl. The reaction stoichiometry (as reflected in the “equiv” column) that we will use is shown in the table below. The IR and 1H NMR of vanillin are shown below after the Conclusion section. It will be helpful to assign the major peaks in the IR and all peaks in the 1H NMR. Check your assignments with your instructor. Name formula MW (g/mol) vanillin 500. 1.0 _____ ___ ___ NaBH4 _____ 0.50 _____ ___ ___ ___ 1M NaOH _____ 1M aq. solution _____ 1.2 _____ 4.0 ___ ___ Theor. mass = _____ _____ ___ Lit. mp ___ vanillyl alcohol mass (mg) mmol equiv d (g/mL) vol (mL) mp (°C) bp (°C) Experiment 1: NaBH4 Reduction of Vanillin Pre‐Lab Notebook (to be completed prior to the start of lab) Enter date, experiment number and title on notebook page and in TOC with corresponding page number. Copy the reaction and the reaction table above (in your own writing) into your notebook and complete the table. Make sure to check your calculations with your instructor before starting the experiment. Find the molecular formulas, molar masses, and melting points for vanillin and vanillyl alcohol, and include that data in your data table. Safety Precautions Sodium borohydride is corrosive and particularly toxic if ingested. Wear gloves and handle it with care. If any comes in contact with your skin, rinse the affected area thoroughly with water. Ethyl acetate, hexanes, and sodium borohydride are flammable. Procedure Weigh out ca. 0.5 g of vanillin, transfer to the 25‐mL Erlenmeyer flask, and add a stir bar (NOTE: always record the actual mass that you measured to the precision of your balance). Add 1.2 equivalents of 1 M NaOH and stir the solution until the vanillin is dissolved. Cool the reaction mixture in an ice bath with stirring, and then added 0.5 equiv. of solid NaBH4 slowly in three (3) portions (NOTE: NaBH4 reacts exothermically; therefore it is added slowly in order to keep the mixture from getting too hot). When all of the sodium borohydride has been added, stir for 5 minutes; then remove the ice bath from the flask and continue stirring at room temperature. While the reaction mixture is stirring, determine the best TLC mobile phase in order to monitor your reaction. Think about whether or not the product is expected to be more or less polar than vanillin. You want the spots to be sufficiently off the baseline to achieve separation but not all the way at the top with the solvent front. Prepare your TLC plate as follows: spot a solution of the vanillin starting material in ethyl acetate and develop the TLC plate in different mobile phases (try at least two different mobile phase combinations – one more polar than the other). Show your results to your instructor before moving on. After the reaction mixture has stirred at room temperature for 15‐20 minutes, check the reaction progress by TLC. Prepare your TLC plate with 3 tick marks at the baseline and spot as follows: spot the left lane with just your vanillin starting material solution; spot the middle “co‐spot” lane with your vanillin solution and then with your reaction mixture (NOTE: you can obtain an aliquot from the reaction mixture by dipping the tip of a disposable Pasteur pipet o the reaction, and then dip your micro‐capillary spotter into the Pasteur pipet tip); and then spot the right lane with just your reaction mixture. Develop the TLC plate in the optimized mobile phase that you determined above and ascertain the extent of reaction. Show your results to your instructor. If your reaction still has starting material, cool it back down in the ice bath, and then add another 0.25 equivalents of NaBH4 in two (2) portions. Once the addition is complete, warm the reaction mixture back to room temperature and stir for another 10 minutes, after which time check the progress again by TLC. Repeat this process as necessary. Once your reaction is complete as judged by TLC, cool the reaction mixture in an ice bath and very slowly add chilled 3 M HCl dropwise by pipet. Continue adding the HCl solution until H2 gas no longer evolves, and check the pH with pH paper to ensure that the reaction mixture is acidic. Collect the resulting solids by vacuum filtration with a Buchner funnel and rinse with two small portions of ice cold water. Pull air through the funnel for at least 10‐15 minutes, and be sure to break up the solids so that they fully dry. Experiment 1: NaBH4 Reduction of Vanillin Data (collect and record in your notebook during the lab) Describe product and calculate % yield Obtain melting point and list with literature value TLC data (draw in TLC plate(s), list mobile phase, and show all Rf calculations) Obtain IR (solid or thin film from CH2Cl2 solution), staple in notebook, and assign major functional group peaks Obtain 1H NMR (CDCl3) and assign all peaks in the spectrum NOTE: you may need to obtain the IR and/or 1H NMR spectra the following week if your product isn’t fully dry Conclusion (record in your notebook) State outcome, yield, proof of success and purity, possible sources of error, and any other information deemed necessary and relevant IR of vanillin (thin film on KBr disc) 1H NMR of vanillin (90 MHz in CDCl3)