* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry 3719L – Week 9 Reduction of Benzil with Sodium
Survey
Document related concepts
Kinetic resolution wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Discodermolide wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Stille reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Transcript
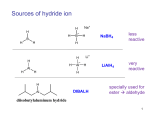
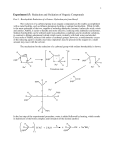
Spring 2004 Chemistry 3719L – Week 9 Reduction of Benzil with Sodium Borohydride Pre-lab reading from Zubrick: Chapter 13: Chapter 12: Whole Chapter – review recrystallization Pages 87-92 – review melting points Aims The use of metal hydride reagents (e.g. NaBH4 and LiAlH4) for the reduction of aldehydes and ketones is the most widely used method for conversion to primary and secondary alcohols respectively. This experiment will give you experience in using the milder NaBH4 reagent that readily converts the diketone benzil into the corresponding diol hydrobenzoin. Reaction Benzil (1) is treated with sodium borohydride (NaBH4, 2) to give the diol hydrobenzoin (3) via a nucleophilic addition reaction. Since the NaBH4 reaction is much less basic than LiAlH4 we can run the reaction in a protic solvent such as ethanol, which also serves as the proton source to generate the final alcohol product. . OH O NaBH4 (2) O EtOH 1 OH 3 Procedure • In a 125 mL Erlenmeyer flask add benzil (1.5 g) and 95% ethanol (15 mL). Cool the flask under running water such that the benzyl forms a fine suspension in the ethanol. • Weigh out sodium borohydride (0.3 g – careful, this fine powder is dangerous if accidentally inhaled) and add this to the reaction mixture in one portion. • Swirl the flask gently for 15 minutes, making notes about any color or temperature changes. • Carefully add DI water (15 mL) and bring the mixture to a boil. If the mixture is not clear then gravity filter it whilst hot into a second Erlenmeyer flask. • Add more DI water (~30 mL) to the filtrate and allow the solid to crystallize (scratching the glass with a glass rod might help here). • Filter the solid, dry for 15 minutes then weigh the product and obtain the melting point. • If possible you should obtain an IR spectrum of your product to compare with that of the benzil starting material. This information will be used in your report to prove that the functional group conversion (i.e. ketone to alcohol) actually occurred. • You should hand the product to your TA in a sealed, labeled test tube for grading. Synthesis Report = 10 pts Adapted from Williamson, Macroscale and Microscale Organic Experiments, Houghton Mifflin.